Hyphessobrycon frickei Guimar, 2020
|
publication ID |
https://doi.org/10.5852/ejt.2020.723.1145 |
|
publication LSID |
lsid:zoobank.org:pub:10B6A707-DDA2-4D2F-9087-394933776459 |
|
DOI |
https://doi.org/10.5281/zenodo.14370781 |
|
persistent identifier |
https://treatment.plazi.org/id/6FECEAF1-EBFC-4978-9861-E502AB12A5C5 |
|
taxon LSID |
lsid:zoobank.org:act:6FECEAF1-EBFC-4978-9861-E502AB12A5C5 |
|
treatment provided by |
Valdenar |
|
scientific name |
Hyphessobrycon frickei Guimar |
| status |
sp. nov. |
Hyphessobrycon frickei Guimar ães, Brito, Bragança, Katz & Ottoni sp. nov.
urn:lsid:zoobank.org:act:6FECEAF1-EBFC-4978-9861-E502AB12A5C5
Fig. 1 View Fig , Table 2 View Table 2
Morphological Diagnosis (PAA)
Hyphessobrycon frickei Guimar ães, Brito, Bragança, Katz & Ottoni sp. nov. clearly differs from most of its congeners, except members of the “Rosy tetra” clade, by the presence of a dark brown or black blotch on the dorsal fin (vs absence) and the absence of a midlateral stripe on the body (vs presence). The new species differs from most of its congeners within the “Rosy tetra” clade by possessing a conspicuous small, slightly vertical and elliptical humeral spot ( Fig. 1 View Fig ) (vs inconspicuous vertically elongated) humeral spot in Hyphessobrycon bentosi Durbin, 1908 , H. caru Guimar ães, De Brito, Feitosa, Carvalho-Costa, Ottoni, 2018 , H. hasemani Fowler, 1913 , H. piorskii Guimar ães, De Brito, Feitosa, Carvalho-Costa, Ottoni, 2018 ; an approximately rounded humeral spot in H. erythrostigma (Fowler, 1943) , H. jackrobertsi Zarske, 2014 , H. minor Durbin, 1909 , H. pando Hein, 2009 , H. paepkei Zarske, 2014 , H. pyrrhonotus Burgess, 1993 , H. roseus (Géry, 1960) , H. socolofi Weitzman, 1977 , and H. sweglesi (Géry, 1961) ; a conspicuous horizontal or posteriorly elongated humeral spot in H. epicharis Weitzman & Palmer, 1997 , H. khardinae Zarske, 2008 , and H. werneri Géry & Uj, 1987 ; a large vertical conspicuous humeral spot at least on males in H. eques (Steindachner, 1882) , H. haraldschultzi Travassos, 1960 , H. micropterus (Eigenmann, 1915) , H. megalopterus (Eigenmann, 1915) , H. simulatus (Géry, 1960) and H. takasei Géry, 1964 ; and the absence of a humeral spot in H. compressus (Meek, 1904) , H. dorsalis Zarske, 2014 , H. georgettae Géry, 1961 , H. pulchripinnis Ahl, 1937 , and H. rosaceus Durbin, 1909 (see Guimar ães et al. 2019: fig. 2). The new species furthermore differs from H. copelandi Durbin, 1908 and H. haraldschultzi Travassos, 1960 by the number of scales on the lateral series (33–37 vs 29–31, 28–30 lateral line scales, respectively); and from H. geryi Guimar ães, Brito, Bragança, Katz & Ottoni sp. nov. by the number of horizontal scale rows between the lateral line and pelvic-fin origin (3 vs 4–5, modally 4). Furthermore, H. frickei sp. nov differs from H. geryi sp. nov. by the absence of conspicuous dark chromatophores on opercular zone (vs presence); the absence of conspicuous dark chromatophores on the region posterior to the humeral spot (vs presence, extending to the end of the caudal peduncle); the absence of a thin vertical line, formed by a concentration of dark chromatophores at the middle of the humeral spot, extending to one to two scales above and below the spot (vs presence); and dorsal-fin base less pigmented (vs conspicuous pigmentation on dorsal-fin base) (see Figs 1 View Fig , 4–5 View Fig View Fig ).
