Parasitylenchus myiophagus, Poinar & Runyon, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5072.1.5 |
|
publication LSID |
lsid:zoobank.org:pub:460D6662-A08E-437E-B256-67FC8E24DB78 |
|
DOI |
https://doi.org/10.5281/zenodo.5729858 |
|
persistent identifier |
https://treatment.plazi.org/id/BA3B87AB-FFC6-FFCC-18D3-F9F1FB3BFE84 |
|
treatment provided by |
Plazi |
|
scientific name |
Parasitylenchus myiophagus |
| status |
sp. nov. |
Parasitylenchus myiophagus n. sp.
( Figs 1–12 View FIGURE 1 View FIGURES 2–3 View FIGURE 4 View FIGURES 5–8 View FIGURES 9–12 )
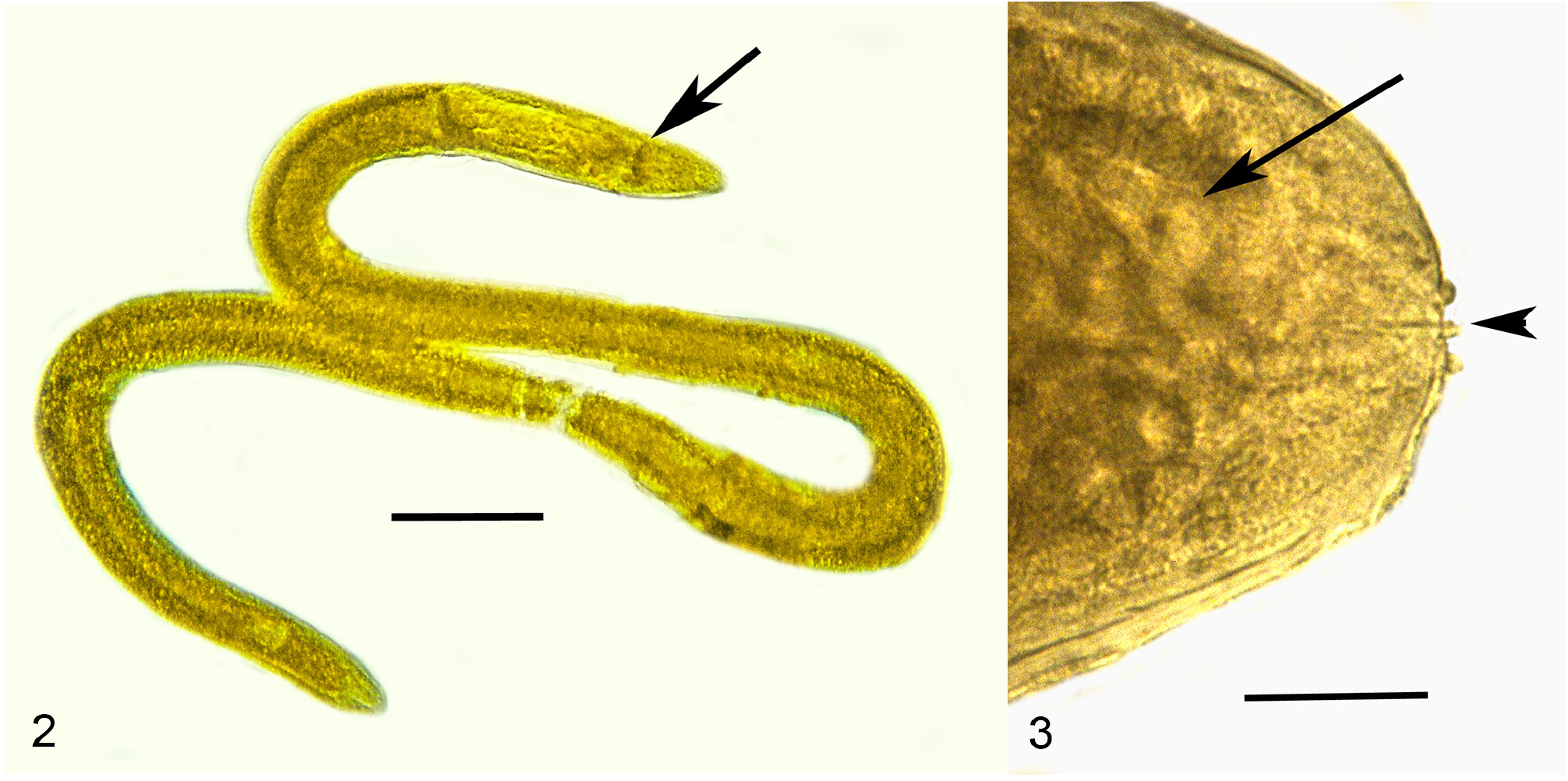
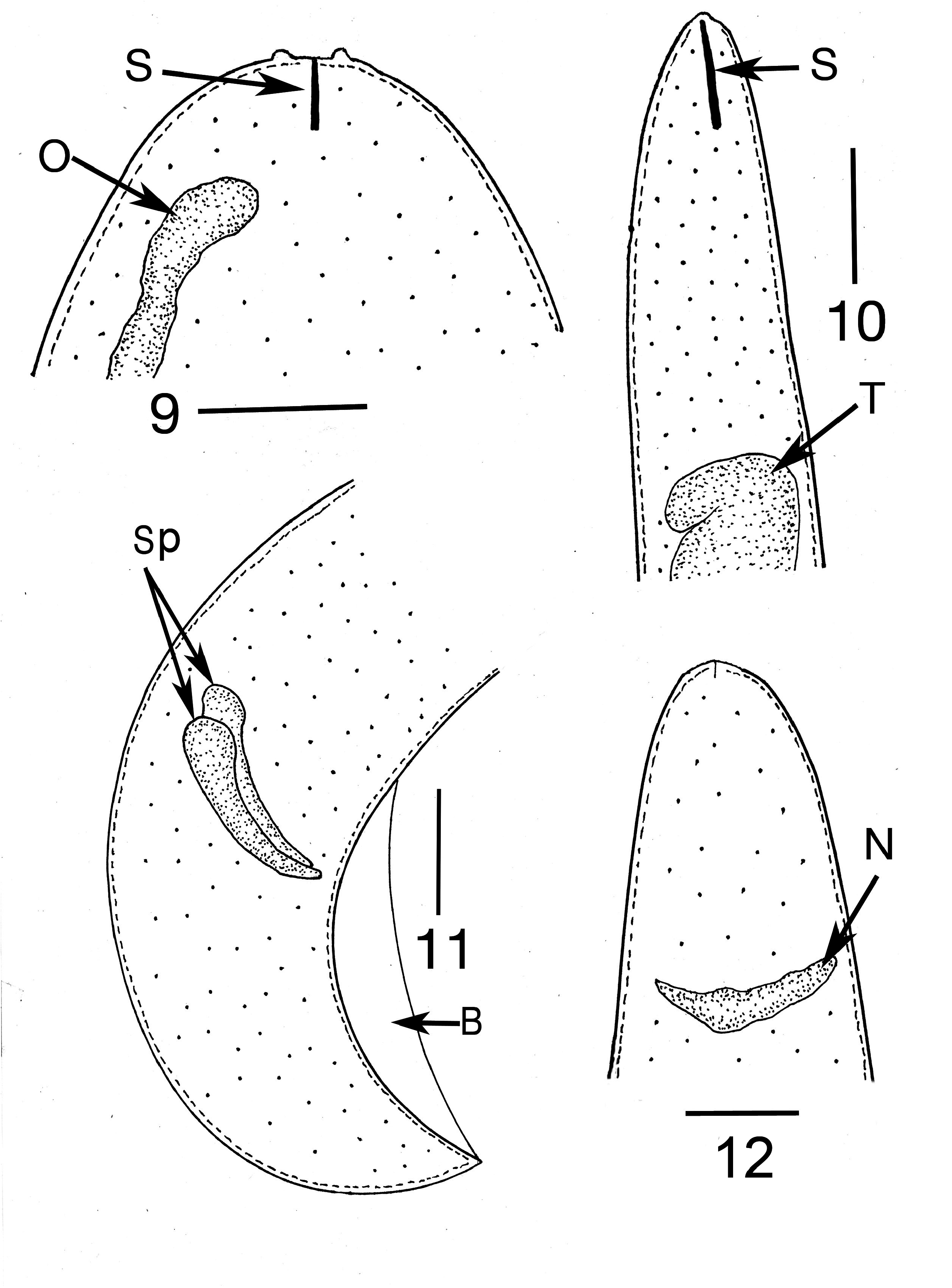
Description ( Table 2 View TABLE 2 ). First generation parasitic female (n = 1): Ovoviviparous, yellow, body ventrally coiled with 3 bends, filled with reproductive and gut cells; body length, 5.4 mm; greatest body width, 205 µ m; stylet thin, narrowing toward apex, lacking basal thickenings; stylet length, 26 µ m; length head to nerve ring, 176 µ m; length tip of intestine to tip of tail, 112 µ m; excretory pore and anus not visible; vulva sub-terminal; tail rounded.
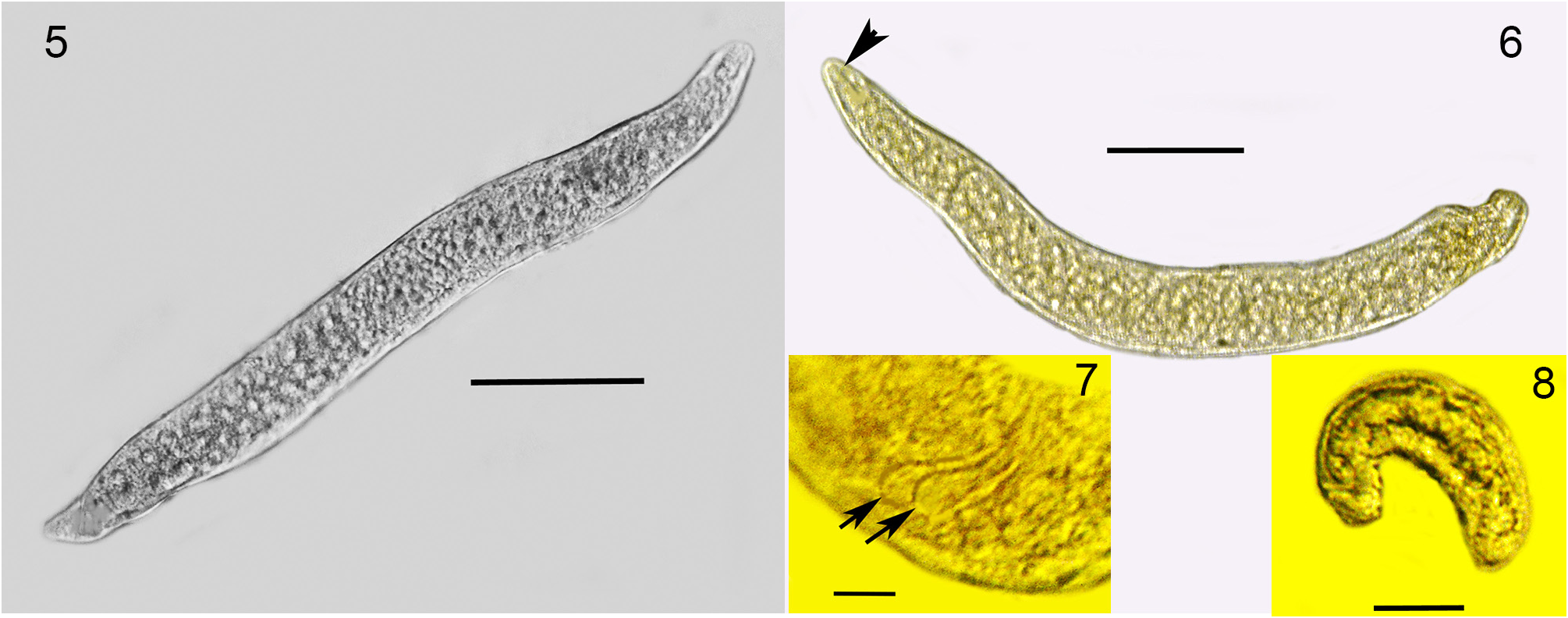
Second generation parasitic females (n = 10): Ovoviviparous, body yellowish-brown, slightly curved but without bends; body mostly filled with reproductive and gut cells; body length, 370–400 µ m; body width, 36–42 µ m.
Second generation parasitic males (n = 5): Body yellowish brown, slightly curved to straight (some individuals have a constriction near the tail tip); body length, 320–330 µ m; body width; 34–40 µ m; distance tail tip from cloaca, 160 µ m; spicules paired, separate, length 12–16 µ m; bursa faint, length 14 µ m; gubernaculum absent.
Developing juveniles (n = 10): Length, 46–190 µ m; width, 10–16 µ m.
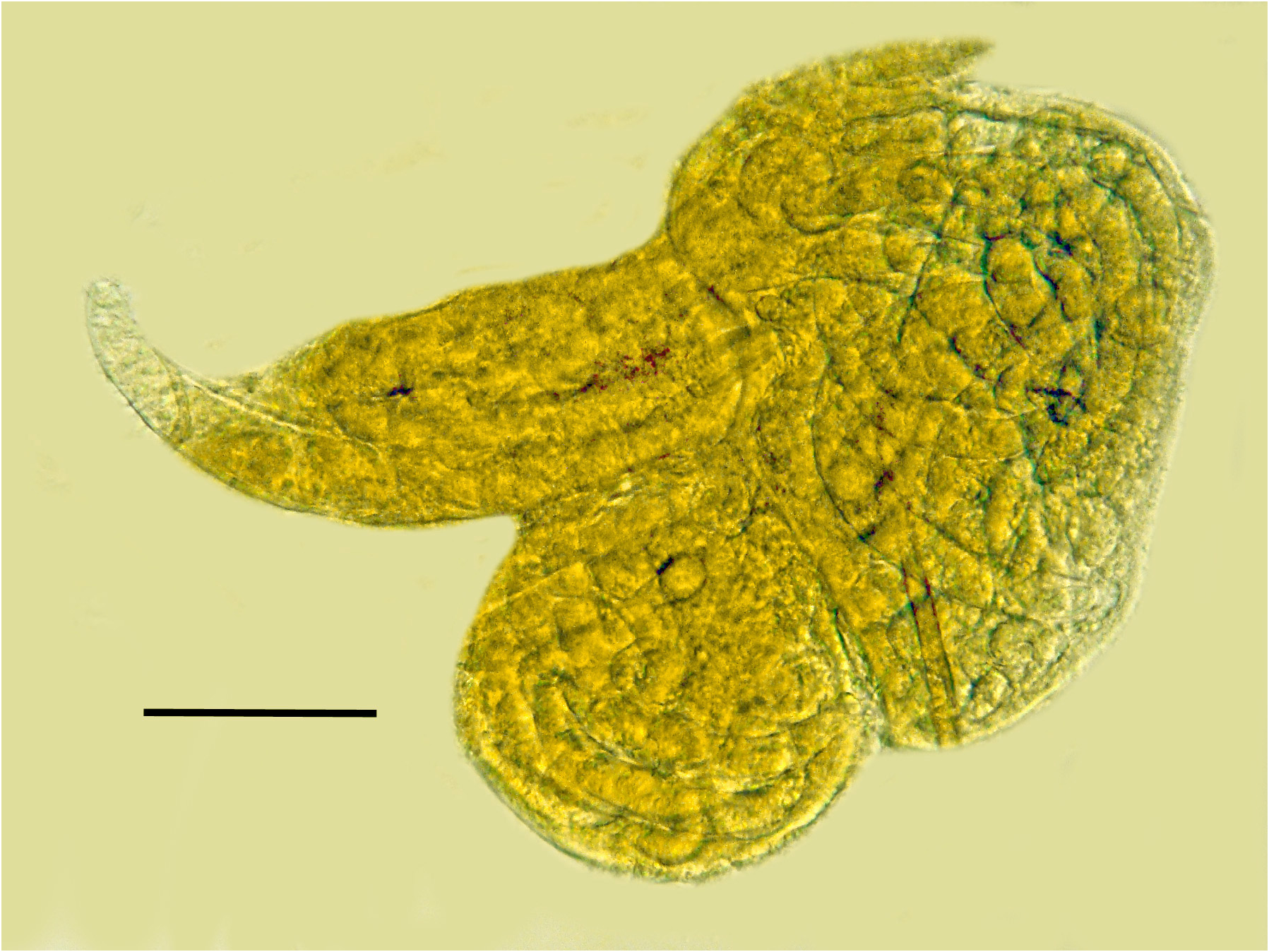
Comments. The first generation parasitic female (Figs 2,3,9,12), second generation parasitic females ( Fig. 5 View FIGURES 5–8 ), second generation parasitic males (Figs 6,7,10,11) and early developmental stages ( Fig. 8 View FIGURES 5–8 ) occur in the host’s body cavity. First generation parasitic female yellowish, body extended with 3 bends, ovoviviparous, containing faint stylet lacking knobs or basal thickenings; second generation parasitic females and males light yellow with indistinct stylets lacking knobs or basal thickenings; males with paired, separate spicules with cephalated heads; faint bursa; gubernaculum lacking. Development of the second generation stages occurs in clusters ( Fig. 4 View FIGURE 4 ), which include eggs, developing juveniles and adults.
Typological characters and diagnosis of P. myiophagus . Placement of P. myiophagus in the family Parasitylenchidae Siddiqi, 1986 and subfamily Parasitylenchinae Siddiqi,1986 is based on the presence of a primary heterosexual generation alternating with a secondary heterosexual generation in the host, parasitic females ventrally curved, slender simple, ventrally arcuate spicules, the presence of a bursa, the absence of a gubernaculum and a dipteran host ( Siddiqi, 2000).
Placement in the genus Parasitylenchus Micoletzky, 1922 is based on three types of adults in the host (primary heterosexual generation female, secondary heterosexual generation female, and male), with the secondary heterosexual forms mating in the host.
Parasitylenchus myiophagus can be distinguished from other species in the genus Parasitylenchus by its dipteran host, a character shared only by P. diplogenus Welch, 1959 and P. nearcticus Poinar, Jaenike & Dombeck, 1997 . Parasitylenchus myiophagus can be distinguished from the former species by its short, straight first and second generation oviparous females that are subequal in size and the longer stylet of the first generation parasitic females. From P. diplogenus , P. myiophagus differs in having relatively short, swollen second generation oviparous females that are subequal in size and by the absence of a stylet in the male.
Etymology. The specific epithet is derived from the Greek “myia”= fly and the Greek “phagos”= to eat.
Type-locality. USA, Montana, Gallatin County, Bridger Mountains, Johnson Canyon , large hillside spring, 30 July 2010, N 45°57.73’ W111°01.61’, JB Runyon GoogleMaps .
Type material. Holotype (T- 560t) first generation parasitic female deposited in the USDA Nematode Laboratory , Beltsville, Maryland, USA . Paratypes (parasitic females and males) deposited in the Montana Entomology Collection, Montana State University, Bozeman, Montana, USA .
Host. Males of Tachytrechus sanus Osten Sacken, 1877 ( Diptera : Dolichopodidae ). Four demasculinized males were collected at the type locality. One was dissected for this study and the remaining three were pinned and deposited in the Entomology Collection at Montana State University, Bozeman.
Effect on host. Most tylenchid parasites do not kill their hosts outright, but many can cause a variety of behavior and developmental host changes. This is normally expressed as a reduction in body size but can involve sterility and even death. In T. sanus , P. myiophagus causes demasculinization (a reduction in development of male primary and secondary sexual characters) that is similar to effects caused in other male dolichopodids by mermithid nematode infection ( Kahanpää, 2008). The most obvious sign of infected males is the much smaller and poorly rotated genitalia (cf. Figs 13,14). Other P. myiophagus -induced changes to male T. sanus include having a wider face that is light yellow to silver (face very narrow and golden in uninfected males), arista of antenna shorter with reduced apical lamella, and darker legs especially the fore coxa and fore femora (which are yellow in uninfected males) ( Fig. 14 View FIGURES 13–14 ). Demasculinization also occurs in other dolichopodids infected with nematodes, including several species of Dolichopus in the Palearctic ( Kahanpää, 2008; Germann et al. 2010) and some Dolichopus and Rhaphuim species in the Nearctic (Runyon, in review), but these cases are so far known to be caused by mermithids.
Microbial parasites of Parasitylenchus myiophagus n. sp.
The second generation females of Parasitylenchus myiophagus n. sp. have uteri filled with eggs, however very few developing juveniles could be found. In a normal Parasitylenchus life cycle, juveniles emerging from the second generation females would be common and eventually leave the host through the digestive or reproductive openings. After maturing and mating in the environment, the fertilized infective stage females would search out a new host, which would probably be a larva or pupa. The developing nematodes would then be carried into the adult stage.
The rarity of juvenile stages of second generation females may be due to a microsporidial infection that was prevalent throughout the population of both second generation parasitic males and females. Cysts (pansporoblasts) ranging up to 10 µ m in diameter contained developing round spores measuring roughly between 2.7 and 3.6 µ m in diameter (Figs 15,16). Microsporidians (Division Microsporidia), especially members of the genus Dubosquia Pérez but also representatives of the genera Thelohania Henneguy , Nosema Nägeli , Pleistophora Gurley and Microsporidium Balbiani are known to infect nematodes ( Dollfus, 1946; Kudo, 1954; Sprague, 1977). The present infection in Parasitylenchus myiophagus sp. n, which may have initially been obtained from the fly host, could account for the absence of juveniles in the second generation parasitic females.
This infection could represent a case of environmental or habitat host selection, where a parasite infects new hosts based on availability (versus phylogenetic or physiological host selection, where the parasite develops in a new host phylogenetically related to its present host). It is known that some species of microsporidia have a wide host range. For instance, the mosquito parasite, Nosema algerae , is capable of infecting members of the orders Lepidoptera, Hemiptera, Odonata and Orthoptera , aside from its natural host ( Undeen & Maddox, 1973).
| USDA |
United States Department of Agriculture |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Parasitylenchinae |
|
Genus |