Oecomys sydandersoni, Carleton & Emmons & Musser, 2009
|
publication ID |
https://doi.org/ 10.1206/612.1 |
|
DOI |
https://doi.org/10.5281/zenodo.5671105 |
|
persistent identifier |
https://treatment.plazi.org/id/B603DD14-6D6F-FF91-03EA-8931FED6A4E5 |
|
treatment provided by |
Felipe |
|
scientific name |
Oecomys sydandersoni |
| status |
sp. nov. |
Oecomys sydandersoni , new species
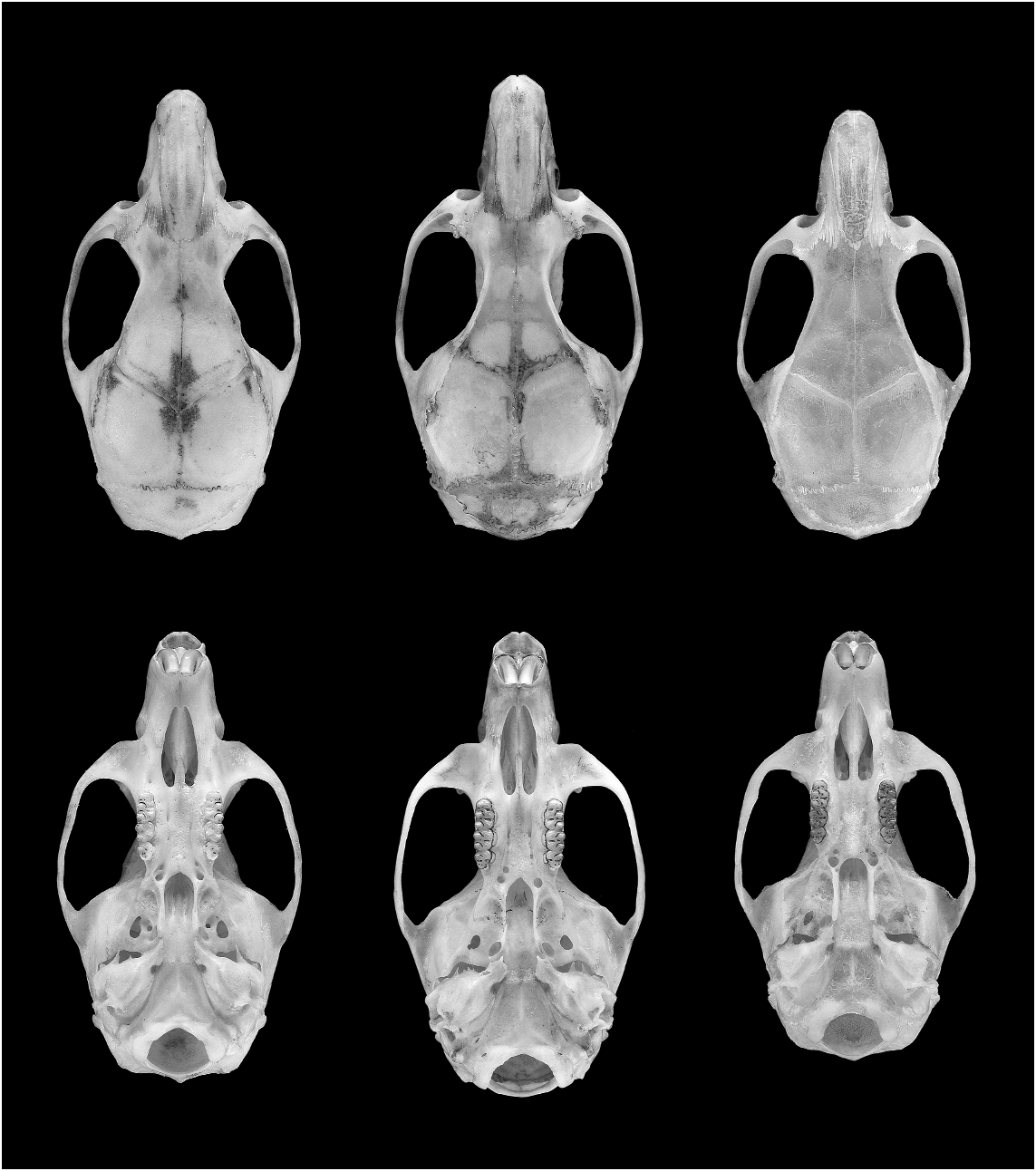
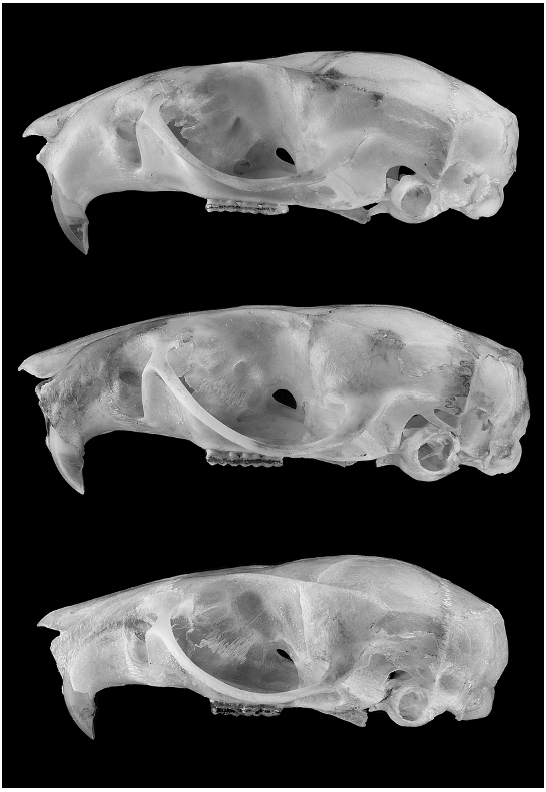
Figures 4 View Fig , 5 View Fig ; tables 4 View TABLE 4 , 6 View TABLE 6
Oecomys concolor (part): Musser and Carleton, 1993: 716 View Cited Treatment ; Anderson (1997: 389).
HOLOTYPE: Museo de Historia Natural Noel Kempff Mercado number 2679, an adult male prepared as round skin and skull; collected 30 July 1997 by Louise H. Emmons (original field number LHE 1415 ).
External data recorded on the skin tag include: TOTL, 242 mm; TL, 124 mm; HFL, 23 mm; EL, 17 mm; WT, 45 g (see table 4 View TABLE 4 for craniodental measurements of the type). The animal was noted as having scrotal testes (11 X 7 mm) and was captured ‘‘in pampa brush on vines.’’
TYPE LOCALITY: Bolivia, Departamento de Santa Cruz, Provincia Velasco, El Refugio Huanchaca, 210 m; 14 ° 46 ' 01 " S /61 ° 02 ' 02 " W (field coordinates as given by the collector; GPS, map datum WGS84) GoogleMaps .
On older maps, the locality now known as El Refugio Huanchaca appears only as Huanchaca, a biological station with a few buildings and an airstrip on private property but now partly within the park. The present owners of the estancia renamed it El Refugio, the place name that appears on specimen labels, but in 2004, they appended Huanchaca to their former designation of El Refugio. ‘‘El Refugio,’’ ‘‘Huanchaca,’’ and the combined form ‘‘El Refugio Huanchaca,’’ as applied in eastern Santa Cruz, are one and the same locality.
