Botrylloides pizoni, Brunetti & Mastrototaro, 2012
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3258.1.2 |
|
persistent identifier |
https://treatment.plazi.org/id/BF3F8794-B136-BF32-FF34-FE68124CE83F |
|
treatment provided by |
Felipe |
|
scientific name |
Botrylloides pizoni |
| status |
sp. nov. |
Botrylloides pizoni View in CoL n. sp.
Etymology. The species is named after Antoine Pizon—the great French zoologist author of the fundamental work on the blastogenesis of the botryllids (1893).
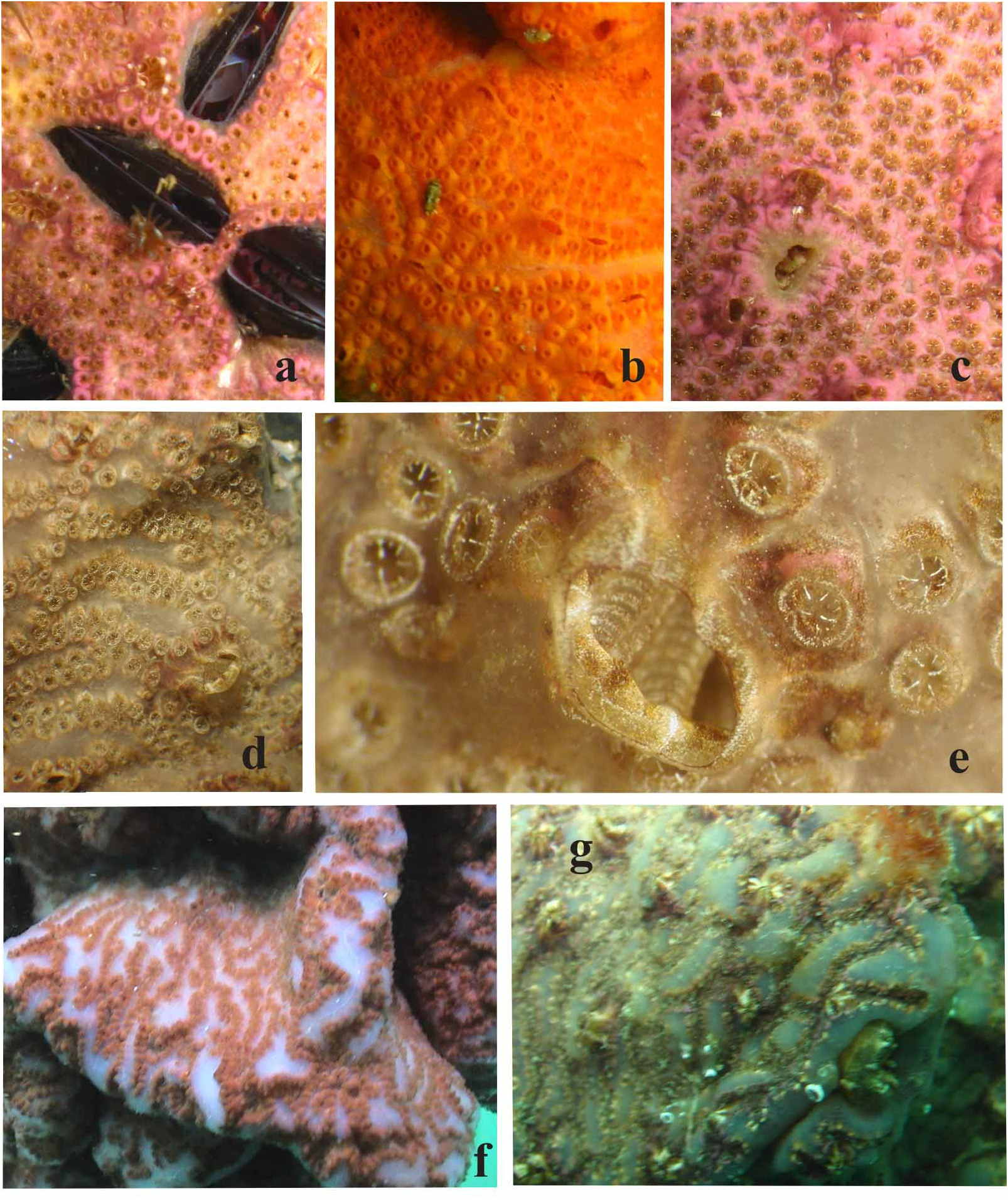
Colonies. Living specimens are mainly violet or from lilac to violet in colour, but red and orange colonies were also found ( Figs 2a–e View FIGURE 2 ). The investing colonies are often lobed, and in the eutrophic waters of the collection site; they may reach large dimensions (several square decimetres) and thicknesses of up to 2-3 cm. Zooids are arranged in “ leachii type ” systems ( Brunetti, 2009). Zooid systems are usually in close proximity ( Figs 2a–d View FIGURE 2 ) but in some colonies (here called mammillated) there are areas, between them without zooids, where the colonial test forms prominences ( Figs 2 f–g View FIGURE 2 ) (see below).
