Acanthicus hystrix Agassiz
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4088.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:7C9F7AA3-B20E-4F5D-908D-3C9404BC1EBC |
|
DOI |
https://doi.org/10.5281/zenodo.5662327 |
|
persistent identifier |
https://treatment.plazi.org/id/B95BCF3A-942D-B529-FF0B-FC04FB7AF9D4 |
|
treatment provided by |
Plazi |
|
scientific name |
Acanthicus hystrix Agassiz |
| status |
|
Acanthicus hystrix Agassiz View in CoL
( Fig. 4 View FIGURE 4 )
Acanthicus hystrix Agassiz in Spix & Agassiz 1829: 3, Pl. 1 (figs. 1–2). Type locality: rio Amazonas. Holotype originally housed at ZSM and destroyed in 1944 during World War II (Terofal, 1983).
Rinelepis acanthicus Valenciennes in Cuvier & Valenciennes, 1840: 487 (unjustified replacement name and objective synonymy of Acanthicus hystrix ). Valenciennes miswrote Rhinelepis without the “h”.
—Günther, 1868b: 233 [species authorship for the first time considered belong to Spix; description of one specimen of 22 inches obtained by Mr. Bartlett in Xeberos].—Eigenmann & Eigenmann, 1889: 46 [description of one specimen from lower Amazon,].—Devincenzi, 1933:1–5 [notes about the acquisition of one specimen by the Montevidéu Museum; description with illustration].—Fernández-Yépez, 1949 [description and illustration of one specimen from rio Guárico, Orinoco].—Isbrücker, 1980: 75 [new classification and catalog]. Isbrücker & Nijssen, 1988 [citation; short description] — Burgess, 1989: 438 [identification guide; short description].—Montoya-Burgos et al., 1998: 367 [molecular phylogeny]. Fisch-Muller, 2003 [catalog; authorship for the first time considered to belong to Spix & Agassiz, 1929].—Armbruster, 2004 [phylogeny].—Lujan et al, 2015 [molecular phylogeny].
Diagnosis. Acanthicus hystrix is distinguished from A. adonis ( Fig. 5 View FIGURE 5 ) by its dark brown to almost black color pattern, without spots or dots (vs. color pattern dark brown with white spots covering body and fins); presence of ochre to gray stripes in caudal fin (vs. stripes absent). It also can be differentiated by reaching up to 600.0 mm SL (vs. up to 134.5 mm SL), by the presence of vermicular blotches on abdomen (vs. abdominal blotches absent), and elongate first hypobranchial (vs. first hypobranchial short).
Description. Morphometric and meristic data summarized in Table 1 View TABLE 1 . Dorsal profile of body slightly convex from tip of snout to vertical through dorsal-fin origin; nearly straight from that point to caudal-fin origin. Ventral profile of body straight from snout tip to caudal-fin origin. Ventral surface from cleithrum region to urogenital papillae with small plates. Greatest body width at pectoral girdle. Trunk strongly ornamented with five rows of keels; each one along each body plate series. Greatest body depth at dorsal-fin origin; body most slender at caudal peduncle.
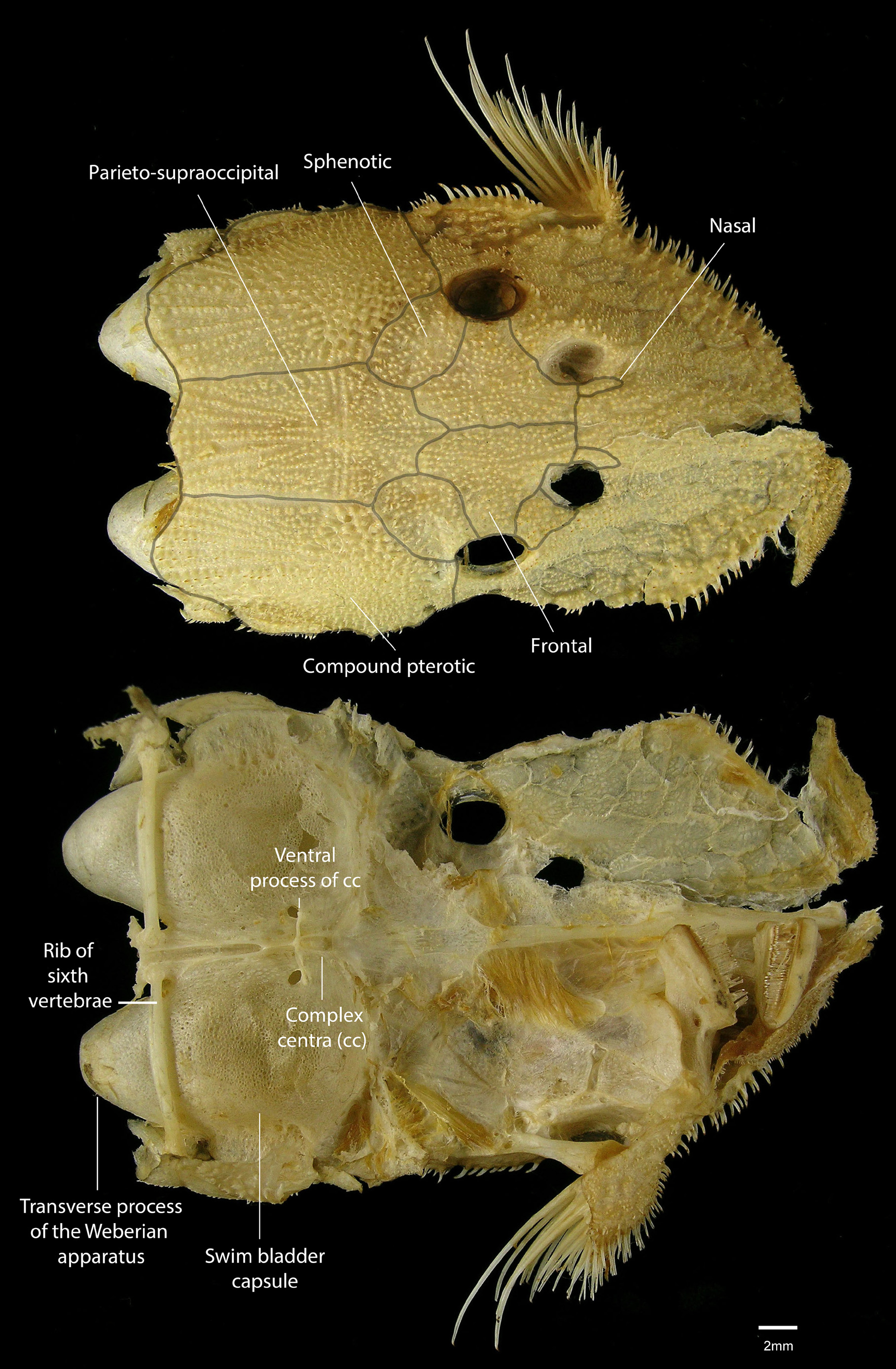
Head short, rounded anteriorly; snout and cheek completely covered by numerous small plates with sharply odontodes. Nasal bone rectangular, thin and elongate ( Fig. 2 View FIGURE 2 . Frontal bone elongated, contacting nares anteriorly and orbit laterally ( Fig. 2 View FIGURE 2 ). Anterior margin of frontal elongated, surpassing posterior margin or half nare length ( Fig. 2 View FIGURE 2 ). Parieto-supraoccipital elongate, its posterior edge wide without crest ( Fig. 2 View FIGURE 2 ). Sphenotic elongated ventrally, with little contact with IO6, without conspicuous odontodes ( Fig. 2 View FIGURE 2 ). Pterotic-supracleithrum expanded and with many fenestrae. Posterior area of pterotic-supracleithrum with one median plate or with several large plates. Orbit positioned dorsolaterally. Iris with small, dorsal flap over pupil. Infraorbital series with 7–8 bones and pores of laterosensorial system. Infraorbital 6 associated with posteroventral margin of orbit. Lateral line pores extend beyond the hypural plate.
Mouth moderate to large in size; lips large with width nearly long as length, covered with papillae; size of papillae decreasing towards posterior margin of lower lip; central buccal papilla present and very developed. Labial filaments absent. Upper lip folded over itself. Maxillary barbel short; base of barbel united to lips, with free tip. Lower lip not reaching anterior margin of coracoid. Premaxillae and dentary wide and short. Dentary oblique inwards. Teeth elongated, thin, with lateral cuspid well-developed, its distal edge slightly curved inwards. Medial end of premaxillary teeth curved inwards. Three pairs of large predorsal plates. Cheek plates eversible up to 90o, with hypertrophied odontodes.
Dorsal-fin rays i,8. Dorsal-fin base short, its length equals to 7 dorsal plates. Dorsal-fin spinelet V-shaped with locking mechanism. Seven furcate neural spines supporting dorsal fin. Pectoral and pelvic fins well developed, medial portion conspicuously expanded relative to base; distal margin rounded. Pectoral-fin rays i,6; unbranched ray covered with well-developed odontodes. Tip of adpressed pectoral fin reaching almost the end of unbranched pelvic-fin ray. Pelvic-fin rays i,5; pelvic-fin spine reaching vertical through anal-fin base when adpressed. Anal-fin rays i,4. Caudal fin i,14,i, truncate; caudal fin-ray filaments present in juveniles; supracaudal plates 7. Adipose fin absent. Three to four (usually three) procurrent caudal-fin rays. Total vertebrae 30, precaudal 13. Sixth rib very thickened, remaining ribs slender. Swimbladder capsule and transverse process of fourth vertebra expanded and surpassing rib of sixth vertebra ( Fig. 2 View FIGURE 2 ).
Color in life. Body color pattern median to dark brown, without spots or blotches on dorsal surface. Ventral surface with vermiculate blotches in most specimens. Some specimens present body color pattern very dark, almost black (rio Madeira, rio Branco and rio Xingu). All fins usually median to dark brown, large specimens (more than 300 mm SL) can present ochre striated blotches poorly defined along the dorsal and caudal fin.
Color in alcohol. Specimens in alcohol usually exhibit the same color pattern of live specimens, but in most cases more faint.
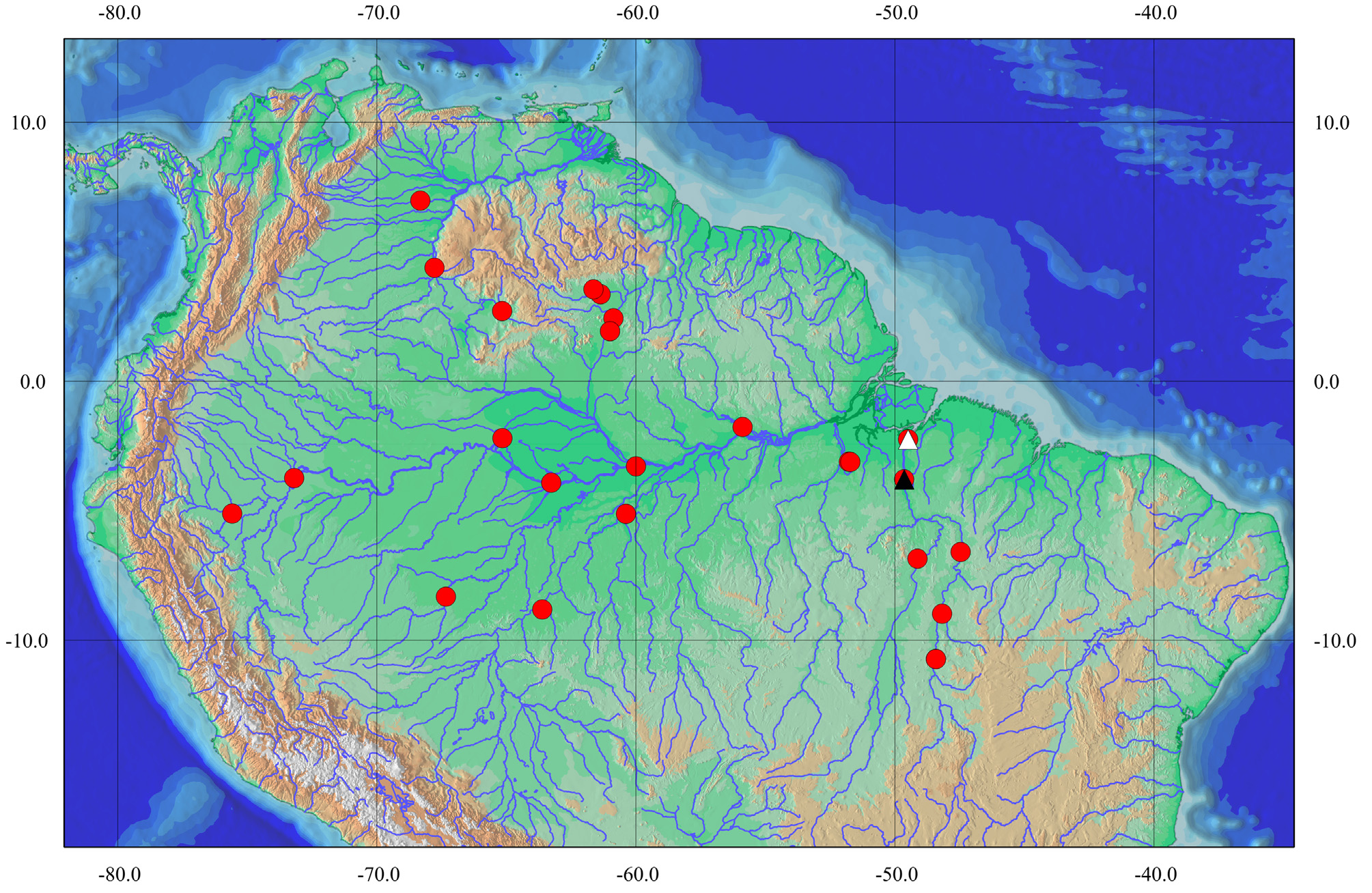
Distribution. Acanthicus hystrix has a wide distribution in the Amazon basin, in drainages of rio Branco, rio Ucayali, rio Solimões, rio Trombetas, rio Japurá, rio Madeira, rio Xingu, and Tocantins-Araguaia basin; also in the Orinoco basin, in the río Orinoco and río Apure. Acanthicus adonis has a distribution restricted to the lower rio Tocantins ( Fig. 6 View FIGURE 6 ).
Ecological notes. Acanthicus hystrix can be found in the main channel of rivers, in moderate to strong currents, in shallow to deep habitats, and are usually collected at 2 to 3 meters in depth, hiding below conglomerate plates of rocks and gravel, or among medium to large bedrocks. In captivity, the species is omnivorous and detritivorous and usually feeds by scraping small particles from the substrate. Mature males usually develop more extensive and shaply odontodes on the pectoral spine, opercle and cheeks; they can also be more aggressive and exhibit territorial behavior (information about feeding and sexual dimorphism from www.seriouslyfish.com).
Fisheries and economical importance. Both species of Acanthicus are important resources as ornamental fishes. Acanthicus hystrix is recognized in the Altamira region by local fishermen as “acari avião” or “guariba” (Xingu) and internationally by aquarists as L155 (Schraml and Schäfer, 2004; Stawikowski et al., 2004; Werner et al., 2005). Acanthicus adonis is internationally recognized as “ adonis pleco”. The specimens are usually captured by diving with the aid of an air compressor, a typical way to capture ornamental fishes in the Altamira region (more about capture techniques in Sousa & Birindelli, 2009).
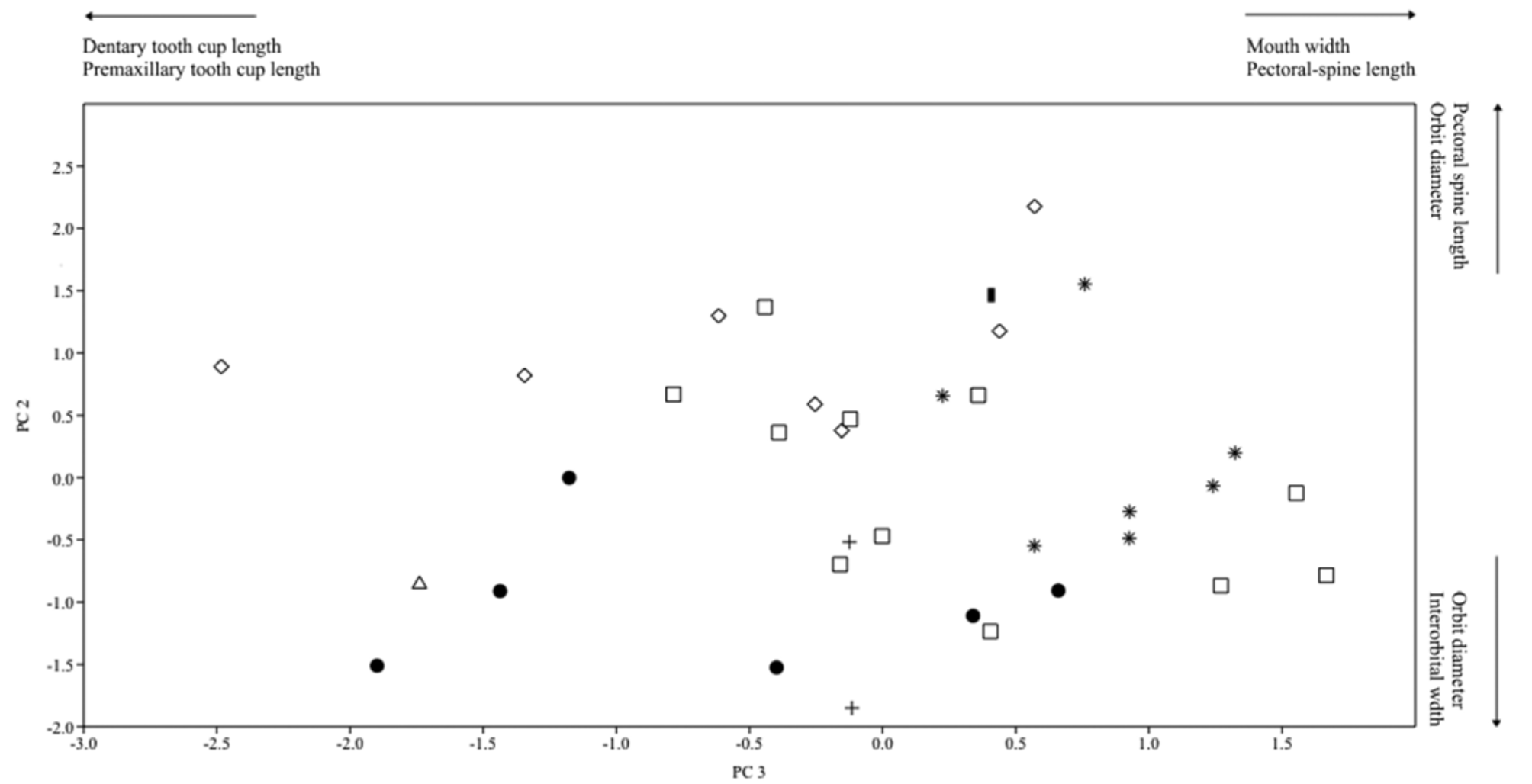
Geographic variation. There are distinct geographical forms among Acanthicus hystrix . Some forms show great variation in color pattern and body shape, as in specimens from the Orinoco basin, rio Branco and rio Takutu, which present a dark color and more depressed body than those from others drainages. These specimens are usually referred to a different species or morphotype by the aquarium trade, Acanthicus sp. L193 (Orinoco, Apure) and Acanthicus sp. L407 (Branco and Takutu). However, is not uncommon that specimens from others drainages, like the Xingu and Madeira, present the same dark color pattern and depressed body shape. Material from several drainages was analyzed and the PCA analysis ( Fig. 7 View FIGURE 7 ) reveled that the morphometric data overlap among subpopulations. Based on this information, the color and body depth differences are here treated as geographical intraspecific variations.
Acanthicus adonis has a distribution restricted to the lower rio Tocantins; however, it probably occurs in Peru (Erlend Bertelsen, pers. com.). Since there is no specific locality, and there are no samples from this locality in ichthyological collections, the map includes only the traditionally known distribution. Furthermore, this occurrence may be due to the introduction of this species by the aquarium trade.
Discussion
Armbruster (2004) proposed 13 autapomorphies for Acanthicus hystrix (the sole congener included in his analysis): accessory process of first ceratobranchial wide (8:2), first hypobranchial elongated (23:1), ridge on hyomandibular absent (49:0), angle of dentaries oblique (69:0), five to six infraorbitals (91:1), lateral line continues beyond hypural plate (92:1), mesethmoid disk relative placement anterior to its main body (101:0), nasal bone very thin (105:0), adipose fin absent (137:1), posterior process of coracoid very elongate (159:1), anterolateral process of basipterygium wide through entire length (169:1), and viliform teeth (205:0). Most of these characters are external morphological features that diagnose the genus. Additional features are herein proposed as diagnostic for the genus, namely: (1) an enlarged and fenestrated compound pterotic associated to the distention of the posterior process of the Weberian apparatus, (2) an enlarged swim bladder capsule, extending beyond the sixth rib; (3) snout completely covered by plates; (4) the presence of abdominal plates; and (5) pectoral-fin spine, when adpressed, reaching almost the end of the unbranched pelvic-fin ray.
Megalancistrus parananus was proposed as sister-taxa of Acanthicus hystrix by Armbruster (2004, 2008). Even though M. barrae and A. adonis were not included in both analyses, both genera are probably sister taxa because most characters used by Armbruster (2004) as synapomorphies of A. hystrix are present in A. adonis and the same occurs in M. parananus and M. barrae (pers. obs.). Recent hypothesis based on molecular data corroborated the monophyly of the Acanthicus clade. However, in a molecular hypothesis (Lujan et al., 2015) with the inclusion of A. adonis the conformation of the Acanthicus clade change to ( Megalancistrus ( Acanthicus ( Pseudacanthicus + Leporacanthicus ))). In Armbruster's (2004) morphological hypothesis, the clade Acanthicus hystrix plus Megalancistrus parananus is supported by several synapomorphies, including the presence of an enlarged compound pterotic, associated to the distention of the posterior process of the Weberian apparatus and the enlarged swim bladder capsule. However, in Megalancistrus , the compound pterotic is moderate in size, and the swim bladder capsule is rounded and does not extend beyond the sixth rib ( Fig. 3 View FIGURE 3 ). This condition also occurs, independently, in Panaque nigrolineatus (Armbruster, 2004; Lujan et al, 2010). These taxa have the highest encapsulated bladders reported in Loricariidae .
Alexander (1964) suggested that, in Loricariidae , the reduction and consequent encapsulation of the swim bladder has occurred as an adaptation to a bottom-dwelling life style, and the importance of the Weberian apparatus has probably prevented the complete loss of the swim bladder. Furthermore, in groups with aerial respiratory organs, it is possible that the reduction of the swim bladder was in compensation of the hydrostatic effect of the accessory respiratory organs (Alexander, 1964; 1966). More recently, Lechner & Ladich (2008) correlated the morphology of the swim bladder with hearing ability, and suggested that reduced and encapsulated swim bladders do not affect hearing in low frequencies. The authors also indicated that in taxa among the Hypoptopomatinae , the large and well-perforated compound pterotic probably increases sound transmission to the inner ear. Based on this information it is assumed that in Acanthicus , Megalancistrus and Panaque the perception of sound might be more efficient than in other Loricariidae with the same proportion of swim bladder capsule and body size.
Micracanthicus vandragti was described by Lujan & Armbruster (2011), and was considered by Armbruster (2008) sister taxon to all other members of the Acanthicus group by the presence of several characters, including the enlarged compound pterotic, associated to an enlarged gas bladder and bladder capsule whose posterior margin reaches the expanded rib of the sixth vertebra, as observed in species of Acanthicus . However, Micracanthicus vandragti can be distinguished from species of Acanthicus by the presence of an adipose fin, dentary teeth with longer shafts and larger cusps than premaxillary teeth, dentaries with a relatively short tooth cusp, and by having an intermandibular angle of 90o or less. Another character exclusive to Micracanthicus , among Hypostominae, is the reduction of body size to at maximum 42.4 mm SL (Lujan & Armbruster, 2011), while Acanthicus hystrix , one of the largest known loricariid species, can reach more than 600 mm SL.
Concerning the taxonomy of A. hystrix, Isbrücker & Nijissen (1988) suggested that due to the widespread occurrence of the genus in the Amazon basin, the designation of a neotype for A. hystrix was urgent and necessary in order to delimit the type locality of the species. Nevertheless, although the holotype of Acanthicus hystrix is lost, the species is undoubtedly recognized by the figure of the type provided in the original description of the species. According to the International Code of Zoological Nomenclature, “the fact that the holotype specimen no longer exists or cannot be traced does not of itself invalidate the original designation” (ICZN, article 74.1.3). Aiming taxonomical stability, it is best to recognize the species and further support its validity through a comprehensive description than to make a small change in the taxonomy of the group by the unnecessary designation of a neotype.
The authorship of many species described in Spix & Aggasiz, 1829 has generated controversy in the literature. Historically, species authorships were generally considered to belong to Spix (Günther, 1868b; Eigenmann & Eigenmann, 1889; Regan, 1904; Fowler, 1954; Isbrücker, 1980), or more recently to Spix and Agassiz (Fisch- Muller, 2003; Ferraris, 2007). Spix and Agassiz’s “Selecta genera et species piscium quos in itinere per Brasiliam annos MDCCCXVII-MDCCCXX” was published in two fascicles, the first one in 1829 and the second in 1831; Acanthicus was described in the first one. According to Kottelat (1988), Spix collected the specimens and supervised the execution of the drawings, while Agassiz arranged the species in the systematic order and prepared the anatomical plates. Most plates of the first fascicle were engraved and colored before Spix’s death, and carry the names that Spix intended to give them, but Agassiz did not follow Spix's ideas in several cases when he judged that some names were inappropriate or misidentifications. Thus, Agassiz is the unique author of the species name in that case, including Acanthicus hystrix .
Concerning the distribution of the genus, the type locality for Acanthicus hystrix is “flumine Amazonum” = [rio Amazonas]” and for A. adonis it is the lower rio Tocantins, Cametá. In the present contribution, the material of several drainages was examined and the distribution of the species highly expanded, showing that it has a widespread distribution in the Amazon, Tocantins-Araguaia, and Orinoco basins. Acanthicus hystrix occurs in whitewaters of Amazonian lowlands (rio Amazonas, rio Solimões, rio Madeira, rio Juruá, and rio Ucayali) as well as on Amazonian clearwater tributaries draining the Brazilian Shield, like the rio Xingu and Tocantins-Araguaia basin; and in the Guiana Shield, in rio Orinoco and rio Apure. On the other hand, Acanthicus adonis was only reported from the lower rio Tocantins, region of Cametá, occurring in sympatry with A. hystrix that has a wider distribution along its upper, median and lower course.
There are several differences between upland shield areas and lowland foreland basins that may have determined fish fauna distribution patterns. While the lowland rivers carry suspended solids and rich nutrients from the Andes (eg. whitewaters rivers: Madeira, Purus, Juruá, Ucayali), rivers originated in the crystalline shields of Brazil and Guyana (e.g. clearwater rivers: Tapajós, Xingu, Tocantins) are poor in nutrients (Goulding et al., 2003; Lima & Ribeiro, 2011). According to Lima & Ribeiro, (2011), there are several cases of restricted taxa distribution, either in lowlands (e.g. Hypoptopoma spp., Otocinclus vittatus , Lamontichthys spp., and Pterygoplichthys pardalis ) or in shields (e.g. Hypancistrus spp., Leporacanthicus spp., Parancistrus spp., Scobinancistrus spp.), whereas taxa with widespread distribution in the Amazon and Orinoco basins plus the Guyanese river systems, draining lowlands and shield areas, display a distribution pattern that should be called “northern cis-Andean South American”. Since Acanthicus hystrix is distributed both in lowlands and shields areas, but with no records in Guyanese river systems and in the rio Tapajós, it does not show any of the aforementioned distribution patterns. The lack of data of the species in the Guyanese systems could be related to the absence of information or deficient sampling efforts. In the rio Tapajós, on the other hand, even with recent expeditions to collect fishes and abundance of material in museum collections, Acanthicus spp. has not been reported from this drainage. Despite the occurrence of A. hystrix in geologically and chemically different systems (lowlands and shields), the species always inhabits river channels with strong currents and rock bottoms.
Key to identification of species of Acanthicus View in CoL View at ENA
1a. Color pattern dark brown or black with white spots distributed along the body; all fins usually with white spots; body length about 150.0 mm SL, and usually up to 300.0 mm SL................................................... A. adonis View in CoL 1b. Color pattern dark brown or black without white spots along the body; all fins usually median to dark brown (large specimens more than 300 mm SL may present ochre striated blotches poorly defined on dorsal and caudal fins); body length about 220.0 SL, and usually up to 600.0 mm SL................................................................ A. hystrix View in CoL Material examined. Brazil, Amazonas State, Amazon drainage: INPA 587, 1, 285.0 mm SL, Manaus, Catalão, rio Solimões, 3°16'53.25"S, 59°59'48.70"W (approximated coordinate), 0 1 Nov 1984, W. Boeger. INPA 2423, 1, 112.7, rio Japurá, Paraná Jaraua, 2°11'49.34"S, 65°9'9.20"W, 31 Jan 1980, Equipe Sr. José. INPA 6376, 1, 145.22 mm SL, rio Solimões Porto da balsa do Careiro, 3°54'54.25"S, 63°16'4.84"W, 10 Sep 1980, S. Freitas. INPA 32226, 7, 387.0–600 mm SL, rio Purus, 8°18'53.44"S, 67°19'58.25"W (approximated coordinate), no data of collection. MZUSP 103858, 1, 182.5 mm SL, rio Trombetas, Oriximiná, confluence with rio Solimões, 1°45'52.05"S, 55°52'51.90"W, 14 Sep 1983, R. Barthem. Brazil, Roraima State, Branco drainage: MZUSP 34244, 4, 1 c&s, 95.0 mm SL, 1 skeleton, 174.6 mm SL, 154.8–185.9 mm SL, rio Branco, cachoeira do Bem Querer, 1°55'57.83"N, 60°59'54.52"W, 8 Jan 1984, M. Goulding. INPA 1970, 3, 56.7–140.3 mm SL, Alto Alegre, rio Uraricoera, Furo Santa Rosa, 3°21'34.03"N 61°21'45.32"W, 12 Mar 1988, M. Jégu. INPA 6305, 2, 168.3–190.0 mm SL, rio Mucajaí, tronco caído, bedrock, 2°26'6.95"N 60°51'57.70"W, 20 Feb 1987, E. G. Ferreira & M. Jegu. ZMA 120.196, 1, 184.1 mm SL, rio Branco, Cachoeira do Bem Querer, remanso, 061°00'00"; W01°56'00"N, 0 8 Jan 1984, M. Goulding. Brazil, Mato Grosso and Rondônia States, Madeira drainage: MZUSP 82210, 1, 522.0 mm SL; rio Madeira, mouth of rio Aripuanã, Mato Grosso, 5° 7'4.07"S, 60°23'0.90"W, 1 Jan 1980, M. Goulding. UFRO-I 0 0 0 689, 4, 54.6–182.3 mm SL, rio Madeira, ensecadeira da cachoeira de Santo Antônio, Porto Velho, Rondônia, 8 48' 30" S; 63 36' 53" W, 1 Nov 2008, Equipe LIP/UNIR. UFRO-I 0 0 0 695, 1, 251.7 mm SL, same locality of UFRO-I 0 0 0 689, 0 1 Nov 2008, Equipe LIP/UNIR. Brazil, Pará State, rio Xingu drainage: INPA 31803, 4, 139–187.7 mm SL, Belo Monte, Ilha do Merencio (Pontão), 03°06'17"S, 051°43'33"W, 0 5 Nov 2008, L. Rapp Py-Daniel et al. MZUSP 107203, 1, 576.3 mm SL, Altamira, locality of Pontão, Belo Monte (property of Dona Maria e Sr. Waldomiro), 3°6'49.00"S 51°43'23.00"W, 12 Jul 2010, O. T. Oyakawa, J. Muriel-Cunha, C. C. Chamon, I. Fichberg, L. Rossi and A. Sawakushi. MZUSP 108571, 3, 80-228.7 mm SL, same data of MZUSP 107203. Brazil, Tocantins State, Tocantins-Araguaia drainage: UNT 478, 2, 244.6–289.4 mm SL, rio Araguaia at the locality of Pontão, Santa Fé do Araguaia, 6°50'52.19"S, 49°07'48.37"W, 12 Oct 2003, EL Beerli. UNT 858, 1, 170.5 mm SL, rio Tocantins near the confluence with rio Sono, Pedro Afonso, 8°58'28.00"S, 48°10'46.00"W, 22 Sep 2000, Núcleo de Estudos Ambientais (NEAMB). UNT 7184, 1, 279,7 mm SL, rio Tocantins, Porto Nacional, 10°43'15.00"S, 48°25'14.00"W, 26 May 2006, NEAMB. UNT 11378, 1, 201.1 mm SL, rio Tocantins near the bridge of road TO-255, Porto Nacional, 10°43'29.00"S, 48°24'59.00"W, 10 Feb 2009, N. C. Assis. Brazil, Maranhão State, Tocantins drainage: MNRJ (PHE2010000001), 1, 332.0 mm SL, rio Tocantins, cofferdam to the dam construction, Estreito, 6°35'21.01"S, 47°27'51.23"W, 31 Jan 2010 to 14 Mar 2010, D. F. Moraes, E. Dubauskas, M. Sena, W. D. Bandeira. MNRJ (no catalog number, Field # PHE2010000002), 1, 321.0 mm SL, same data of MNRJ (PHE2010000001). MNRJ (PHE2010000003), 1, 242.0 mm SL, same data of MNRJ (PHE2010000001). MNRJ (PHE2010000004), 1, 220.8 mm SL, same data of MNRJ (PHE2010000001). MNRJ (PHE2010000005), 21, 188.6–61.4 mm SL, same data of MNRJ (PHE2010000001). Brazil, Pará State, Tocantins drainage: INPA 2487, 2, 98.3–260 mm SL, rio Tocantins, Tucuruí, 3°45’39.16”S, 49°39’50.85”W, 31 Oct 1987, F. Martinho. INPA 6306, 6, 114.4–240 mm SL, lago Tucuruí, 3°45’39.16”S, 49°39’50.85”W (approximate coordinate), 2 Aug 1985, Equipe de Ictiologia do INPA (Lucia Rapp Py Daniel et al.). INPA 6307, 4, 38.8–101.2 mm SL, downstream Tucuruí dam, 3°46’2.84”S, 49°39’36.68”W, 9 Oct 1984, Equipe de Ictiologia do INPA. INPA 6312, 1, 171.5 mm SL, Tucuruí, igarapé Jatobal, 4°28’26.61”S, 49°27’18.23”W, 31 Oct 1980, Equipe de Ictiologia do INPA. INPA 31850, 22, 43.3–209.4 mm SL, downstream Tucuruí Dam, 3°46’2.84”S, 49°39’36.68”W, 0 1 Sep 1984, G. M. dos Santos. ZMA 119.969, 3, 93.0–125.7 mm SL, Cametá, 0 2 14’ S; 49 30’ W, 1987, A. Werner. Peru, Ucayali drainage: AMNH 9934, 1, 628.3 mm SL, Iquitos, Loreto, 3°44’6.23”S, 73°11’28.52”W, 1925 (no data of collector). BMNH 1867.6.13.28, 1, 400,7 mm SL, Jeberos, 5 6’23.59S, 7535’1.18W, (no data of collector). Venezuela, Orinoco basin: ANSP 162384, 3, 518.0-576.3 mm SL, El Burro, rio Orinoco, 2°42’41.38”N, 65° 9’49.61”W, 26 Nov 1985, B. Chernoff et al. AUM 42135, 1, 26.1 mm SL; Pasaganado, 38 km N of San Fernando de Atabapo, on Rio Orinoco, 4 23’ 3.9120N; 67 46’ 38.7841 W. MCNG 50865, 2, rio Apure, 6°58’, 68°19 (approximate coordinates), no data of collector. MCNG 53135, 1 skeleton, 247.4 mm SL, rio Apure, 6°58’N, 68°19’W (approximate coordinates), no data of collector. No locality data: ANSP 189661, 1, 57.4 mm SL. BMNH 1997.6.26.70, 1, 247.0 mm SL; donation by M. Hardman; INHS 36803, 1 c&s, 146.4 mm SL.
TABLE 1. Morphometric and meristic data of Acanthicus hystrix. N = number of specimens; SD = Standard deviation.
| Character | N | Minimum | Maximum | Mean SD |
|---|---|---|---|---|
| Standard length (SL) | 40 | 57.4 | 600.0 | 222.9 - |
| Percents of SL | ||||
| Predorsal length | 40 | 41.5 | 79.0 | 47.4 5.7 |
| Head length (HL) | 40 | 36.2 | 71.3 | 40.2 5.4 |
| Body length at dorsal-fin origin | 40 | 9.6 | 40.7 | 16.6 4.9 |
| Cleithral width | 40 | 25.9 | 54.5 | 32.5 4.4 |
| Thorax length | 40 | 18.4 | 44.1 | 23.3 3.9 |
| Pectoral-spine length | 40 | 22.5 | 53.2 | 38.4 6.4 |
| Abdominal length | 40 | 18.7 | 36.5 | 21.6 2.7 |
| Pelvic-spine length | 40 | 18.7 | 36.1 | 24.0 2.9 |
| Postanal length | 40 | 27.2 | 62.2 | 36.8 5.1 |
| Anal-fin spine length | 36 | 12.8 | 23.0 | 18.3 2.2 |
| Dorsal-fin spine length | 34 | 21.6 | 41.1 | 27.0 3.3 |
| Dorsal-fin base length | 40 | 16.9 | 37.3 | 21.9 3.1 |
| Caudal peduncle depth | 40 | 5.7 | 14.3 | 7.9 1.3 |
| Percents of HL | ||||
| Orbit diameter | 40 | 4.1 | 13.6 | 8.3 2.3 |
| Snout length | 40 | 49.7 | 57.3 | 53.2 2.0 |
| Internarial width | 40 | 9.2 | 24.2 | 14.6 2.8 |
| Interorbital width | 40 | 27.6 | 34.6 | 30.2 1.5 |
| Head depth | 40 | 24.9 | 46.5 | 37.5 4.6 |
| Dentary tooth cup length | 35 | 9.0 | 17.9 | 12.9 2.0 |
| Premaxillary tooth cup length | 37 | 7.5 | 15.7 | 12.1 2.2 |
| Mouth length | 37 | 30.2 | 51.0 | 38.9 4.9 |
| Mouth width | 37 | 31.7 | 49.8 | 42.2 5.5 |
| Meristics | Mode | |||
| Dorsal plate series | 40 | 22 | 27 | 24 - |
| Mid-dorsal plate series | 40 | 22 | 26 | 23 - |
| Median plate series | 40 | 22 | 27 | 24 - |
| Mid-ventral plate series | 40 | 23 | 36 | 24 - |
| Ventral plate series | 40 | 13 | 23 | 19 - |
| Precaudal plates | 40 | 5 | 7 | 6 - |
| Premaxillary teeth | 35 | 28 | 79 | 33 - |
| Dentary teeth | 34 | 36 | 87 | 36 - |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.