DIAGNOSIS: A species of Oecomys ( Sigmodontinae : Oryzomyini ) characterized by a combination of medium size (HBL <115– 135 mm, HFL <23–25 mm, ONL <29– 31 mm), relatively short tail (TL <125– 140 mm), absolutely and relatively very wide incisive foramina, smaller molars (CLM <4.4–4.7), presence of alisphenoid struts, and a derived carotid circulatory pattern (skull lacking squamosal-alisphenoid groove, sphenofrontal foramen, and posterolateral groove on the parapterygoid plate; posterior opening to the alisphenoid canal compressed; stapedial foramen absent; groove dorsally crossing the parapterygoid plate present).
REFERRED SPECIMENS: BOLIVIA: Beni, Río Iténez , ca. 4 km above Costa Marques, 12 ° 29 ' S / 64 ° 15 ' W ( AMNH 210023 About AMNH ) GoogleMaps ; Río Iténez, bank opposite Costa Marques , 12 ° 29 ' S / 64 ° 17 ' W ( AMNH 209987 About AMNH ) GoogleMaps ; Bahía de los Casara, 20 km W Larangiera, Río Iténez , 13 ° 13 ' S / 62 ° 21 ' W ( AMNH 210012 About AMNH ) GoogleMaps . Santa Cruz, El Refugio , 210 m, 14 ° 46 ' 01 " S / 61 ° 02 ' 02 " W ( USNM 588189 About USNM , 588190 About USNM ; MNK- LHE 1412 ) GoogleMaps ; 3 km NE El Refugio, Pampa , 14 ° 44 ' 35 " S /61 ° 01 ' 20 " W ( MNK 3763 View Materials , 3765 View Materials , 3766 View Materials , 3772 View Materials , 3776–3778 View Materials , 3782 View Materials , 3788 View Materials ; USNM 584554–584663. GoogleMaps
DISTRIBUTION: Extreme eastern Bolivia (fig. 6).
DESCRIPTION: Size of O. sydandersoni medium for the genus (e.g., larger than O. bicolor or O. auyantepui , smaller than O. mamorae or O. roberti —see fig. 4 and tables 4 View TABLE 4 , 6 View TABLE 6 herein; and table 28 in Voss et al. 2001). Fur texture soft and fine; pelage moderately short, hairs about 5–7 mm long over the middle rump. Dorsal pelage ochraceous brown to pale tawny, finely intermixed with black over the middle dorsum and generally bright in tone; more grayish showing on head and flanks. General appearance of ventral pelage a pale to medium gray; hairs of chin, throat, and around inguinum entirely white to base in most specimens; hairs over chest and abdomen gray basally with long white tips. Dorsalventral pelage transition moderately defined, without ochraceous lateral line. Eyelids black but no eye-ring per se. Pinnae dark brown to brownish gray, thinly covered with short, ochraceous hairs. Upper surfaces of metatarsals and phalanges covered with whitish to pale ochraceous hairs, general appearance of hind foot a dirty white. Hind-foot conformation typical of the genus—relatively short and broad; bright white ungual tufts developed on digits II–V; digit V nearly as long as digits II– IV; plantar surface smooth with six large, closely positioned pads, the thenar and hypothenar well developed. Tail slightly longer than head and body; color medium to dark brown all around, slightly paler underneath at the base; caudal hairs short, scarcely visible to the unaided eye and not obscuring fine scale pattern; rudimentary pencil expressed at tip of tail.
Skull ruggedly built for its size, with short rostrum and relatively broad interorbit. Supraorbital shelves present, converging forward such that the least interorbital width is projected relatively anteriad between the orbits; free ledge over orbit heavy, approaching an incipient bead; low temporal ridging continues across the lateral parietals in older individuals. Zygomatic arches noticeably expanded at rear and strongly tapered rostrally; dorsal notch of zygomatic plate well defined, plate relatively broad. Posterolateral wall of braincase consistently perforated by small subsquamosal fenestra, shape slitlike or narrowly ovoid, about one-half the area of the postglenoid foramen; tegmen tympani reduced, touching the ventrolateral squamosal in some specimens but not overlapping it. Alisphenoid struts typically present, delineating a discrete foramen ovale accessorius and masticatory-buccinator foramen. Incisive foramina medium long but very broad, posteriorly terminating just anterior to the anterior root of the M1s; foramina widest toward the rear, posterior ends obtuse (blunt) and anterior ends acute, in some specimens the transition from wide to narrow abrupt. Hard palate basically flat, the palatal gutters shallow and barely evident to the naked eye; palate extends slightly beyond posterior margin of M3s; posterior palatine foramen exits about the middle of M2. Posterolateral-palatal pits well expressed, usually as one large pit with interior perforations plus one or two supernumerary foramina emerging immediately anterior. Mesopterygoid fossa broad and wide, its anterior margin bluntly U-shaped, lacking medial spine or blunt protrusion; roof of mesopterygoid fossa entirely osseous, not perforated by sphenopalatine vacuities. Ectotympanic bulla small, revealing much of the medial periotic, which contributes (as viewed ventrally) to the rear border of the carotid canal.
Upper incisors opisthodont, enamel colored dull yellow-orange to a moderately saturated orange. Molars brachyodont and cuspidate as per the genus, uppers with three roots and lowers with two. M1 with well-formed anteroloph and M1–2 with mesoloph. Anterocone broad, anterolingual and anterolabial conules joined medially, not cleft by anteromedian fold; conules distinct in juveniles and young adults, but definition lost with wear. Mesolophid consistently present on m1–2; ectolophid variably developed, absent in some individuals.
In one-way ANOVAs, none of the 18 craniodental variables demonstrated significant secondary sexual dimorphism (14 ³, 11 ♀; F = 0.025 –3.391; P = 0.975 –0.054).
MORPHOLOGICAL COMPARISONS: Specimens of O. sydandersoni can be separated from most species of Oecomys based solely on the osseous character-state differences associated with the complete versus derived carotid arterial patterns (see above descriptions under Results). Other features must be consulted for discrimination from O. concolor and O. mamorae , the other species so far known to possess a derived carotid plan. We encountered slight variation in the expression of the derived carotid pattern among the entire series examined of these three species. In some individuals of O. mamorae (UMMZ 125456, 133793; UCONN 19187–19189) and O. sydandersoni (USNM 584554, 584558), but none of O. concolor , a vestige of a squamosal-alisphenoid groove can be detected on the inner wall of the braincase, but no sphenofrontal foramen is present; in these cases, the stapedial foramen persists as a minute pinhole, a tiny aperture in comparison with the full foramen observed in those Oecomys with a complete carotid circulatory pattern. Notwithstanding these individual exceptions, a large majority of specimens of O. mamorae and O. sydandersoni display the typical derived condition as described under Results. Such occasional atavistic reminders of the ancestral character state are to be expected in view of the evolutionary polarity established for carotid arterial patterns in muroid rodents ( Bugge, 1970; Carleton, 1980; Voss, 1988; Weksler, 2006).
As currently documented, a wide geographic gap separates the ranges of O. sydandersoni , in eastern Bolivia, and O. concolor , in southern Venezuela and northwestern Brazil (fig. 6). Examples of O. sydandersoni and O. mamorae , however, have been collected in close proximity in eastern Bolivia (fig. 6), and their identification may pose greater difficulty. Individuals of O. sydandersoni are smaller than those of O. concolor and O. mamorae , a contrast readily appreciated from variable loadings derived for the first principal component (fig. 2; table 2 View TABLE 2 ) or simple inspection of univariate means ( table 4 View TABLE 4 ). Length of tail is absolutely and relatively shorter in specimens of O. sydandersoni (TL <106% of HBL) compared with those of O. concolor (TL <118% of HBL) and O. mamorae (TL <113% of HBL). The dorsal pelage of O. sydandersoni is only slightly longer (5–7 mm), as measured on the rump, than that possessed by the shortfurred O. concolor (4–5 mm); dorsal pelage of O. mamorae is the longest of the three (7– 9 mm). Oecomys sydandersoni and O. concolor resemble one another in dorsal pelage coloration, albeit somewhat darker in tone in O. concolor . More grayish hues are evident over the head and flanks of O. sydandersoni , compared with a dominant fulvous-brown color in O. concolor . The dorsal pelage of O. mamorae shows the most gray, ranging from gray to grayish buff, and a buffy to bright ochraceous lateral strip demarcates the upper- and underparts in most specimens. The ventrum of O. sydandersoni appears gray, in contrast to dull white, from the chin to inguinum, as observed in most specimens of O. concolor and O. mamorae ; some individuals of the latter two exhibit encroachment of basal gray hairs over the middle abdomen. The dorsal-ventral pelage contrast thus tends to be more sharply marked in O. concolor and O. mamorae .
Besides cranial size ( table 4 View TABLE 4 ), subtle but consistent differences in shape provide other reliable means to distinguish the three species (fig. 4). The condition of the supraorbital shelf and interorbital region is similar in O. sydandersoni and O. concolor , and both differ in the same ways from O. mamorae . In the latter species, the interorbit is narrower, and the free edge of the shelf is thinner, less prominent, and confined to the rear of the orbit. Hence, the least interorbital constriction of O. mamorae occurs about the middle of orbits (more amphoral), whereas the more prominent shelves in O. sydandersoni and O. concolor extend farther forward such that the least interorbital width appears more anteriad (more cuneate). The zygomatic plate is broadest in specimens of O. mamorae ( table 4 View TABLE 4 ), and its dorsal notch appears more deeply excised compared with O. sydandersoni and O. concolor . Posterior termination of the incisive foramina is approximately the same in all three species, reaching the level of the anterior root of the first molars, but their typical shape differs notably among them: anterior and posterior ends acute in O. concolor , gently curving along the lateral edges and widest near their middle; foramina also widest at the middle in O. mamorae , but outward bowing less pronounced, the foramina appearing more nearly parallel sided and narrower; the foramina in O. sydandersoni are noticeably widest toward the rear, the posterior ends obtuse (blunt) and anterior ends acute. The bony palate noticeably projects beyond the posteri- or margins of the M3s in examples of O. sydandersoni and O. concolor , as in most oryzomyines, but the rear termination of the hard palate in O. mamorae is more or less even with the caudal margin of the M3s. Perhaps in correlation with their longer palates, the posterolateral palatal pits are well developed in the former two—typically as one large pit with interior perforations plus one or two supernumerary foramina emerging immediately anterior—whereas pit construction in O. mamorae is simpler, usually consisting of a single opening. Most of these size and shape contrasts figure prominently in the phenetic patterns derived from the various multivariate analyses and are reflected in the signs and strengths of variable loadings (figs. 2, 3; tables 2 View TABLE 2 , 3 View TABLE 3 ; see Results).
The occurrence of the alisphenoid strut, the slim bony column that delineates foramina at the base of the alisphenoid, varies within and among species of oryzomyines ( Carleton and Musser, 1989; Voss and Carleton, 1993; Musser et al., 1998), including those of Oecomys ( Weksler, 2006) . Among the three species with a derived carotid pattern, we observed an alisphenoid strut in most specimens of O. concolor and O. sydandersoni but rarely in those of O. mamorae ( table 5 View TABLE 5 ). In some individuals, the strut exists as a slender thread (which we counted as present) or is found on only one side of the skull. Used in conjunction with other measurements and qualitative traits, the presence/absence of the alisphenoid strut is useful for identifying specimens of O. mamorae and O. sydandersoni where their ranges approach one another.
Three other species of Oecomys — O. bicolor , O. roberti , and O. trinitatis —have been documented to date in the Parque Nacional Noel Kempff Mercado ( Emmons et al., 2006), albeit not in syntopy with O. sydandersoni (see below). The three can be easily differentiated from the new species by a combination of size and qualitative features of the skin and skull. Foremost, they all possess a complete carotid circulatory plan, in contrast to the derived pattern exhibited by specimens of O. sydandersoni . Oecomys bicolor is a diminutive species compared with O. sydandersoni and averages appreciably smaller in every external and cranial dimension quantified ( table 6 View TABLE 6 ). The dorsal pelage of O. bicolor is shorter and closely cropped, 3–4 mm long at rump (pelage longer in O. sydandersoni , 5–7 mm), and its ventral pelage is uniformly bright white (mostly gray in O. sydandersoni ). Furthermore, O. bicolor possesses a relatively shorter tail, only about as long as the head and body (longer, absolutely and relative to the head and body in O. sydandersoni ), and the caudal hairs are longer, forming a more distinct terminal pencil (pencil indistinct in O. sydandersoni ). Specimens of O. sydandersoni approach those of O. roberti and O. trinitatis in size but average smaller in most dimensions ( table 6 View TABLE 6 ). Noteworthy are the longer, broader rostra in examples of O. roberti and O. trinitatis (truncate in O. sydandersoni ), heavier supraorbital shelf with a distinct bead (incipient bead in O. sydandersoni ), and longer bony palate that extends forward beyond the M1s (shorter palate terminates approximately equal with the anterior border of the M1s in O. sydandersoni ). The expansive bony palate in these two species inversely correlates with their shorter incisive foramina, which end anterior to the level of the front root of the M1s. Their incisive foramina are also shaped differently, lacking the posterior widening and blunt ends characteristic of O. sydandersoni . The distinctive dorsal pelage of O. trinitatis — deep (8–10 mm), palpably luxuriant, and lustrous—at once separates it from examples of O. sydandersoni , as well as those of O. bicolor and O. roberti . Moreover, the ventrum of O. trinitatis is darker gray, the hairs often tipped with buff, its caudal hairs are longer, and, as in O. bicolor , the tail possesses a more noticeable terminal pencil compared with O. sydandersoni . The degree of caudal hairiness and pencil development are comparable in O. sydandersoni and O. roberti , but the tail of the latter is longer and its dorsal pelage is shorter (3–5 mm in contrast to 5–7 mm in O. sydandersoni ).
ECOLOGICAL NOTES: At the type locality of El Refugio Huanchaca, Emmons and associates captured 22 of 23 individuals of O. sydandersoni in open savanna characterized by a mosaic of long grasses and smaller clumps of woody vegetation (fig. 7). The grassland is deeply flooded by standing water (ca. 0.5 m) for 1–4 months during the wet season (December–March), and the woody vegetation grows on elevated mounds or hummocks originally formed around termite nests within the grassland. Because cattle no longer graze within the park and the wet grasslands tend to suppress fires, the woody vegetation on these hummocks is dense and well developed, with tall trees in the center and many woody vines. Examples of O. sydandersoni were uniformly collected within the large forested hummocks in snap traps or Sherman live traps placed above ground, up to 2.5 m high on vines, trunks, or branches within arm’s reach. A single individual was captured in closed riverine forest, taken at 1.5 m height on a vine in a shrub along the river’s edge.
Most O. sydandersoni were captured in 1998, when 21 individuals were obtained in the woody hummocks during 1430 trap-nights of collecting effort, along with the forest species Hylaeamys acritus (13), Proechimys longicaudatus (16), and Marmosa murina (1). Four other oryzomyine species ( Cerradomys maracajuensis , Holochilus sciureus , Oligoryzomys microtis , Pseudoryzomys simplex ) and one akodont ( Necromys lenguarum ) were captured in the surrounding grassland matrix. We find it noteworthy that O. sydandersoni was the only arboreal cricetid rodent caught in these woodland islands, that it was the most abundant rodent in that habitat, and that its occurrence was nearly confined to that patchy habitat. Emmons and Bolivian colleagues have tallied over 30,000 trap-nights of survey in nearby habitats, including over 11,000 trapnights in contiguous semideciduous forests, and captured only a single O. sydandersoni . They have failed to secure the species in dryground savanna woodland that lacks dense tree clumps (Cerrado proper), or in sporadically flooded ‘‘termite’’ savannas that contain many small shrubs and only small wooded hummocks (<10 m across). Three other species of Oecomys — O. bicolor , O. roberti , and O. trinitatus —were obtained in the adjacent, continuous-canopied, semideciduous forests.
These observations portray O. sydandersoni as a fairly narrow habitat specialist with a geographically restricted distribution. Such characteristics would perhaps account for its heretofore escape from the eyes of taxonomists and present rarity in collections. As its distribution is so far known, O. sydandersoni joins other species with limited geographic ranges that have been recently documented from extreme eastern Bolivia, in the Parque Nacional Noel Kempff Mercado or its vicinity. These include the forest oryzomyine Hylaeamys acritus ( Emmons and Patton, 2005) , the akodonts Juscelinomys huanchacae and J. guaporensis ( Emmons, 1999) , and the rare marsupial Cryptonanus unduaviensis ( Voss et al., 2005) , which has been collected in the grassland matrix that surrounds the woodland patches inhabited by O. sydandersoni ( Emmons et al., 2006) . Whether the restricted ranges apparent for this group of species reflect a common historical response to the interplay of ecological change and biogeographic events in eastern Bolivia invites further research ( Emmons et al., 2006).
REMARKS: The condition of the basal carotid arterial circulation in O. sydandersoni , O. concolor , and O. mamorae offers a morphological synapomorphy that suggests their closer relationship relative to other species of Oecomys . Such a provisional kinship hypothesis is encouraged by independent and combined analyses of IRBP sequences and morphological traits among oryzomyines ( Weksler, 2003, 2006), in which the sistergroup pairing of O. concolor and O. mamorae was consistently and strongly supported among the five exemplars of Oecomys studied (other species included O. bicolor , O. catherinae , and O. trinitatis ). The molecular voucher that Weksler called O. mamorae (MVZ 155005 from Peru, Amazonas, Río Cenepa), however, proves to be an example of O. roberti (identified by GGM and reconfirmed by J.L. Patton), a species with a complete carotid circulatory pattern (table 1). Confidence in the sister-group relationship of O. concolor and O. mamorae is thus eroded based on Weksler’s data. Our foremost purpose in documenting the carotid pattern displayed by all Oecomys type specimens (table 1) was to constrain the differential diagnosis of the new species from Bolivia with respect to the most morphologically similar species currently recognized ( Musser and Carleton 2005). In addition to possession of the same carotid circulatory plan, the resemblance of O. concolor and O. sydandersoni is striking, as conveyed by the earlier tentative identification of the few known Bolivian specimens as O. concolor ( Musser and Carleton, 1993) . The fine series later obtained by Emmons allowed morphological and morphometric confirmation of the two as valid species and appreciation of their approximately equivalent level of differentiation from O. mamorae (fig. 3), the third species of Oecomys known to possess the apomorphic carotid condition. Whether the derived carotid arterial pattern constitutes a synapomorpy of these three species or evolved independently will require further molecular and morphological studies based on broader taxon sampling within the genus and among other oryzomyines.
ETYMOLOGY: The first three examples of O. sydandersoni were collected by Sydney Anderson (fig. 8) and members of his field team in 1964 and 1965, along the Río Iténez in eastern Bolivia. As a fresh-faced assistant curator in 1963, he had revived the earlier natural history explorations in Bolivia undertaken for the American Museum of Natural History, notably those of Anthony and Tate in the 1920s (see chronology of mammalian inventory in Anderson, 1997). Over the next three decades, Syd and his field collaborators amassed impressive series of Bolivian mammals that he utilized in numerous taxonomic reports on this biotically rich but poorly understood country, culminating in his weighty treatise ( Anderson, 1997) on the ‘‘Mammals of Bolivia, Taxonomy and Distribution.’’ All the while, he made these collections available to any serious scientist who needed to examine Bolivian specimens and was especially kind in allowing us unrestricted access to oryzomyine rodents, the results of which found their way into our own publications. Such selflessness and sterling ethics are typical of Syd. In the opening paragraph to his 1997 work, Syd wrote, ‘‘This work is dedicated to the hypothesis-testers of this world. Everything concluded is subject to further testing’’ (his laconic drawl, tinged with his gently prodding humor and punctuated by a trailing chortle, permeates this passage in our mind’s ear). New species descriptions form the keystone to all biological hypotheses, and in the spirit of testing one of his conclusions, we are pleased to name this handsome Bolivian endemic of Oecomys in his honor. The species name is thus a patronym in the genitive singular, sydandersoni formed by combining the individual’s familiar name (‘‘Syd’’) and surname.
TABLE 4 External and Craniodental Measurements for the Type Series of O. sydandersoni, New Species, and Samples of O. concolor and O. mamorae (Sample statistics include the mean, ± 1 standard deviation, and the observed range).
| O. sydandersoni Holotype | O. sydandersoni El Refugio | O. concolor Amazonas, Brazil | O. mamorae Beni, Bolivia | |
|---|---|---|---|---|
| Variable | (MNK 2679) | (N = 21–23) | (N = 23, 24) | (N = 14–16) |
| TOTL | 242 | 257.9 ± 16.7 233–290 | 273.2 ± 11.2 255–296 | 296.0 ± 18.3 270–230 |
| HBL | 118 | 125.0 ± 12.2 109–166 | 125.4 ± 5.8 115–141 | 139.8 ± 13.1 120–170 |
| TL | 124 | 132.8 ± 8.4 115–145 | 147.9 ± 6.9 137–160 | 158.5 ± 10.7 144–180 |
| HFL | 23 | 24.1 ± 1.7 21–27 | 27.4 ± 1.1 26–29 | 27.3 ± 1.8 23–30 |
| EL | 17 | 16.4 ± 1.1 15–19 | 17.1 ± 1.3 15–20 | 18.0 ± 1.2 17–20 |
| WT | 45 | 44.9 ± 7.8 30–57 | 57.8 ± 9.5 41–80 | 73.9 ± 29.9 48–120 |
| ONL | 30.7 | 29.8 ± 1.2 27.7–32.0 | 32.2 ± 0.9 30.2–34.3 | 32.5 ± 1.2 30.9–34.5 |
| ZB | 16.7 | 16.5 ± 0.6 15.3–17.6 | 17.7 ± 0.6 16.6–19.1 | 17.1 ± 0.7 15.6–18.1 |
| BBC | 11.8 | 11.8 ± 0.3 11.3–12.4 | 12.5 ± 0.4 11.8–13.3 | 12.6 ± 0.3 12.0–13.2 |
| DBC | 9.5 | 9.1 ± 0.3 8.5–9.7 | 9.4 ± 0.3 8.8–9.9 | 9.3 ± 0.5 7.7–10.0 |
| BOC | 6.6 | 6.5 ± 0.1 6.3–6.8 | 6.8 ± 0.2 6.4–7.3 | 7.3 ± 0.4 6.6–7.8 |
| IOB | 5.4 | 5.3 ± 0.3 4.8–5.7 | 5.5 ± 0.2 5.1–6.0 | 4.9 ± 0.2 4.6–5.2 |
| LR | 8.2 | 8.4 ± 0.5 7.5–9.1 | 9.1 ± 0.4 8.4–9.9 | 9.1 ± 0.4 8.6–10.0 |
| BR | 5.4 | 5.5 ± 0.3 4.7–6.2 | 6.1 ± 0.3 5.5–6.7 | 5.8 ± 0.3 5.4–6.2 |
| PPL | 10.4 | 10.3 ± 0.5 9.2–11.4 | 10.6 ± 0.4 9.9–11.4 | 11.3 ± 0.5 10.4–12.0 |
| BPL | 5.7 | 5.6 ± 0.3 4.9–6.0 | 6.6 ± 0.3 5.9–7.3 | 6.1 ± 0.4 5.4–6.9 |
| LIF | 5.6 | 5.4 ± 0.3 4.9–5.8 | 5.3 ± 0.3 4.5–6.2 | 5.8 ± 0.3 5.3–6.3 |
| BIF | 2.6 | 2.6 ± 0.2 2.2–2.8 | 2.3 ± 0.2 1.8–2.7 | 2.5 ± 0.3 2.0–2.9 |
| LD | 7.4 | 7.7 ± 0.5 6.8–8.7 | 8.1 ± 0.4 7.6–9.1 | 8.0 ± 0.5 7.2–8.7 |
| BBP | 5.7 | 5.7 ± 0.2 5.2–6.2 | 6.1 ± 0.2 5.7–6.4 | 5.9 ± 0.3 5.4–6.4 |
TABLE 2 Results of Principal Components Analyses Comparing Adult Oecomys sp. novum with O. concolor and O. mamorae (Based on 17 log-transformed craniodental variables; see Materials and Methods and fig. 2.)
| O. sp. novum + O. concolor | O. sp. novum + O. mamorae | |||
|---|---|---|---|---|
| Correlations | Correlations | |||
| Variable | PC I | PC II | PC I | PC II |
| ONL | 0.96*** | 0.10 | 0.97*** | - 0.07 |
| ZB | 0.86*** | 0.13 | 0.83*** | 0.29 |
| BBC | 0.80*** | 0.02 | 0.74*** | - 0.15 |
| DBC | 0.53*** | 0.15 | 0.32 | 0.26 |
| BOC | 0.63*** | - 0.24 | 0.75*** | - 0.28 |
| IOB | 0.54*** | 0.13 | - 0.17 | 0.76*** |
| LR | 0.82*** | 0.26 | 0.84*** | - 0.05 |
| BR | 0.84*** | - 0.04 | 0.74*** | 0.06 |
| BZP | 0.76*** | 0.11 | 0.88*** | - 0.03 |
| PPL | 0.73*** | 0.41** | 0.91*** | - 0.06 |
| BPL | 0.87*** | - 0.29* | 0.71*** | - 0.14 |
| LD | 0.84*** | 0.35** | 0.85*** | 0.41** |
| LIF | 0.35** | 0.72*** | 0.81*** | - 0.09 |
| BIF | - 0.18 | 0.91*** | 0.36 | 0.82*** |
| BBP | 0.82*** | 0.11 | 0.78*** | 0.07 |
| CLM | 0.72*** | - 0.40** | 0.59*** | - 0.69*** |
| WM1 | 0.64*** | - 0.37** | 0.38 | - 0.58*** |
| Eigenvalue | 0.033 | 0.011 | 0.045 | 0.012 |
| % Variance | 53.5 | 17.5 | 55.8 | 14.9 |
* = P # 0.05; ** = P # 0.01; *** = P # 0.001.
TABLE 3 Results of Three-group Discriminant Function Analysis of Adult Oecomys sp. novum, O. concolor, and O. mamorae (Based on 17 log-transformed craniodental variables; see Materials and Methods and fig. 3.)
| Correlations | ||
|---|---|---|
| Variable | CV 1 | CV 2 |
| ONL | - 0.31 | 0.66*** |
| ZB | 0.08 | 0.41** |
| BBC | - 0.23 | 0.51*** |
| DBC | 0.33 | 0.24 |
| BOC | - 0.56*** | 0.50*** |
| IOB | 0.75*** | 0.12 |
| LR | - 0.28 | 0.47*** |
| BR | 0.19 | 0.61*** |
| BZP | - 0.37* | 0.35* |
| PPL | - 0.61*** | 0.26 |
| BPL | 0.20 | 0.83*** |
| LD | 0.03 | 0.41** |
| LIF | - 0.63*** | 0.07 |
| BIF | - 0.09 | - 0.45*** |
| BBP | 0.01 | 0.52*** |
| CLM | - 0.47*** | 0.72*** |
| WM1 | - 0.09 | 0.54*** |
| Canonical correlations | 0.93 | 0.86 |
| Eigenvalue | 6.15 | 2.77 |
| % Variance | 68.9 | 31.1 |
* = P # 0.05; ** = P # 0.01; *** = P # 0.001
TABLE 5 Occurrence of Alisphenoid Struts (2/2 5 struts absent both sides; 2/+ 5 strut present on one side; +/+ 5 struts present both sides.)
| Alisphenoid Struts | |||
|---|---|---|---|
| Species and Country | - / - | - /+ | +/+ |
| O. concolor | |||
| Brazil Colombia Venezuela Totals | 1 0 0 1 | 0 1 0 1 | 25 0 18 43 |
| O. mamorae | |||
| Bolivia Brazil Paraguay Totals | 29 1 7 37 | 1 0 0 1 | 0 0 0 0 |
| O. sydandersoni | |||
| Bolivia | 1 | 2 | 23 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.