Molecular diagnosis (CBB)
Hyphessobrycon frickei Guimar ães, Brito, Bragança, Katz & Ottoni sp. nov. is a member of the H. copelandi clade, possessing the following 25 nucleotide substitutions: COI 90 (C+A), COI 126 (A+G), COI 138 (C+T), COI 189 (C+T), COI 192 (T+A), COI 237 (C+T), COI 264 (T+C), COI 282 (C+T), COI 285 (C+A), COI 312 (T+C), COI 384 (C+T), COI 402 (A+G), COI 429 (A+G), COI 435 (A+G), COI 486 (T+C), COI 522 (A+G), COI 525 (C+T), COI 547 (C+T), COI 582 (T+C), COI 621 (T+C), COI 624 (A+G), COI 678 (T+C), COI 684 (A+C), COI 690 (T+C), COI 696 (A+G). In addition, it differs from H. geryi sp. nov. by possessing the following nine nucleotide substitutions: COI 231 (T+C), COI 315 (A+G), COI 345 (A+C), COI 375 (G+A), COI 402 (G+A), COI 405 (T+C), COI 528 (A+G), COI 558 (A+G), COI 609 (A+G); and from A. copelandi by possessing the following eleven nucleotide substitutions: COI 126 (G+A), COI 141 (G+C), COI 291 (A+G), COI 300 (A+G), COI 345 (C+G), COI 366 (C+T), COI 435 (G+A), COI 510 (T+C), COI 633 (T+C), COI 657 (C+T), COI 672 (T+C).
Etymology
The new species is named after the ichthyologist Ronald Fricke, in recognition of his contribution to ichthyology.
Material examined
Holotype
BRAZIL • 18.8 mm SL; Maranhão State, Maracaçumé municipality, Maracaçumé River ; 2°3′14″ S, 45°57′16″ W; 29 Jan. 2017; E.C. Guimarães and P.S. Brito leg; CICCAA 02363 GoogleMaps
Paratypes
BRAZIL • 10 specs; 14.8–19.6 mm SL; Maranh ão State, Maracaçumé municipality; stream tributary to the Maracaçumé River basin; 2°08′38″ S, 45°47′22″ W; 29 Jan. 2017; Guimarães E.C., Brito P.S. leg.; CICCAA 02362 GoogleMaps • 5 specs; 15.5–17.7 mm SL (C&S); same collection data as for preceding; collected with holotype; CICCAA 02388 GoogleMaps • 1 spec.; 17.8 mm SL; same collection data as for preceding; UFRJ 6918 GoogleMaps .
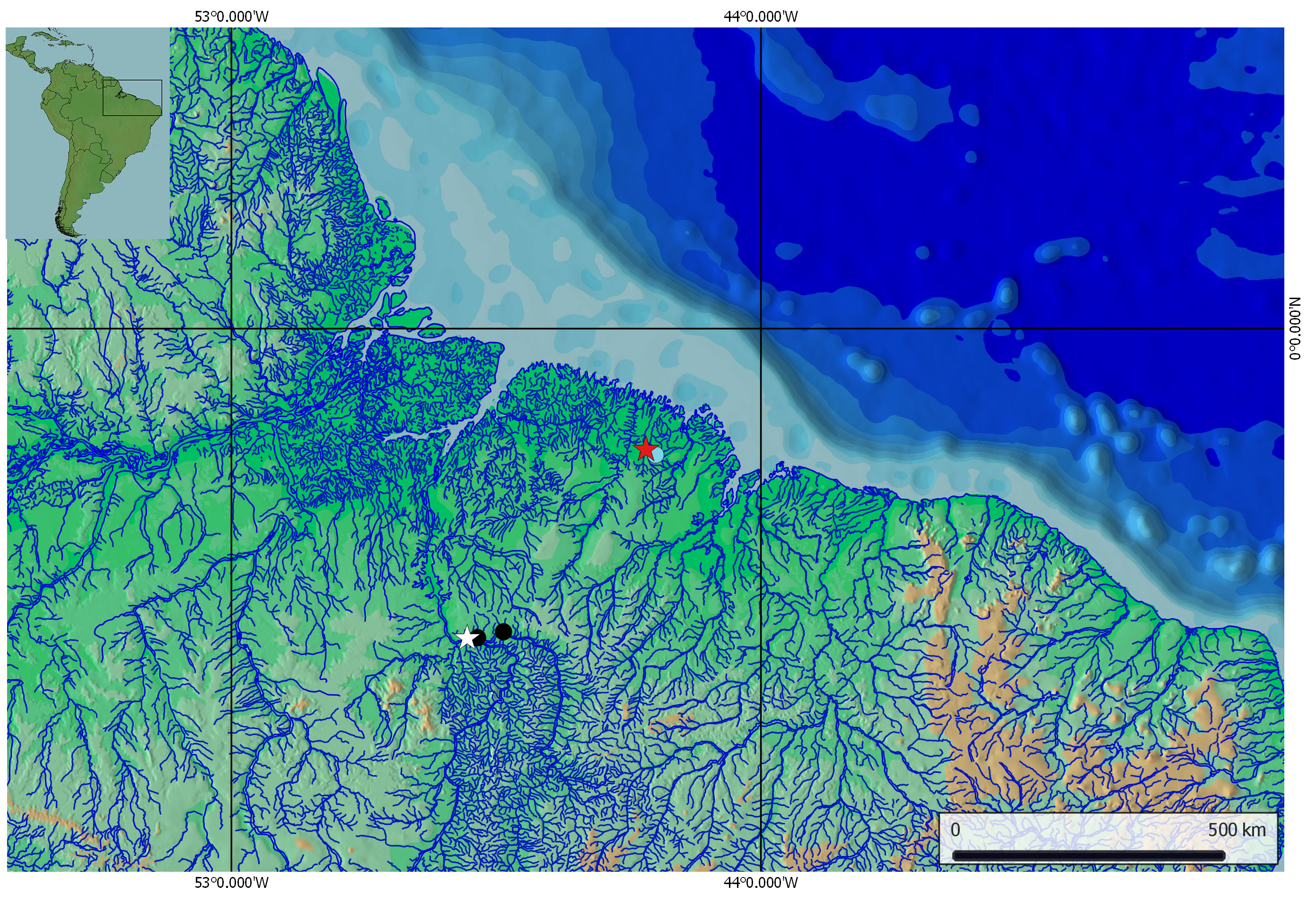
Type locality
Maracaçumé River basin, a coastal river in the Maranh ão state, northeastern Brazil ( Fig. 3 View Fig ).
Description
Morphometric data of holotype and paratypes are presented in Table 2. View Table 2
BODY. Small (larger specimen with 19.6 mm of SL), laterally compressed, moderately deep, greatest body depth slightly anterior to dorsal-fin base; body profile straight and downward directed from the end of dorsal fin to adipose fin, straight or slightly convex between latter point and origin of dorsal most procurrent caudal-fin ray; dorsal profile of head convex from upper lip to vertical through eye; predorsal profile of body roughly straight, dorsal-fin base slightly convex, posteroventrally inclined; ventral profile of head convex from lower jaw to pelvic-fin origin; straight and posterodorsally slanted along anal-fin base; and slightly concave on caudal peduncle. Jaws equal, mouth terminal. Maxilla reaching vertical to anterior margin of pupil.
TEETH. Premaxillary teeth in two rows. Outer row with two tricuspid teeth; inner row with 3(10), pentacuspid teeth and 3(7) or 2(3) tricuspid teeth. Maxilla with 2(5) or 3(6) tricuspid teeth. Dentary with five (10) larger pentacuspid teeth followed by five (8) or six (2) smaller tricuspid teeth tooth ( Fig. 2 View Fig ).
SCALES. Scales cycloid, three to eight radii strongly marked, circuli well-marked anteriorly, weakly marked posteriorly; lateral line incompletely pored, with 7(6), 8(7)*, 9(3) or 10(1) perforated scales. Longitudinal scales series including lateral-line (perforated) scales 33(1), 34(1), 35(3), 36(7)* or 37(1). Longitudinal scales rows between dorsal-fin origin and lateral line 5(1) or 6(16)*. Horizontal scale rows between lateral line and pelvic-fin origin 3(17)*. Scales in median series between tip of supraoccipital spine and dorsal-fin origin 8(3) or 9(14). Circumpeduncular scales 10(12)*, 11(3) or 12(2).
FINS. Dorsal-fin origin at midbody. Base of last dorsal-fin ray at vertical through first third of anal-fin. Dorsal-fin rays ii + 9(10)*, iii + 9(5), ii + 10(2). First dorsal fin pterygiophore main body located behind neural spine of 4 th vertebrae. Adipose fin present. Anal-fin origin aligned with vertical line through middle of dorsal-fin, between 6 th and 8 th dorsal-fin rays. Anteriormost anal-fin pterygiophore inserting posterior to haemal spine of 11 th vertebrae. Anal-fin origin aligned with vertical line through middle of dorsal fin (between base of 6 th and 8 th dorsal-fin rays). Anal fin iii + 22(10)* or iii + 23(7); anterior anal-fin margin slightly convex, with anteriormost rays more elongate and slightly more thickened than remaining rays, forming a distinct lobe. Remaining rays smaller with straight distal margin. Pectoral-fin rays 12(17)*. Tip of pectoral fin surpassing pelvic-fin base. Pelvic-fin rays 8(17)*, surpassing anal-fin origin. Caudal fin forked, upper and lower lobes similar in size. Principal caudal fin rays 11+10(10)* or 10+9(7); dorsal procurrent rays 8(2) or 9(3) and ventral procurrent rays 7(2) or 8(3).
OSTEOLOGICAL COUNTS. Branchiostegal rays 4(5). First gill arch with 1(4), 2(1) hypobranchial, 11(1), 12(3), or 13(1) ceratobranchial, 1(5) cartilage between ceratobranchial and epibranchial, and 5(1) or 6(4) epibranchial gill-rakers. Supraneurals 4(4) or 5(1). Precaudal vertebrae 11 (5) and caudal vertebrae 19 (5). Total vertebrae 30(5).
Coloration in alcohol ( Fig. 1 View Fig )
Ground coloration light yellowish brown. Humeral spot conspicuous, slightly vertically elliptical. Flank with inconspicuous chromatophores, more concentrated on dorsal and ventral regions; middle region without or with inconspicuous chromatophores. Ventral region lacking dark brown chromatophores. Dark brown chromatophores present on dorsal portion of head and tip of snout, becoming sparser or absent on opercular region, and absent on cheek. Dorsal-fin ground coloration hyaline, with conspicuous black or dark brown spot located on anterior portion of fin, reaching about 6 th ray, approximately between half to two thirds of fin depth. Dorsal-fin base with inconspicuous scattered chromatophores. Anal and caudal fins hyaline. Anal and caudal fins with a darker, usually dark brown, posterior margin. Adipose fin hyaline to light brown, with dark chromatophores at margin and posterior portion. Pectoral and pelvic fins hyaline; pelvic fin with variable amounts of dark brown pigmentation remaining depending on specimen preservation state.
Sexual dimorphism
Bony hooks on fins are absent in all examined specimens. According to Malabarba & Weitzman (2003), the presence of bony hooks is a common dimorphic feature among characid species. Although this sexual dimorphism is not observed in all characid species, as in the case of the species described here.
Color pattern is not sexually dimorphic either.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |