Nediphya, Marusik & Omelko, 2017
|
publication ID |
https://doi.org/ 10.1515/vzoo-2017-0027 |
|
DOI |
https://doi.org/10.5281/zenodo.6461799 |
|
persistent identifier |
https://treatment.plazi.org/id/B56DA71D-DE64-FF93-D691-FF00FED29FC1 |
|
treatment provided by |
Felipe |
|
scientific name |
Nediphya |
| status |
gen. nov. |
Nediphya gen. n.
urn:lsid:zoobank.org:act:55D4DFDB-361F-4E62-AD8F-BB81FDD1FC12
T y p e s p e c i e s. Nediphya lehtineni View in CoL sp. n.
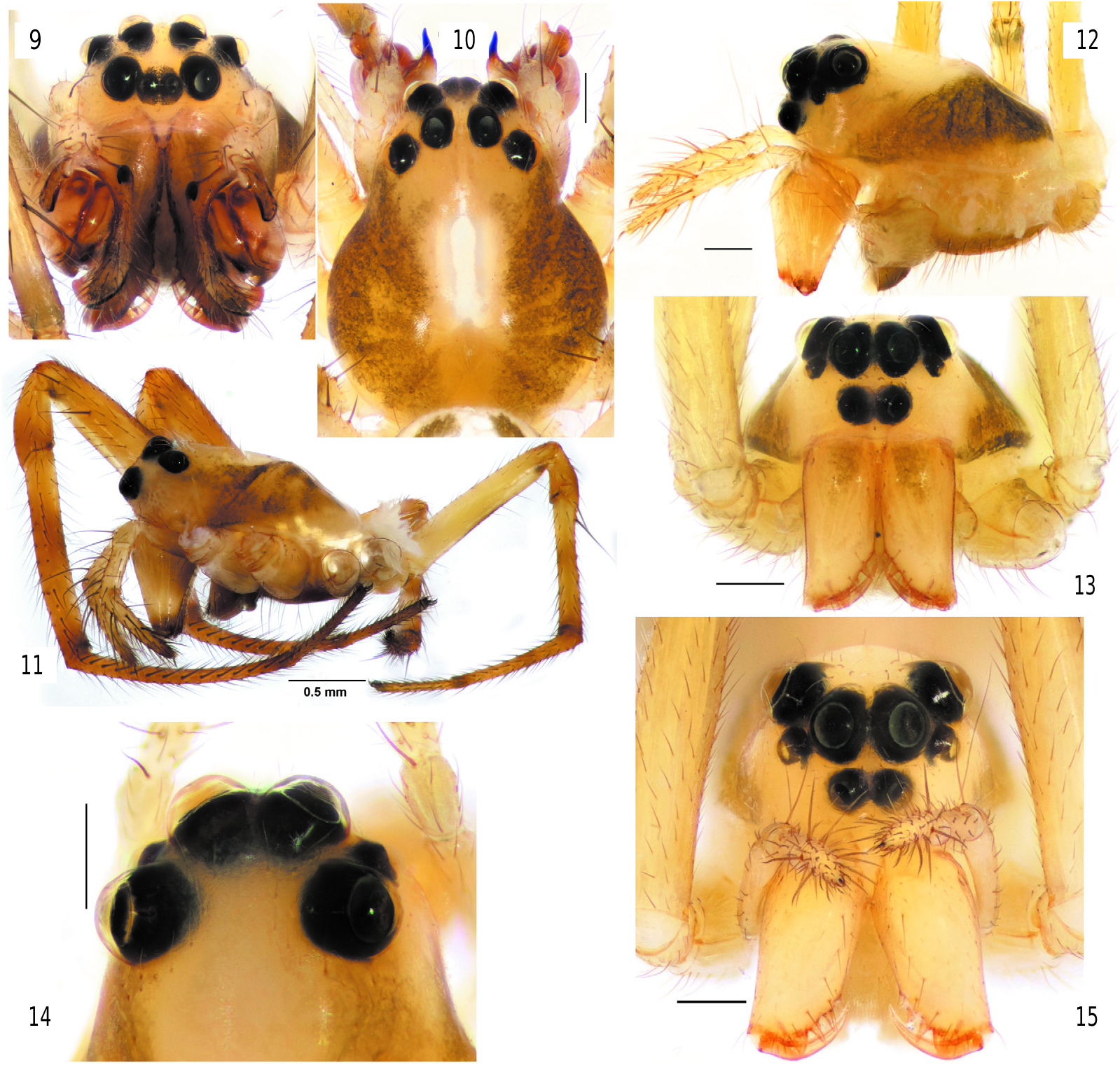
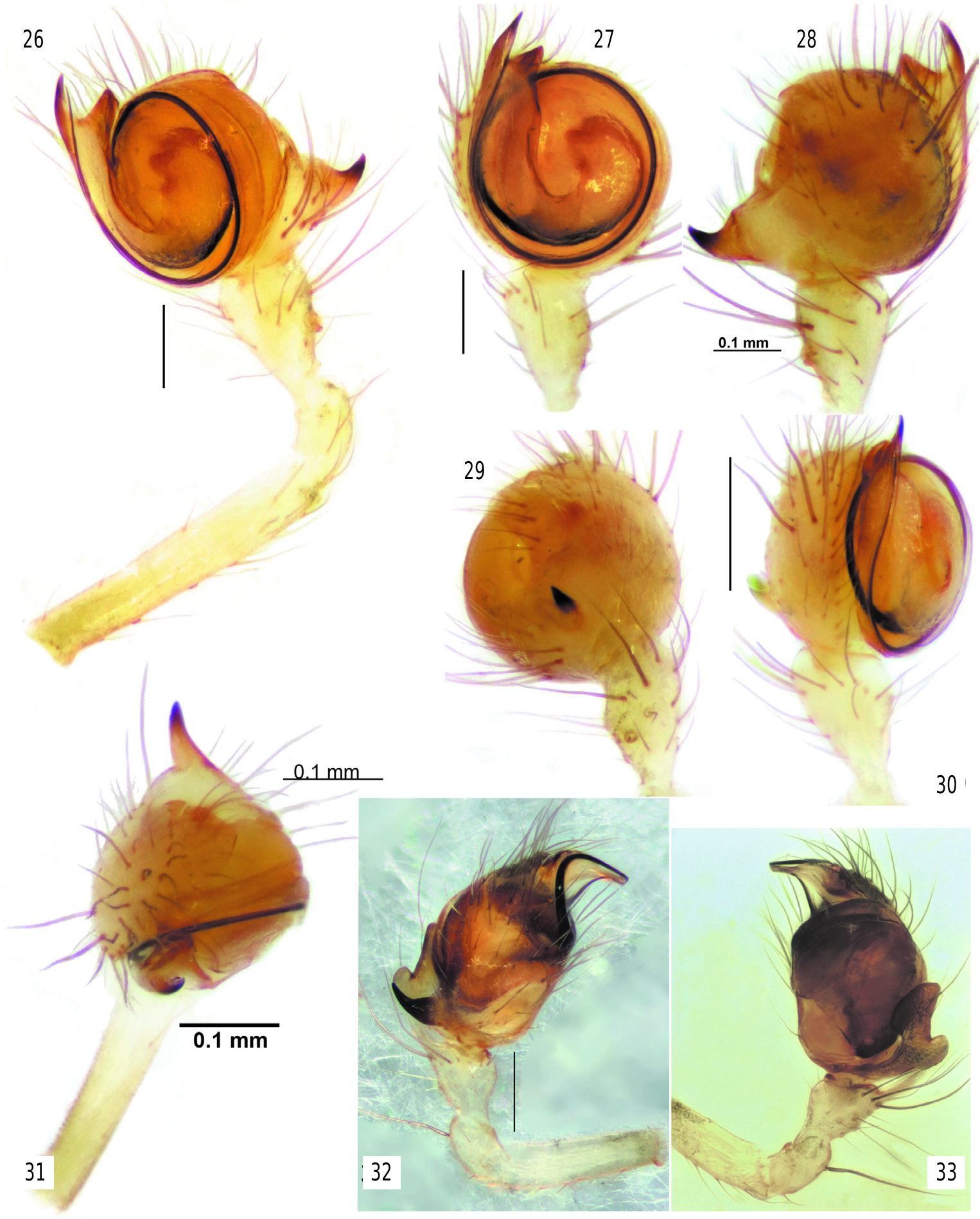
Diagnosis. The new genus differs from all Tetragnathidae by having eyes arranged in 3 rows ( figs 12–17 View Figs 9–15 View Figs 16–25 ). Nediphya gen. n. is most similar to Diphya ( figs 9–11 View Figs 9–15 ) by having heterogeneous eyes, a prolateral row of stiff setae on the tibia-tarsus of legs I–II, and a strong, large dorsal claw-like branch of the paracymbium. New genus can be distinguished by small anterior lateral eyes, not spaced with posterior eyes, low clypeus (less than diameter of AME), vs. ALE equal in size to PLE and PME, lateral eyes widely spaced, clypeus higher than diameter of AME in Diphya . In addition, the cephalic part of the carapace in Nediphya gen. n. is unmodified ( fig. 12 View Figs 9–15 ) (slanted in Diphya ( fig. 11 View Figs 9–15 )). The two genera can also be distinguished by the shape of the epiandrous plate and the number of fusules (only 2 pairs of fusules located in 2 pits in Nediphya gen. n. ( fig. 25 View Figs 16–25 ) vs. about 2 dozen arranged in a transverse row in Diphya (cf. Marusik & Omelko, 2017)). Males of Nediphya gen. n. can be recognized by having a strongly reduced ventral branch of the paracymbium (large and bilobed in Diphya , fig. 33 View Figs 26–33 ) and the presence of a cymbial lobe (lacking in Diphya ( figs 32– 33 View Figs 26–33 )), a filamentous and gradually rounded embolus (broad and twisted in Diphya ). Females of two genera can be easily distinguished by the epigyne weakly sclerotized in new genus and well sclerotized in Diphya ).
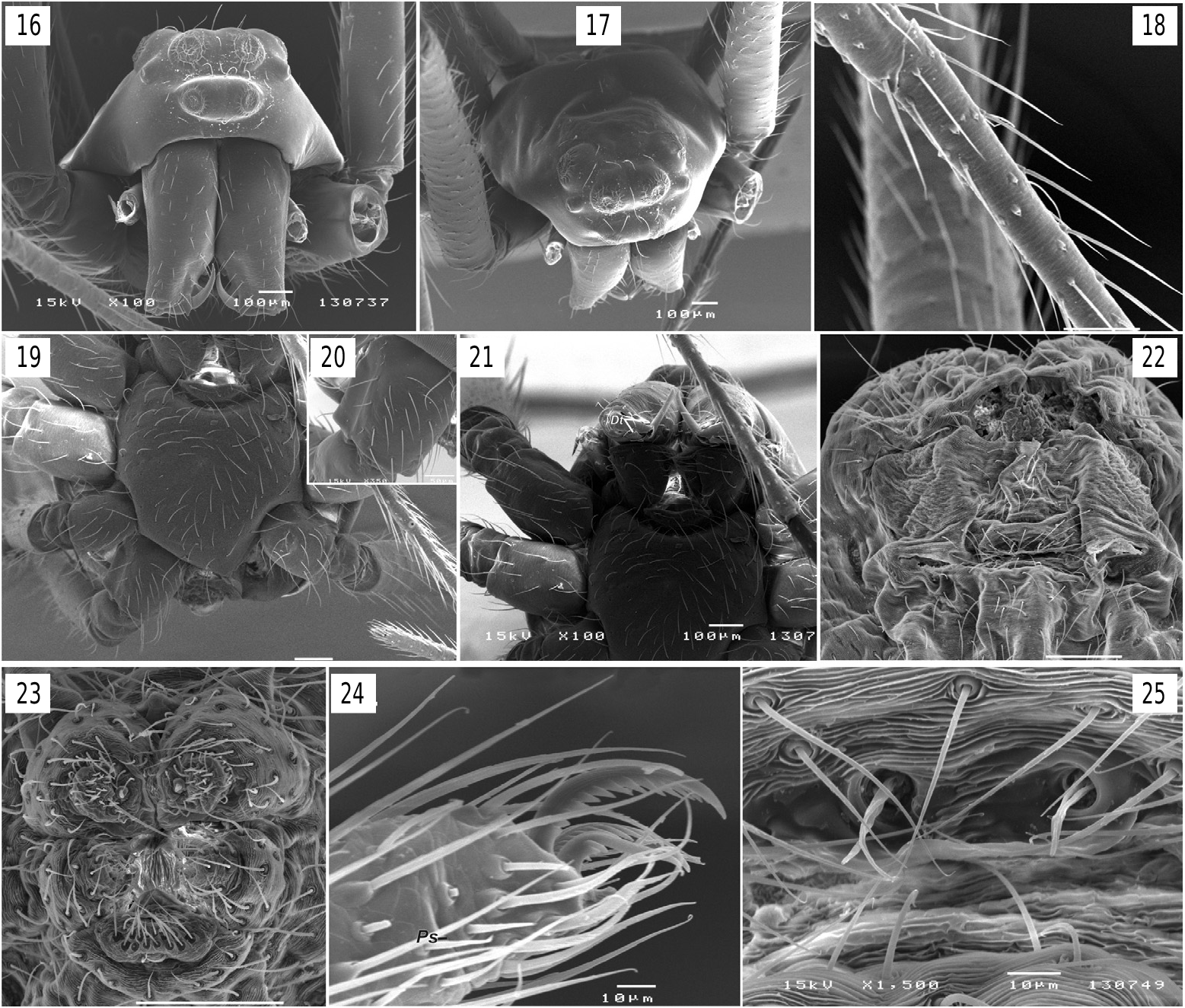
D e s c r i p t i o n. Small, male 2.50, females 2.42–3.10; carapace 1.14 long in male, 1.05–1.23 in females. Carapace pear-shaped, rather high ( figs 1–2, 6–8 View Figs 1–8 , 12–13,15 View Figs 9–15 ), with pattern composed of lateral or sublateral dark bands. Eyes in 3 rows ( figs 12–17 View Figs 9–15 View Figs 16–25 ), AME in first row, ALE and PME in second, and PLE in third. ALE and AME subequal in size, ALE 1.5–2 times smaller than PME, clypeus small, less than 1 diameter of AME. Sternum shield like ( figs 3 View Figs 1–8 , 19 View Figs 16–25 ) with slightly darkened margins. Chelicerae not enlarged, with 3 prolateral and 2–3 retrolateral teeth; distal teeth (Dt) large ( fig. 21 View Figs 16–25 ). Legs with annulations, tibia-tarsus of legs I and II with rows of stiff subdecumbent setae ( fig. 18 View Figs 16–25 ) forming a kind of “catching basket” ( figs 6–8 View Figs 1–8 ). Few macrosetae, 0–5 on each segment. Tarsi pseudosegmented ( fig. 24 View Figs 16–25 ). Coxae IV in male unmodified, lacking stridulatory teeth or ridges. Female palp with straight, untoothed claw ( fig. 12 View Figs 9–15 ). Abdomen patterned, pattern partly composed of white guanine spots in 2 species. Book lung opercula unmodified, lacking stridulatory ridges. Male spinnerets as in fig. 23 View Figs 16–25 . Colulus well developed with 4 setae ( fig. 23 View Figs 16–25 ). Epiandrous plate with 2 pits, each pit with pair of fusules.
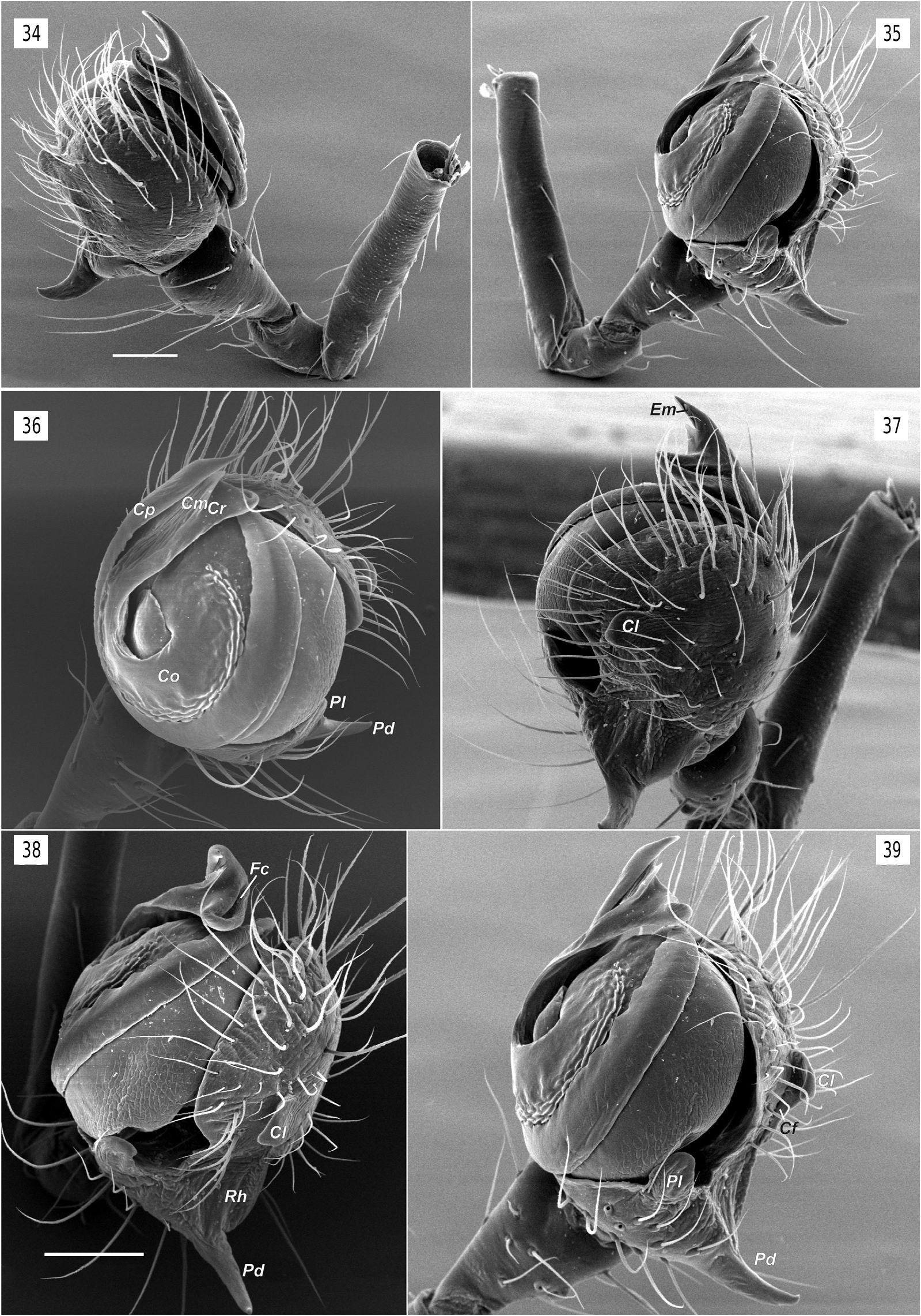
Copulatory organs. Male palp with long femur (6 times longer than wide and about 1.4 times longer than patella + tibia); patella and tibia unmodified; cymbium almost round with retrolateral hollow (Rh), small antero-retrolateral lobe (Cl) connected by shallow fold (Cf) to paracymbium; paracymbium composed of small lateral branch (Pl) and large dorsal claw-like branch (Pd); bulb round in ventral view, hemispherical in lateral view, ventral side of bulb flat, almost entirely covered with broad ribbon-like semitransparent conductor (Co); tip of conductor with 3 processes (rounded retrolateral (Cr), sharply pointed prolateral (Cp) and weakly sclerotized median (Cm )); dorso-anterior part of conductor with furrow (Fc); embolus (Em) very long, filamentous, making 1.5 loops (ca 540°), and entirely enclosed by the conductors fold.
Epigyne weakly sclerotized, with distinct median plate (Mp), copulatory opening indistinct; copulatory ducts (Cd) visible through integument, subparallel; 1–3 pairs of weakly sclerotized receptacles.
R e l a t i o n s h i p s. Although the modified eyes, spination of legs I and II with peculiar stiff setae forming a catching basket, lack of sexual dimorphism, small size and unmodified chelicera in Nediphya gen. n. are similar to these in Diphya , the morphology of the copulatory organs is significantly different between the two genera.
Highly heterogeneous eyes are also known in Pinkfloydia Dimitrov et Hormiga, 2011 , but in that genus only the PME are strongly enlarged and larger than the lateral eyes.
The epiandrous plate in Nediphya ( fig. 25 View Figs 16–25 ) is similar to that in Nanometa (cf. fig. 87E in Álvarez-Padilla & Hormiga (2011)) and Dolichognatha pentagona (Hentz, 1850 (cf. fig. 31G View Figs 26–33 in Álvarez-Padilla & Hormiga (2011)) with 2 isolated pit each bearing 4 fusules.
To date, prolateral rows of stiff setae on tibia-metatarsi of legs I and II are well documented in tetragnathids only in Diphya (Tanikawa, 1995; Marusik, 2017, Marusik et al., 2017), but can also be found in Metellina orientalis (Spassky, 1932) and M. kirgisica (Bakhvalov, 1974) (personal data) and in an unidentified genus and species from Papua New Guinea ( figs 52–54 View Figs 52–54 ).
The bulb in Nediphya is very similar to those illustrated of “ Orsinome ” sarasini Berland, 1924, Nanometinae sp. and Nanometa sp. illustrated by ( Álvarez-Padilla & Hormiga (2011), as well as “ Orsinome ” lagenifera (Urquhart, 1888) . Those species are from either Australia, New Zealand or Tasmania and all have a broad conductor hiding the tegulum as in Nediphya lehtineni and a filamentous embolus hidden partly or entirely by the fold of the conductor. In addition, those taxa all possess an anterolateral lobe of the cymbium (= CEMP or cymbial ectomedian process sensu Álvarez- Padilla and Hormiga (2011)) and a cymbial fold between the lobe and the dorsal branch of the paracymbium (= CEBP or cymbial ectobasal process sensu Álvarez-Padilla and Hormiga (2011)). In addition to the similar bulb and cymbium morphology in the four species, they each have a well-developed ventral branch of the paracymbium bearing few setae; in Nediphya lehtineni sp. n. the ventral branch of paracymbium is strongly reduced and lacks setae ( figs 35–39 View Figs 34–39 ). None of these four species has a modified eye pattern.
Females of “ Orsinome ” sarasini and Nanometa sp. illustrated by Álvarez- Padilla and Hormiga (2011) have epigynes rather similar to that of Nediphya lehtineni sp. n. The complicated morphology of the copulatory organs reflects the phylogenetic relationships between taxa much better than does somatic morphology and thus we consider that Nediphya gen. n. belongs to Nanometinae Forster & Forster, 1999 sensu Álvarez-Padilla & Hormiga (2011) . Nanometinae is currently composed of the monotypic genera Nanometa Simon, 1908 (known from the female only (WSC 2017)) and Pinkfloydia Dimitrov et Hormiga, 2011 ( Álvarez-Padilla & Hormiga (2011).
Status of Nanometinae Forster & Forster, 1999
Forster & Forster (1999) considered Nanometinae to be composed of Nanometa , Orsiella lagenifera (Urquhart, 1888) (Orsiella is a nomen nudum and currently species misplaced in Orsinome ) and Eryciniolia Strand, 1912 . Álvarez-Padilla & Hormiga’s (2011) concept of Nanometinae included only Nanometa , Pinkfloydia , misplaced “ Orsinome ” sarasini, and a single unplaced “ Nanometinae sp.” It is unclear how Álvarez-Padilla & Hormiga (2011) recognized “ Nanometa sp. ” or “ Nanometinae sp.” without studying the type species, N. gentilis Simon, 1908 . The type species is known only by the verbal description of Simon (1908) from Western Australia and figures in Dalmas (1917) of the eye region and epigyne of a New Zealand specimen (WSC 2017). Dalmas (1917) studied Simon’s type and mentioned some differences between specimens from New Zealand and Australia. It is worth noting that Roewer (1942: 1013) erroneously indicated that Nanometa gentilis was described based on the female and known only from Western Australia, although Simon (1908) described both sexes and Dalmas (1917) reported specimens from New Zealand. These errors are repeated in Platnick (2000 –2014) and the World Spider Catalog (2017) which are based on Roewer’s incorrect data.
The morphology of the copulatory organs of Pinkfloydia , Eryciniolia , and two misplaced Orsinome species differ considerably from Nanometa sensu Álvarez-Padilla & Hormiga (2011) and, to our mind, cannot be considered in Nanometinae .
Distinguishing species of Nediphya gen. n. Some species can be recognized by carapace pattern ( figs 2, 4, 6–8 View Figs 1–8 , 12–15 View Figs 9–15 ). All species differ by spination and shape of epigyne (see diagnoses of the individual species).
Composition: Nediphya lehtineni sp. n. (♂, ♀), N. hippai sp. n. (♀), N. lyleae sp. n., and N. padillai sp. n. (♀), all from Papua New Guinea.
Etymology. The genus name is a combination of two letters from terra typica Papua New Guinea with Diphya and, in most Slavic languages, meaning “not Diphya ”. The gender is feminine.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |