ZYGAENIDAE IN FLUX
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2005.00139.x |
|
persistent identifier |
https://treatment.plazi.org/id/BA07932D-7111-FFDA-1B82-F94FCB95F914 |
|
treatment provided by |
Diego |
|
scientific name |
ZYGAENIDAE IN FLUX |
| status |
|
THE FAMILY ZYGAENIDAE IN FLUX View in CoL View at ENA
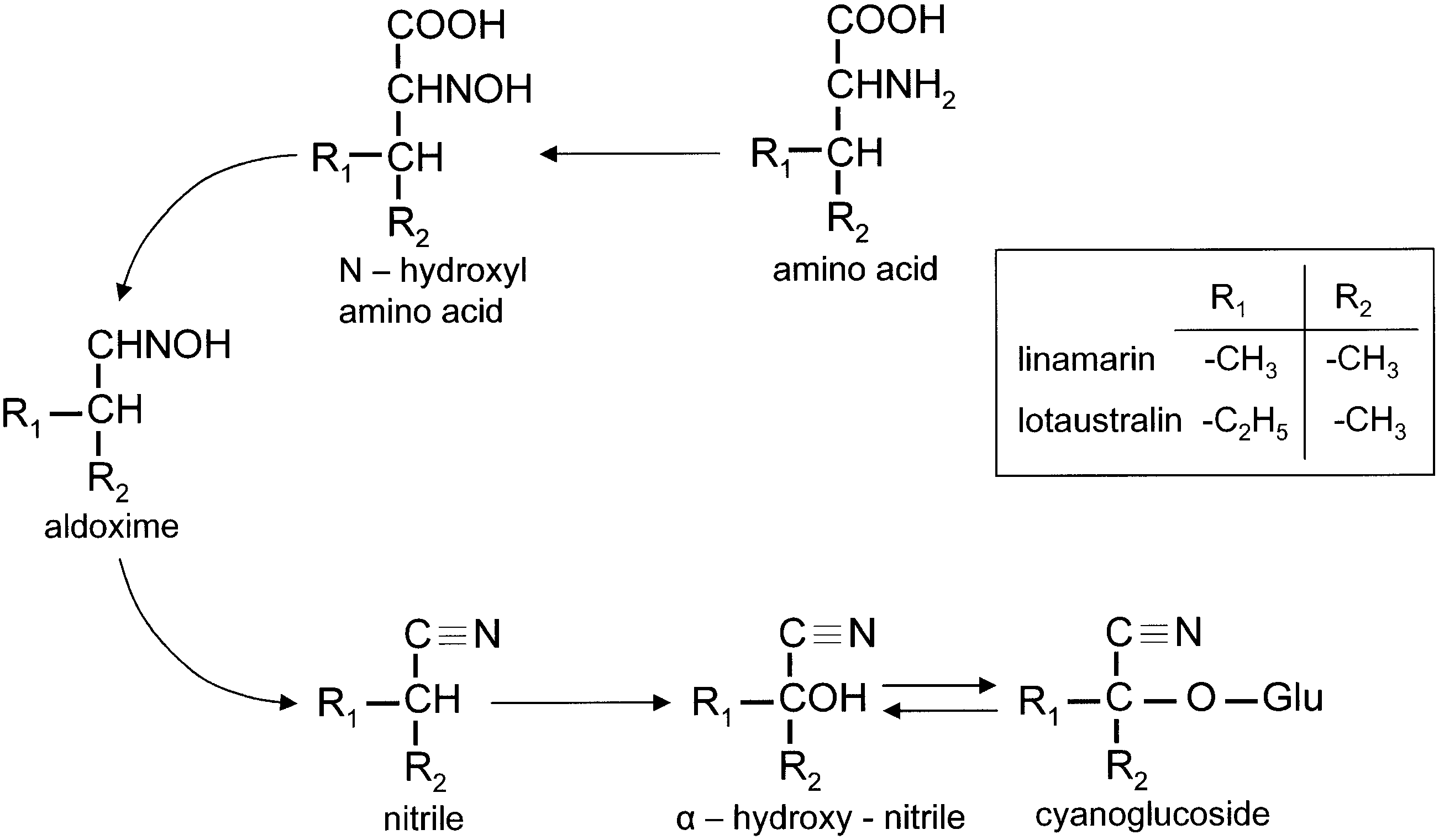
The Zygaenidae View in CoL , colloquially Burnet moths ( Zygaeninae ) and Forester moths (European Procridinae View in CoL ), is one of the largest families within the Zygaenoidea, estimated to include c. 1200 species worldwide ( Bryk, 1936, Epstein et al., 1999). According to Naumann Tarmann & Tremewan (1999) and Epstein et al. (1999), the current concept of zygaenids is not supported by general morphological features but rather by their ability to synthesize two cyanogenic compounds from the amino acids valine and isoleucine ( Witthohn & Naumann, 1984a, b, 1987a, b; Epstein et al., 1999; Naumann et al., 1999).
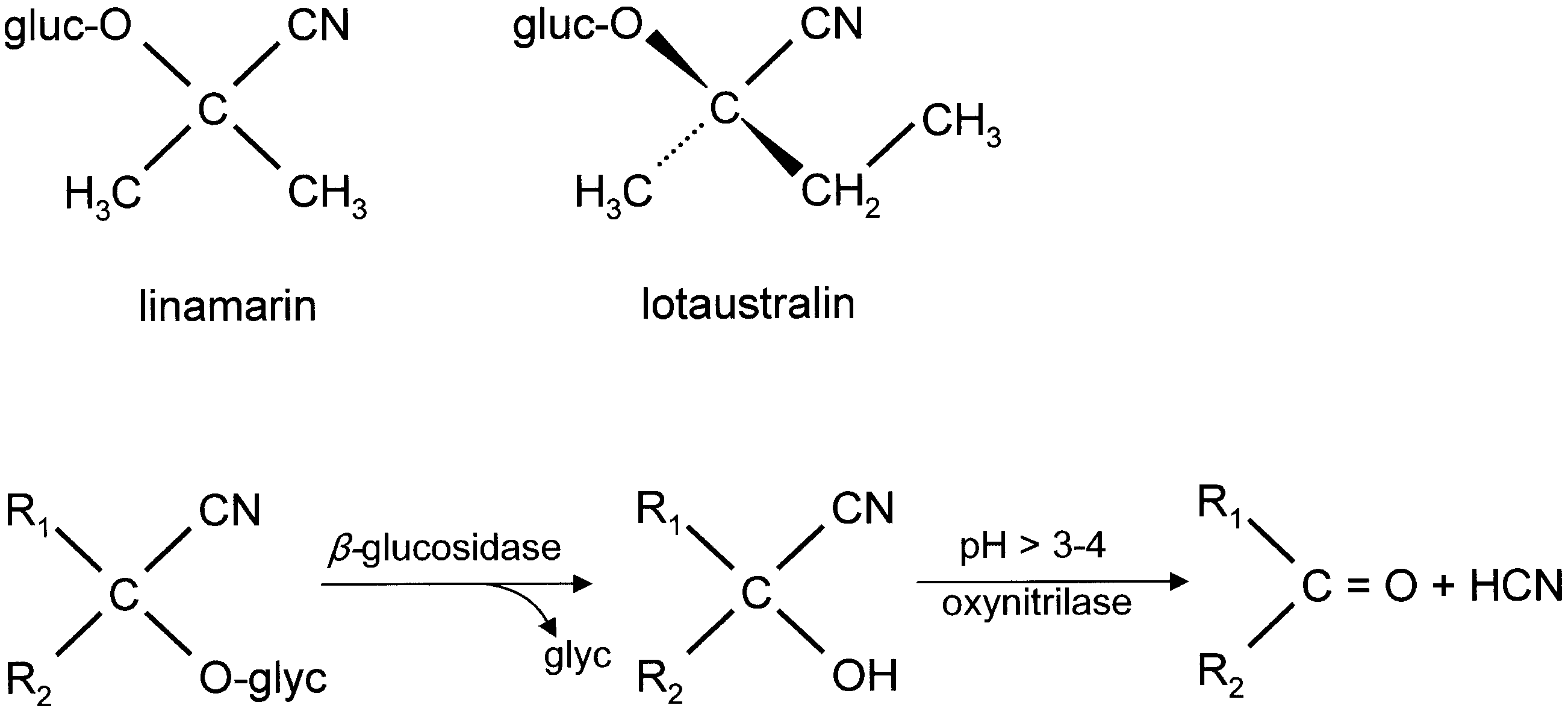
The chemical defence ecology of Zygaenidae View in CoL was first reported by Jones et al. (1962) who demonstrated that HCN is released from crushed tissues of all instars of Zygaena filipendulae View in CoL , and that the highest concentrations of HCN precursors are found in the eggs. Subsequently, various studies on the biochemical mechanisms, interactions amongst hostplants, moths and their parasitoids, and the relevant morphological structures of several representative genera of Zygaenidae View in CoL were conducted by a number of research teams. The chemical source of HCN in Burnet moths remained unknown until Davis & Nahrstedt (1979) demonstrated that it is derived from two cyanoglucosides, linamarin and lotaustralin. Linamarin and lotaustralin have long been known to occur in a number of plant families, e.g. Fabaceae View in CoL , widely utilized as a host plant by the species of Zygaena View in CoL (see Figs 5 View Figure 5 , 6 View Figure 6 ). However, the fact that some Zygaeninae live on acyanogenic host plants, but remain cyanogenic, suggests that these insects are capable of synthesizing these compounds de novo.
Although cyanogenesis in the surveyed zygaenid species has been considered to be autapomorphic for this family (e.g. Naumann et al., 1999), a similar chemical defence mechanism is known to exist in its potential sister group, the Heterogynidae ( Zilli, 1987; Zilli & Racheli, 1989; Epstein et al., 1999; Fänger & Naumann, 2001), and the current taxonomic composition of the Zygaenidae remains problematic ( Yen, 2003c). The consistency of apomorphies recognized in previous studies has yet to be tested in a comprehensive study.
The chaotic taxonomic history of Zygaenidae has involved most family groups within the superfamily, as well as various non-zygaenoid groups. When established by Latreille (1809) as Zygaenides, only the wellknown western Palaearctic genus Zygaena Fabricius, 1775 was included in Zygaenidae . Subsequently, the following groups were included in the family, and its concept varied between different authors: Heterogynis (in Zygaenides by Walker, 1854), Syntominae and Ctenuchini of Arctiidae (by Walker, 1854), Procridinae Boisduval, 1828 (as Procridae = Pyromorphina Herrich-Shaffer, 1855), Chalcosiinae Walker, 1864 [1865] (as Chalcosiidae), Charideinae Butler, 1876 (as Charideinae in Arctiidae = Glaucopidae Harris, 1839, Pompostolinae Jordan, 1907 ), Himantopteridae Rogenhofer, 1884 , Phaudinae Kirby, 1892 and Anomoeotidae Hering, 1937 . There were, in addition, two little-known groups, Lactura -group (sensu Kyrki,
Boradia 2 Sciodoclea 3 Isocrambia 4' Aphantocephala 87 1' ' Anarbudas Trypanophora 5 Docleopsis 2' Isocrambia 7' Phlebohecta 5 ' Aphantocephala 8 Thaumastophleps 7 Scotopais 10 Arbudas 10 14 1 Caprima Boradiopsis 9 Euxanthopyge 10 0 Phlebohecta Leptozygaena 16 Pollanisus 15' 17 Hestiochora Doclea 16 Levuana ' 19 Eucormopsis ' Trypanophora 20 93 ' ' Cryptophysophilus 82 21 ' Docleopsis ' Pseudoscaptesyle
1
93 Soritia Cyclosia 23 Histia 22 Cadphises Prosopandrophila ' 23 26 ' Docleopsis 25 Allocyclosia 26 Gynautocera
28 15 ' Trypanophora Pollanisus 30 Callizygaena 31 ' Procris Procotes Aphantocephala
16
' 33 Herpolasia ' 34 ' Clematoessa 35 ' Pompelon 48 ' Herpa basiflava
22 ' 73 44 Heteropan 40 90 Herpa 30 42 Rhodopsona ' 45 Aglaope 55 36 ' Pidorus truncatus
36'
73
74
33 77
' 80 ' Doclea 79 ' Prosopandrophila 80 Chalcosia Heteropan Barbaroscia 37 39 Hampsonia 40 ' Corma 41 38 ' Herpa ' 55 Hemiscia 43' Barbaroscia
47
60
36 Pseudonyctemera 46' 52 Pidorus 53 Chalcosia ' 54 ' Pseudoscaptesyle 47 ' Retina 52 Caprima '
56
37 ' ' 57 ' Eumorphiopais
58 ' 84 Elcysma Pseudonyctemera 47 ' Retina 44 52 ' ' Caprima 56 ' Eusphalera 57 ' Eumorphiopais 46 58 ' ' Allocaprima 59 Chalcosia 8497 Philopator Agalope 47 Retina 60 ' Erasmia 61 64 Eucorma ' 63 Chalcosia 64 ' Amesia 62 66 ' Soritia Eusphalera Heterusia 68 ' Chalcosia 63 ' Milleria 67 ' Chalcosia 71 ' 97
Anomoeotini ANOMOEOTINAE Dianeurini
Philopator Agalopini Chalcophaedra
Heteropanini Eumorphiopais
1984; Common, 1990) and Burlacena -group ( Heppner, 1981), which were transferred from Yponomeutidae and Glyphipterigidae sensu lato, respectively. Consequently, the boundary of Zygaenidae had been extended to include seven subfamilies (see Bryk, 1936; Alberti, 1954; Common, 1970; Scoble, 1992) plus some genera without subfamilial attribution.
When Minet (1986, 1991) started to challenge the traditional classification of the Lepidoptera , he transferred Charideinae to Thyrididae (Thyridoidea) ( Fig. 1C View Figure 1 ). He supported Fletcher & Nye’s (1982) viewpoint that Himantopteridae and Anomoeotidae , which are assumed to be related to Megalopygidae + Somabrachyidae ( Fänger et al., 1999) , should be treated as families distinct from Zygaenidae . Eventually, four subfamilies – Zygaeninae , Procridinae , Chalcosiinae and Phaudinae – were retained in Zygaenidae after Minet’s re-arrangement. His proposal that Heterogynidae may be most closely allied to Zygaenidae ( Fig. 1C View Figure 1 ) was later followed by Scoble (1992), Naumann et al. (1999), Fänger et al. (1999) and Epstein et al. (1999).
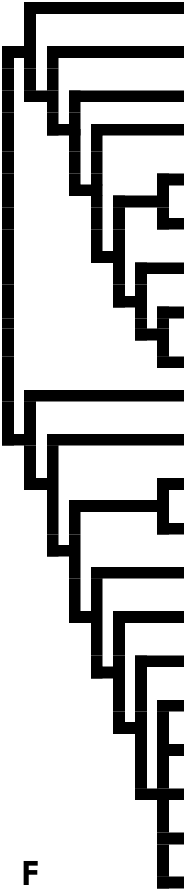
However, the classification proposed by Heppner (1984, 1992, 1998) still lumped Anomoeotidae with Zygaenidae and suggested that the Himantopteridae was the sister group of all the other zygaenid subfamilies ( Fig. 1F View Figure 1 ). The Callizygaeninae , as a newly elevated subfamily based on several features of genitalic structures, were separated from Procridinae by Tarmann (1994). Furthermore, Fänger et al. (1999) pointed out that the Phaudinae would probably have to be excluded from Zygaenidae , since this group obviously shared a number of apomorphic characters with the limacodid families; as a result, the inter-subfamilial relationships of the Zygaenidae have become even more contentious. The Lactura -group, which was suggested as belonging to Zygaenidae by Kyrki (1984) and Common (1990), was later established as a new family, Lacturidae , by Heppner (1995) and regarded as the sister group of Zygaenidae by Holloway et al. (2001). As for the Burlacena -group, its taxonomic association has wandered amongst various families ( Glyphipterigidae , Yponomeutidae , Tineidae , Choreutidae ); the Zygaenidae seems to be its current destination, but even this placement is dubious.
In summary, the monophyly of the current concept of Zygaenidae has never been tested cladistically. Scoble’s (1992) placing of the genera Heterogynis Rambur, 1837 and Janseola Hopp, 1923 in Heterogynidae may well be correct. Of the subfamilies Zygaeninae , Procridinae , Callizygaeninae and Chalcosiinae , only the monophyly of Zygaeninae has been established ( Naumann, 1987a, Naumann et al., 1999), although no cladistic analysis is available for any of them. The historical changes of the relationships of zygaenid groups are shown in Figure 1. View Figure 1
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
ZYGAENIDAE IN FLUX
| Yen, Shen-Horn, Robinson, Gaden S. & Quicke, Donald L. J. 2005 |
Procridinae
| Boisduval 1828 |
Zygaeninae
| Latreille 1809 |
Zygaeninae
| Latreille 1809 |
Zygaena
| Fabricius 1775 |