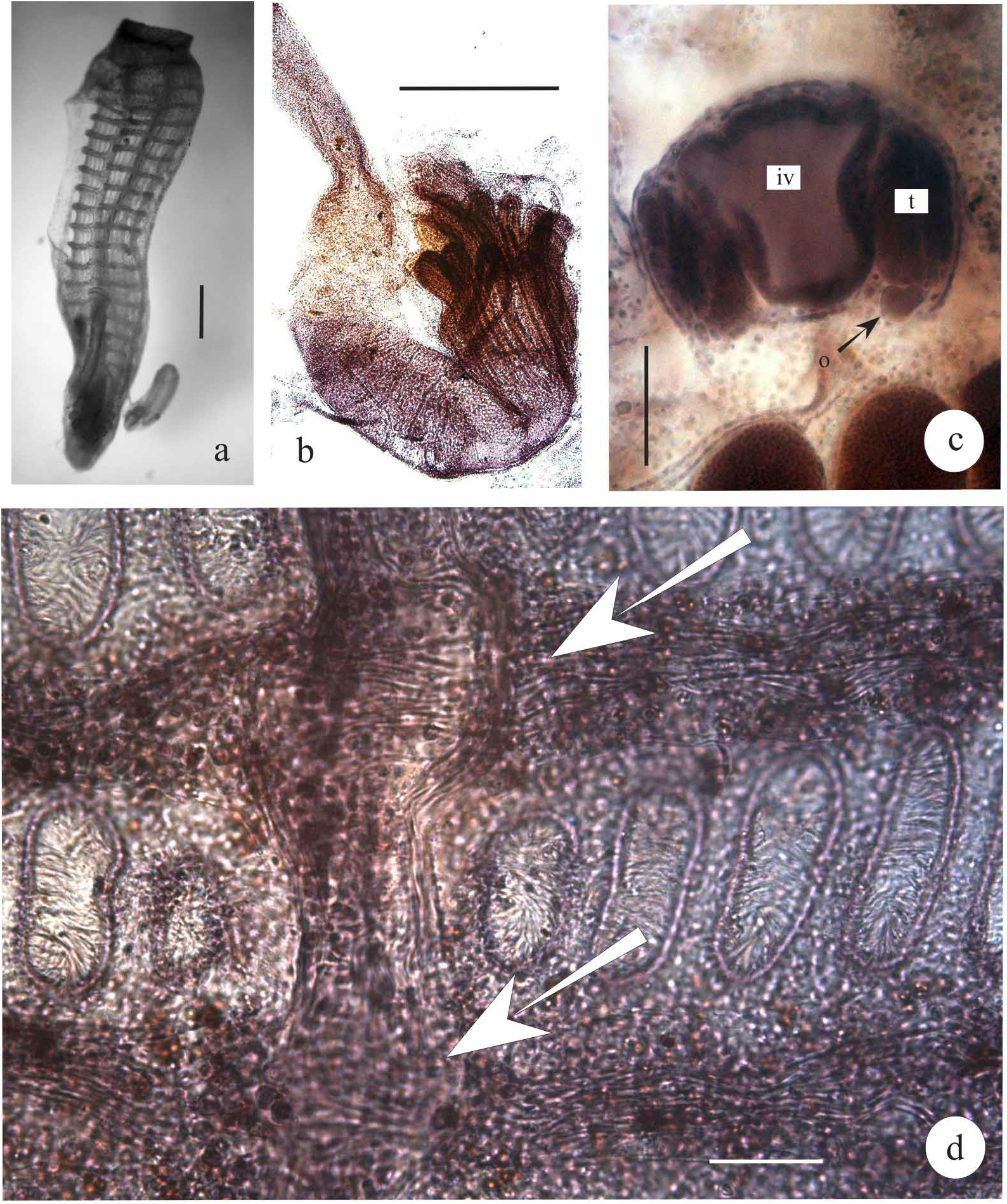
Zooids. Zooids are cylindrical in shape and up to 4 mm long ( Fig. 3a View FIGURE 3 ). The oral siphons are smooth edged; the atrial aperture is wide, exposing much of the branchial sac, as usual in animals with this type of system; however in this species, the posterior third or more of the branchial sac is not exposed, and all abdominal organs including the rectum lie inside a body wall bag which is embedded in the colonial matrix. The anterior rim of the atrial aperture extends in a more or less developed dorsal languet, according, as usual, the position of the zooid in the system.
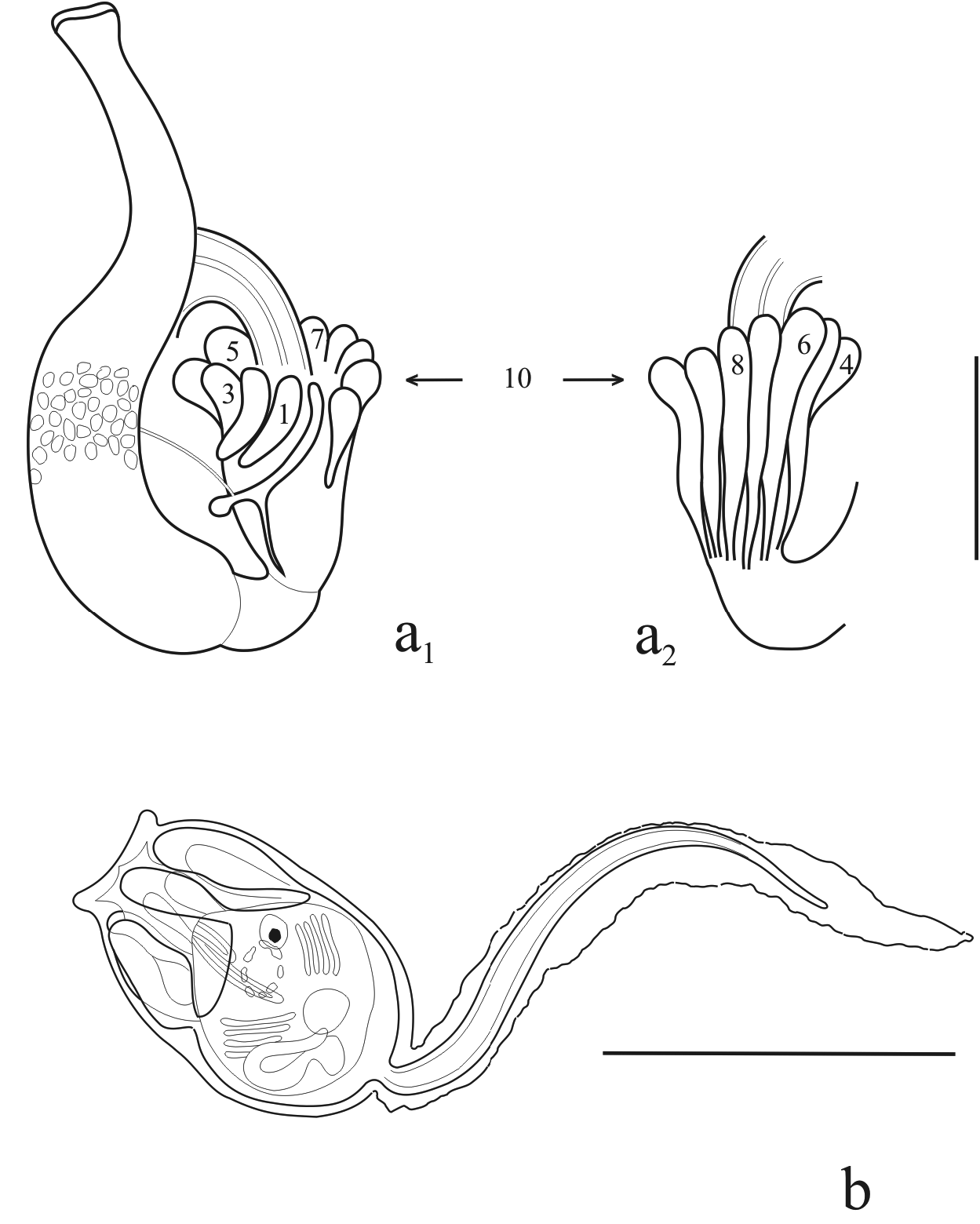
In living specimens, 4 or 5 tentacles appear to be dominant ( Fig. 2e View FIGURE 2 ). However, dissection shows that they number about 30, in 3 order of size regularly arranged according to the following scheme L,S,M,S,L (large, small, medium) ( Fig. 3d View FIGURE 3 ). They rise from a single ring and are finger-like, not differing greatly in diameter from the base to the top. The anterior third of the oral siphons, from the tentacles ring to the edge, is run through by numerous thin circular muscle fibers, the remaining posterior two-thirds have longitudinal fibers ( Fig. 3d View FIGURE 3 ). Thin longitudinal muscles fibers also descend along the whole body wall, approaching the edge of the atrial aperture and forming a band around it.
Branchial sac. The prepharyngeal ring does not form a V below the dorsal tubercle, which presents a simple, oval, vertically arranged aperture ( Fig. 3d View FIGURE 3 ). The neural gland complex is about 200 µm further down; it is oval in shape with the major longitudinal axis shorter than a stigmata. The prebranchial area is about half the height of a stigmata. There are usually 17 rows of stigmata, all complete, plus 3–4 irregular rows at the posterior end. There is a difference in length between the dorsal and endostylar sides of the branchia, because the latter 3–4 irregular rows of stigmata do not reach the dorsal side ( Fig. 3e View FIGURE 3 ). However, on the whole, the branchial sac is cylindrical in shape, the same number of stigmata appearing in every row. The longitudinal vessels are not very large and not very protuberant, with a diameter not greater than the space between two stigmata ( Fig. 3d View FIGURE 3 ). The branchial dorsal (D), lateral (L) and ventral (V) sectors are D>V>L and the stigmata are distributed in a remarkably constant manner, according to the branchial formula: L 4,2,2,5 DL 6,2,2,5 R. The transversal vessels are large, externally protuberant, and filled with pigmented blood cells. Two large muscle bands run on both sides of the dorsal vessel and the endostyle. Other muscle fibers also run along the transversal vessel partly connecting with the longitudinal fibers at the side of the dorsal vessels, and partly passing over the latter and connecting with the fibers at the sides of the endostyle. Muscle fibers also run along the internal longitudinal branchial vessels ( Figs 3d View FIGURE 3 – 5d View FIGURE 5 ).
Gut loop. The oesophagus presents four very evident longitudinal, grooves. The stomach is bell-shaped with 11 folds, excluding the typhlosolis ( Fig. 4a View FIGURE 4 1 –a 2 View FIGURE 2 ). Anteriorly, at the cardiac end, the folds extend in ampullae around the oesophagus. On the right side of the stomach, the folds are as long as the stomach itself, posteriorly thinned, not closed, and with reduced glandular epithelium ( Figs 4a 2 –5b View FIGURE 4 View FIGURE 2 View FIGURE 3 View FIGURE 5 ); on the left side (Nos 1, 2, 10, 11) they are much shorter ( Fig. 4a View FIGURE 4 1). The typhlosolis runs along the first fold and does not extend over the posterior end of the stomach. The pyloric caecum rises about half-way along its length; it is finger-like, about one-quarter the length of the stomach, straight, or only slightly bent, and with an only slightly swollen tip. Between the typhlosolis and the 11th fold, there is a smooth polygonal area ( Fig. 4a View FIGURE 4 1). The major axis of the stomach forms an angle of 45° with the longitudinal axis of the branchial sac, so that the stomach lies almost completely behind the latter and the intestine forms an extensive S-shape ( Figs 3a,b View FIGURE 3 – 4a View FIGURE 4 1). After a short pyloric tract, the intestine is larger in diameter and has a pavement epithelium with cells regularly arranged in transversal rows. The pyloric gland forms a band around the intestine, and its duct opens into the caecum half-way along its length ( Fig. 4a View FIGURE 4 1). The rectum is long, ascending along the dorsal side of the branchial sac as far as the rim of the atrial aperture ( Figs. 3a View FIGURE 3 , 5a View FIGURE 5 ). The anus is smoothedged but when closed, appears to be bilobed ( Fig. 4a View FIGURE 4 1).
Gonads. The testis and ovary lie immediately below the buds, the testis anterior to the ovary. The testis is usually formed of about 10 elongated follicles meeting in a single central deferent ( Fig. 3f View FIGURE 3 ). When the ovary is present the testis is arranged as an arch on the ovary, however when only ripe testes are present the swollen follicles give them a mulberry appearance. In filtering zooids there is only one egg per side, projecting from the body wall. The embryo develops in the same position, enclosed in a brood pouch ( Fig. 3a View FIGURE 3 ) as happens in some Botrylloides species. Sometimes only one embryo develops.
Reproduction. Reproduction takes place from spring to early winter. Like all species of Botryllinae for which the reproductive physiology is known, the present species is protandric ( Satoh, 1994). The first testis primordia and subsequently oocytes develop in buds ( Figs 3b–c View FIGURE 3 , 5c View FIGURE 5 ) but both regress during the change of generation (samples 1, 4, 11); then, if the season is in favour of sexual reproduction, the gonadic primordia of the buds ripen when the latter become filtering zooids: at first only the testis (samples 3, 6, 9, 10, 13), then also the ovary (samples 7), finally only the ovary (sample 8) whereas the testis are atrophic or absent in filtering zooids ( Fig. 3a View FIGURE 3 ). Thus during the reproductive season colonies with “male”, “hermaphroditic” or “feminine” zooids can be found. Some colonies (probably young ones) may of course have no gonads in the reproductive season (samples 2, 14). Lastly, reproduction stops in winter (samples 5, 12). The situation of samples 4 and 11 represents the transition from the reproductive to resting season.
The larvae are liberated from cloacal apertures. However, the dissection of one colony, fixed during the spawning period (sample 8), showed that larvae are discharged from the brood pouch when its external wall breaks into the colonial matrix where many larvae were found. In addition, this colony, which was fixed during the bcg physiological stage (colony immediately preceding regression of filtering zooids or change of generation), the most external layer of the matrix, which is immediately below the cuticle and surrounds the filtering zooids is mucous. Although this may be an artefact due to a fixation error, we presume it may also be a natural, although temporary, condition which makes it easier for the larvae, which leave the maternal body in a such an unusual way, to reach the cloacal channels and thence leave it.
Swimming larvae are about 1.8 mm long (without fins), of which the trunk is about 600 µm. It has 8 ampullae and 3 adhesive papillae, like almost all species of the subfamily. When near to settling in the trunk, the structures of the future oozooids are visible ( Fig. 4b View FIGURE 4 ).
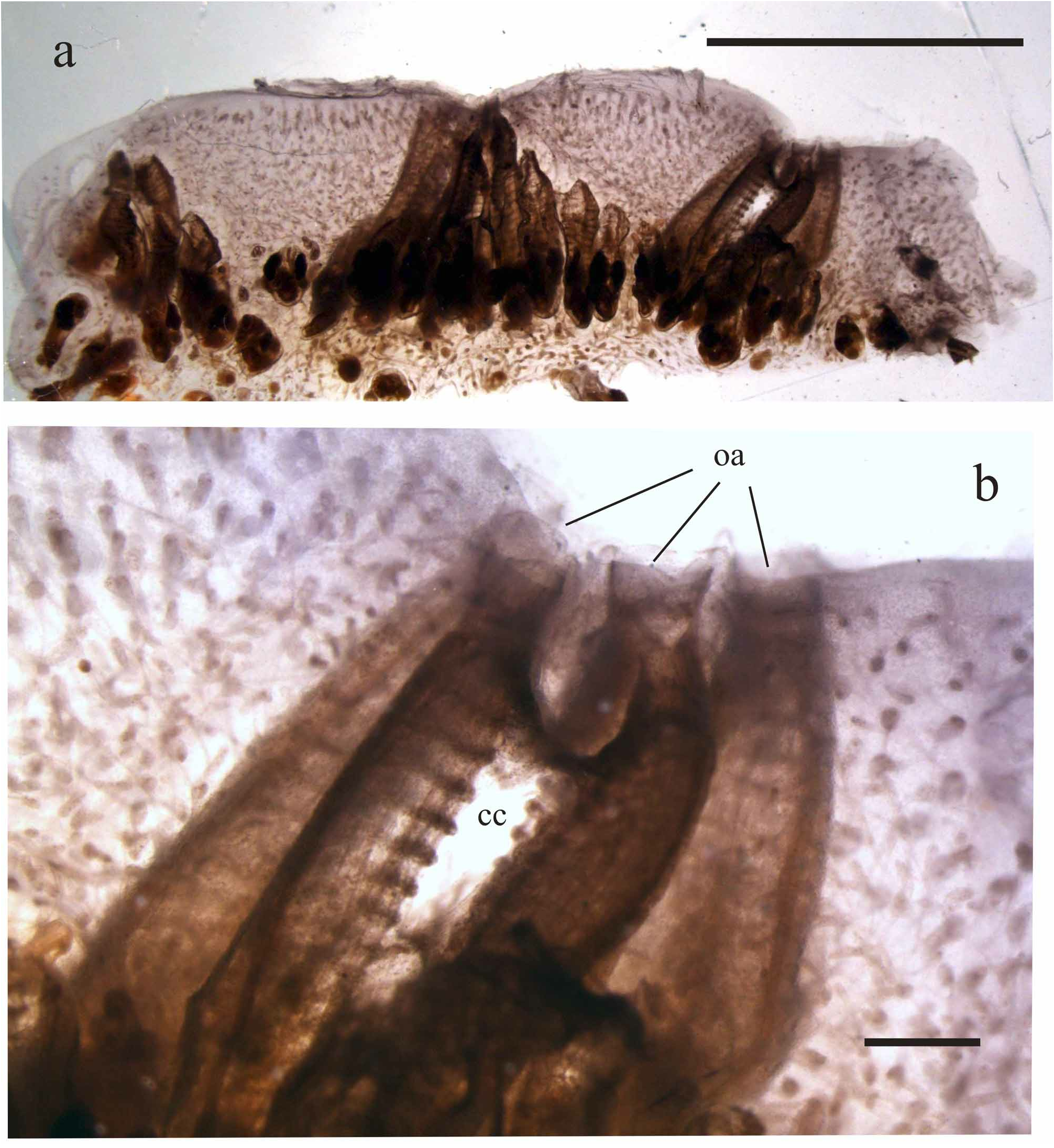
“Mammillated” colonies. The curious appearance of the colonies shown in Figs 2 f–g View FIGURE 2 is not common, since only 4 out of 14 samples present this aspect. As noted above, these colonies have areas of test without zooids between systems. A similar condition is not peculiar, and may be observed in many (or all) species of the Botryllinae . However, in this case, these areas are prominent, and almost enclose the systems in a furrow. In crosssection, these structures appear to be due to an expansion of the matrix, produced by a large number of vascular ampullae, branching out from vessels which rise vertically between the systems from the basal layer of the colony to the surface ( Fig. 6a View FIGURE 6 ) They are not linked to any season or particular physiological stage (e.g. sexual reproduction) of the colony, and the reason for the phenomenon is still unclear. Although the zooids do not appear to be in poor conditions, they are clearly suffocated by the surrounding matrix, which may indicate a pathological condition ( Figs 2g View FIGURE 2 – 6b View FIGURE 6 ).
Remarks. Among Botryllinae with “ leachii type ” systems only one species may be related to the present one: Botryllus perspicuus: Monniot, F. & C., 2001 (originally described as Botryllus firmus (Monniot, F. & C., 1996, where the description is given). Both species are characterised by large zooids, with branchial sacs with numerous rows of stigmata, of which the second is complete, and furnished with a stomach larger at the cardiac than the pyloric end, but devoid of the large ovoid swelling at their cardiac end, which are present in many species of the group (i.e., in the type species Botrylloides leachii ). However, the two species differs in the shape of gut loop and the atrial aperture. In B. firmus the stomach is horizontal, the intestinal loop describes a close loop, the anus opens one row of stigmata forward of the anterior edge of the intestinal loop and the atrial aperture is very large, reaching the level of the oesophageal opening. In addition, the stomach differs in the number and shape of its folds.
Another species with large cylindrical zooid with many rows of stigmata, Botryllus eilatensis ( Shenkar & Monniot, 2006) , must be compared, because the closeness of its type locality, the Gulf of Eilat , and the theoretical possibility of its introduction in the Mediterranean. However the latter species differs in some taxonomically important characters, such as the shape of the stomach and pyloric caecum, the second row of stigmata incomplete, and other minor ones .
The new species described here was first recorded in 2001, but it is difficult to establish whether it is native or an invader. However, although in non-taxonomic studies Botryllinae are usually all assigned to the most famous local species (in this case, Botryllus schlosseri and Botrylloides leachii ), it was probably really absent in the area previously, because its large zooids and the peculiar shape of the gut loop would have been noticed earlier.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.