Mesochaetopterus Potts, 1914
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3974.4.2 |
|
publication LSID |
lsid:zoobank.org:pub:2CBE8AE3-EE5B-4EE3-AE98-94BC534B58CA |
|
DOI |
https://doi.org/10.5281/zenodo.5662120 |
|
persistent identifier |
https://treatment.plazi.org/id/BD368D0C-2437-0E47-FF57-F9F063BF5ABF |
|
treatment provided by |
Plazi |
|
scientific name |
Mesochaetopterus Potts, 1914 |
| status |
|
Genus Mesochaetopterus Potts, 1914 View in CoL
Mesochaetopterus tingkokensis n. sp.. ( Figures 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 , Table 2–3)
Materials examined. 14 specimens ( Table 2). Holotype: IOCAS MBM 240650, one complete specimen with tube. Paratypes: IOCAS MBM 240651 - 240659, AM W.47811-47814. IOCAS MBM 240650 and AM W.47811, complete specimens with tube; AM W.47812-47814, IOCAS MBM 240652 - 240658, incomplete specimens with at least regions A and B. Several notopodia and neuropodia were removed from paratypes 0 2, 12 and 13 for observation of chaetal morphology. IOCAS MBM 240659, incomplete region A, was cut into pieces and preserved in 100% ethanol for molecular analysis. All samples were collected on 28 May 2014.
Etymology. The specific epithet “ tingkokensis ” comes from Ting Kok, a beach in Tolo Harbor, Hong Kong, the type locality of this species.
Diagnosis. Large-sized Mesochaetopterus , exceeding 17.5 cm in body length for complete worms. A pair of palps with two longitudinal orange stripes. Region A with nine chaetigers; modified chaetae of A4 dark brown, 13 to 15 in number. Region B with two chaetigers; B1 with large wide neuropodia; B2 with large wing-shaped notopodia. Region C with 35–41 chaetigers (complete specimens), each chaetiger with club-shaped notopodia bearing 8–10 conspicuous capillary chaetae. Uncini with 8 to 10 teeth in B1, 10 to 12 teeth on ventral lobe and 12– 14 teeth on lateral lobe in B2; uncini with 10 to 12 teeth on ventral lobe and 12–14 teeth on lateral lobe in C1.
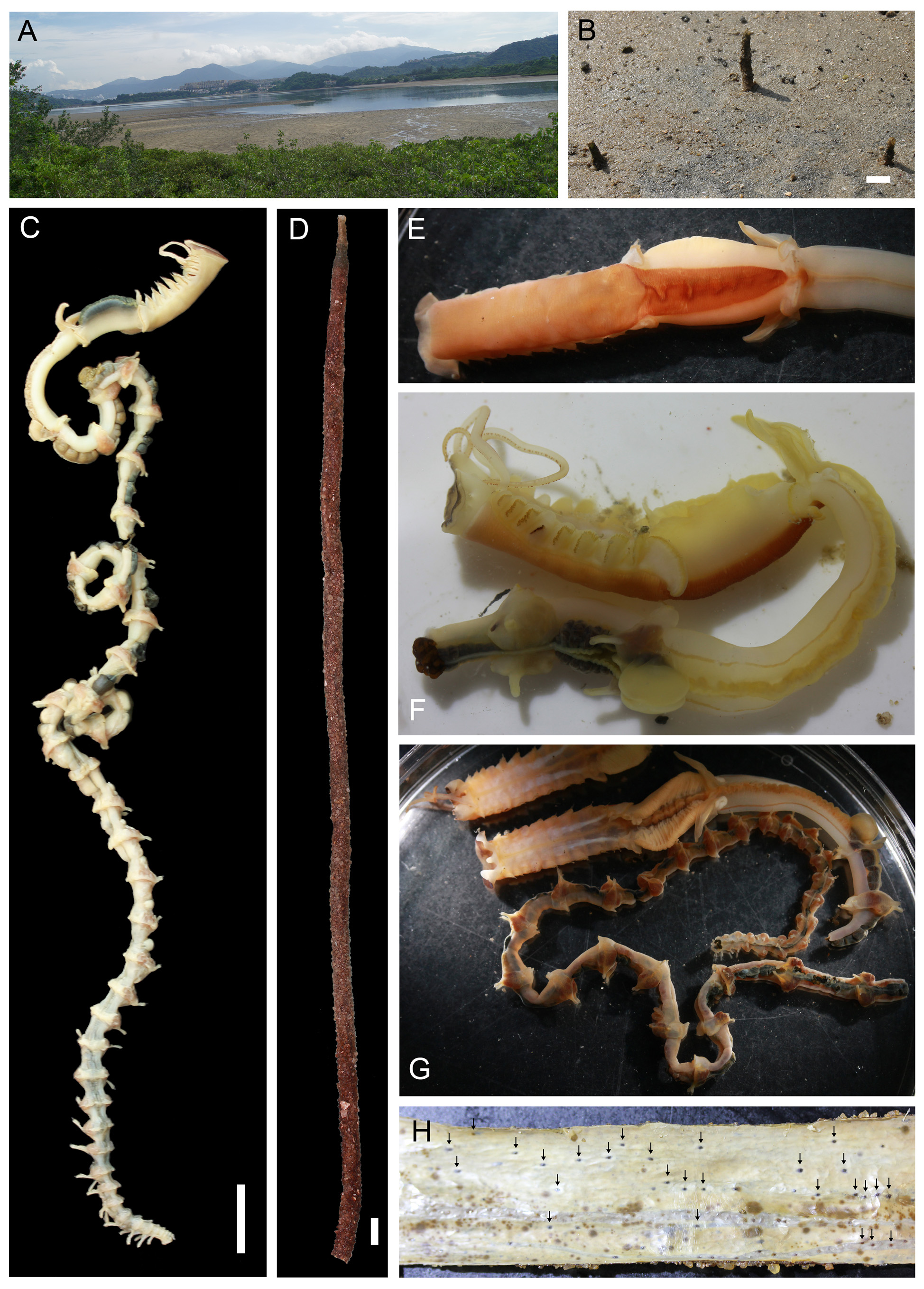
Description. Holotype complete with tube ( Figure 1 View FIGURE 1 C–D). Body width 6.0 to 8.0 mm, total length of complete specimens 17.9 to 24.1 cm, 47 to 52 chaetigers (9 in region A, 2 in region B and 36–41 in region C); all incomplete specimens lacking region C ( Table 2).
Catalogue no. Body Regions Length of region (cm) Body Modified A4 Length of Sex length contained A B C width chaetae (left/ palp (cm) (cm) (mm) right)
Holotype IOCAS MBM 240650 18.3 A1–C36 1.4 2.4 14.5 6.5 14/14 1.2? Paratypes IOCAS MBM 240651 17.9 A1–C37 1.4 2.5 14 8 14 /14 2.2 ♀ AM W.47811 24.1 A1–C41 1.3 2.1 20.7 6.8 15/14 2? AM W.47812 13.6 A1–C9 1.6 3 9.0+n.r. 7.5 14/14 1.4? AM W.47813 2.4 A1–B1 1.4 1 n. r. 7 13/14 1.4? AM W.47814 4.2 A1–C2 1.4 2.2 0.6+n.r. 6 13/ 13 n.r.? IOCAS MBM 240652 3 A1–B2 1.2 1.8 n.r. 7.2 14/14 2? IOCAS MBM 240653 3.2 A1–B2 1.1 2.1 n.r. 6.5 13/15 1.6? IOCAS MBM 240654 2.9 A1–B2 1.2 1.7 n.r. 6.8 13/13 1.5? IOCAS MBM 240655 4 A1–B2 1.5 2.5 n.r. 7.8 14/15 1.8? IOCAS MBM 240656 3.5 A1–B2 1.3 2.2 n.r. 7 14/14 1.9? IOCAS MBM 240657 4.6 A1–C2 1 2.5 1.1+n.r. 6.5 13/ 13 n. r.? IOCAS MBM 240658 1.8 A1–B 1 1 0.8 n.r. 7 14/ 14 n.r.? IOCAS MBM 240659 *
n.r. character not recorded due to loss of the posterior part or the palps. * Region A preserved in 100% ethanol for molecular analysis.
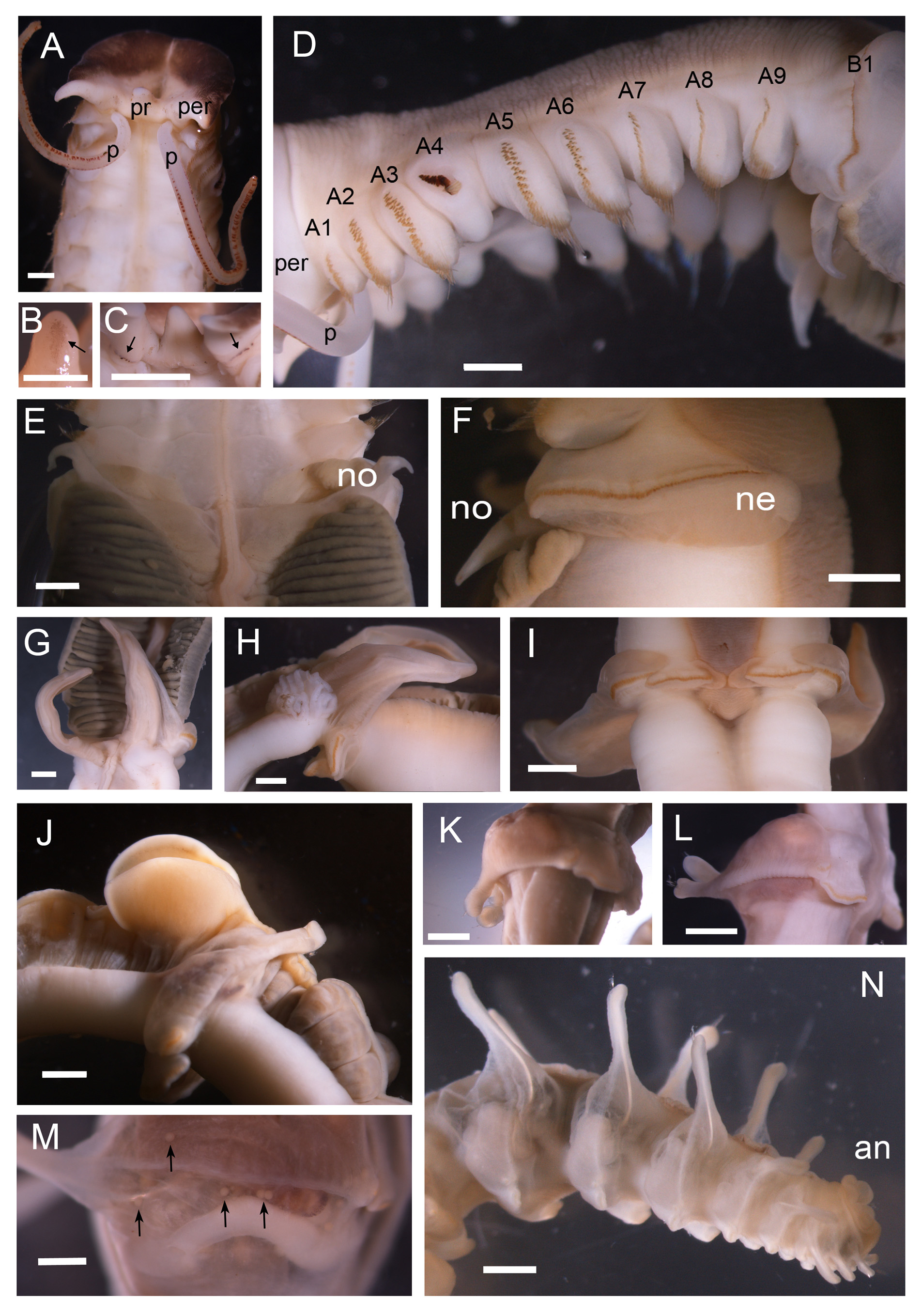
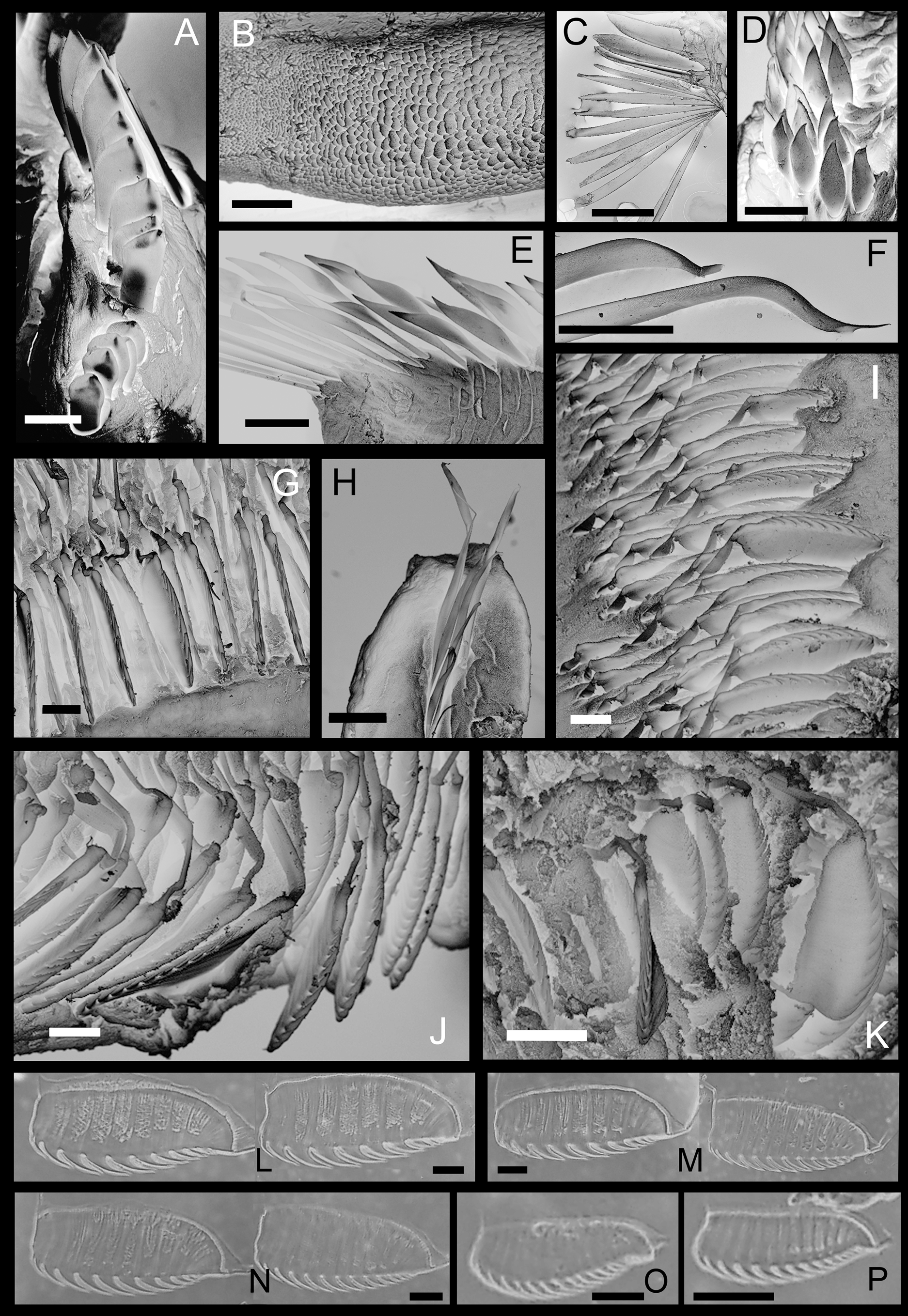
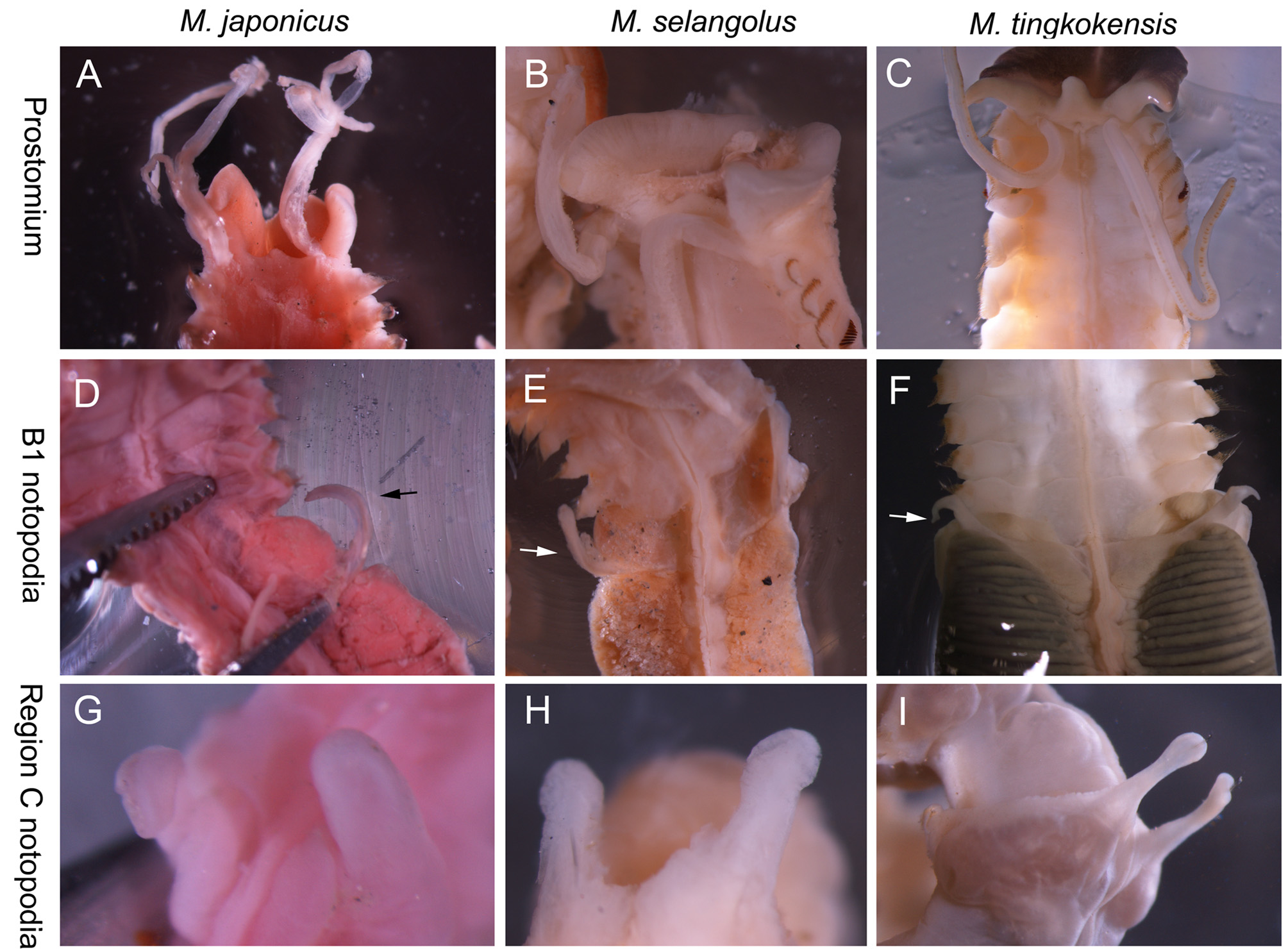
Region A long and narrow with 9 chaetigers, 1.0 to 1.6 cm long in preserved specimens ( Table 2). Living specimens with a reddish orange ventral shield ( Figure 1 View FIGURE 1 E–F); ventral shield color faded in preserved specimen ( Figure 1 View FIGURE 1 C, 2D). Prostomium small, with a patch of light brown pigment on ventral side, without eyespots ( Figure 2 View FIGURE 2 A). Peristomium extended, forming a wide collar surrounding prostomium, with two wing-shaped blackish patches facing peristomium ( Figure 2 View FIGURE 2 A). Two palps arising dorsally from junction of prostomium and peristomium, slender (0.9 to 1.7 times of region A) with a mid-dorsal longitudinal groove and two lateral stripes of suture-like short orange bands. A line of brown pigment spots present dorsally on junction of peristomium and chaetiger 1( Figure 2 View FIGURE 2 C). A mid-dorsal ciliated groove on regions A to C ( Figure 1 View FIGURE 1 E). Region A with 9 chaetigers; parapodia stout, cream-colored, with notopodia only ( Figure 2 View FIGURE 2 D), with chaetae arranged in one row on A1 and A7– A9 and in two or three irregular rows on A2–A3 and A5–A6 ( Figure 2 View FIGURE 2 D). Chaetae becoming gradually longer from lateral to dorsal side; on lateral side A1–A3 and A5–A9 notopodia, oar-like chaetae with short exposed part ( Figure 3 View FIGURE 3 D); on dorsal side, lanceolate chaetae with long exposed part ( Figure 3 View FIGURE 3 E); lanceolate chaetae long, asymmetrical, with rows of small knobs on one side, similar to modified chaetae on A4 ( Figure 3 View FIGURE 3 F). Notopodia of A4 modified, shorter than those of A3 and A5, with chaetae on lower lateral side only; chaetae two types: 13 to 15 dark brown modified chaetae stout, knoblike, with a scaly surface on the top ( Figure 3 View FIGURE 3 A–B), and 11 to 12 slender knife-like simple chaetae ( Figure 3 View FIGURE 3 C), posteriorly to upper stout chaetae ( Figure 3 View FIGURE 3 A).
Region B with two chaetigers, both longer than A and C ones. B2 longer (15.0–19.0 mm vs. 10.0–12.0 mm) and broader (11.0–14.0 mm vs. 5.0– 6.5 mm) than B1. Dorsal luminous gland on B1, yellow in living worms ( Figure 1 View FIGURE 1 G) grey in preserved specimens ( Figure 2 View FIGURE 2 E and G), with transverse elevated ridges. Parapodia of region B biramous. B1 notopodia unilobed, small, pointed, digitiform, with 22 to 25 embedded chaetae but without exposed chaetae ( Figure 2 View FIGURE 2 E–F). B1 neuropodia unilobed, with a row of uncini along ridge ( Figure 2 View FIGURE 2 F). B2 notopodia elongated, large wing-shaped, with 80 to 90 embedded chaetae but without exposed chaetae ( Figure 2 View FIGURE 2 G–I).A pair of white fluffy ball-shaped structures at base of, and immediately behind, B2 notopodia ( Figure 2 View FIGURE 2 H). Associated feeding organ (cupule) on posterior side of B2 and anterior side of C1 ( Figure 2 View FIGURE 2 J). B2 neuropodia bilobed, with ventral and lateral tori ( Figure 2 View FIGURE 2 I) having one row of uncini each. Uncini of B1 neuropodia with 8 to 10 teeth ( Figure 3 View FIGURE 3 F). Ventral uncini of B2 neuropodia with 10 to 12 teeth, and lateral uncini with 12 to 14 teeth ( Figure 3 View FIGURE 3 F, L and M). Length of uncini 104 to 114 Μm.
Region C with 36 to 41 chaetigers in complete specimens. Parapodia all biramous. Notopodia unilobed, digitiform, with 8 to 10 conspicuous simple chaetae ( Figure 2 View FIGURE 2 J–N; Figure 3 View FIGURE 3 H). Neuropodia bilobed as in B2, but lateral tori much smaller (only 1/3 the length of lateral tori of B2). Uncini of C1 100–105 Μm long, smaller than that of B2 but with more teeth: 10–12 for ventral tori ( Figure 3 View FIGURE 3 I) and 12–14, ( Figure 3 View FIGURE 3 N) for lateral tori. Uncini in chaetiger 20 (middle region C, paratype IOCAS MBM 240651) around 65Μm long, with 12–14 teeth in both ventral and lateral tori, much smaller than those of C1 ( Figure 3 View FIGURE 3 I). Uncini in posterior region near anus around 45 Μm long, with 13–15 teeth on both lateral and ventral tori ( Figure 3 View FIGURE 3 J). Oocytes present in neuropodia of IOCAS MBM 240651 ( Figure 2 View FIGURE 2 M).
Tube. Straight ( Figure 1 View FIGURE 1 D), perpendicular to sediment surface. Upper 1–2 cm grey to light brownish, extending out of sediment surface, tapering towards opening (3–4 mm in diameter). Most of the tube buried in sediment, similar throughout (6–8 mm in diameter), dark brown. Outer surface rough, coated with sand ( Figure 1 View FIGURE 1 D). Inner surface smooth, membranous, with perforations ( Figure 1 View FIGURE 1 H) throughout entire tube.
Distribution. Currently only known from Tolo Harbor, Tai Tam Tuk, and Tsing Lung Garden in Hong Kong.
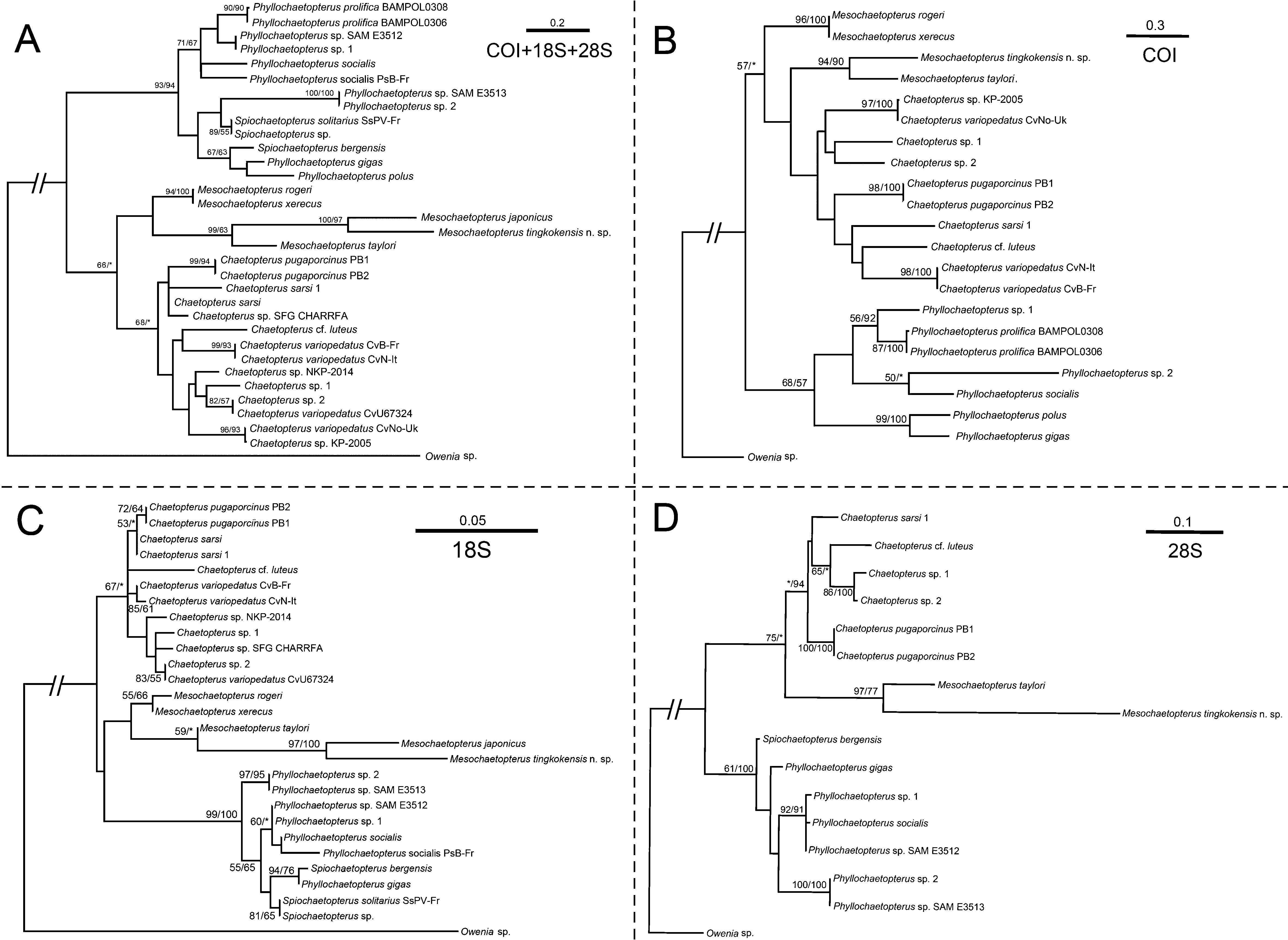
Molecular analysis. Partial sequences of COI (572 bp), 18S rRNA (646 bp) and 28S rRNA (779 bp) and their concatenated sequences (1997 bp) were used for phylogenetic tree construction based on the ML and MP analysis. The results from the concatenated sequences and 18S ( Figure 4 View FIGURE 4 A, C) show that the various Mesochaetopterus spp. form a clade, which is consistent with the finding of Martin et al. (2008). However, the support of the clade is weak, as the bootstrap values are below 50. But the results from COI indicate that genus is paraphyletic ( Figure 4 View FIGURE 4 B). Nevertheless, all results from concatenated and individual gene sequences ( Figure 4 View FIGURE 4 ) show that M. tingkokensis n. sp. is most closely related with at least one other species of Mesochaetopterus , supporting the placement of this new species into the genus.
| IOCAS |
Institute of Oceanology, Chinese Academy of Scineces |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.