Endecous (Ramalhoecous) infernalis, Carvalho & Junta & Castro-Souza & Ferreira, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5263.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:3386FD59-2075-4F6A-8B21-B36C7F463EA9 |
|
DOI |
https://doi.org/10.5281/zenodo.7797743 |
|
persistent identifier |
https://treatment.plazi.org/id/B3578783-FFB0-E853-FF44-F99EFB928A93 |
|
treatment provided by |
Plazi |
|
scientific name |
Endecous (Ramalhoecous) infernalis |
| status |
sp. nov. |
Endecous (Ramalhoecous) infernalis n. sp.
( Figures 2 – 6 View FIGURES 2–6 , 7 – 15 View FIGURE 7–15 , 16 – 17 View FIGURES 16–17 , 18 – 21 View FIGURES 18–21 , 22 – 27 View FIGURES 22–27 , 28 – 31 View FIGURES28–31 ; Table 1 View TABLE 1 )
Material examined– Holotype, ♁, code ISLA 104838, Brazil, Bahia, municipality of Carinhanha, Água Clara cave (43°57′6.24″W, 13°48′4.25″S), 11.x.2017, R. L. Ferreira; condition: right tegmen and left legs I and II were detached and stored alongside the holotype GoogleMaps . Paratypes, 7 ♁♁, ISLA 104839, municipality of Carinhanha , Água Clara cave (43°57′6.24″W, 13°48′4.25″S), 11.x.2017, R. L. Ferreira GoogleMaps ; ISLA 104840, 104841, 104842, municipality of Carinhanha , Água Clara cave (43°57′6.24″W, 13°48′4.25″S), 1.ix.2022, R. L. Ferreira GoogleMaps ; ISLA 104843, municipality of Carinhanha , Lapa dos Peixes I cave (43°57′25.2″W, 13°49′22.08″S), 10.x.2017, R. L. Ferreira GoogleMaps ; ISLA 104844, municipality of Coribe , Baixão da Canoa cave (44° 9′50.35′′W, 13°51′27.69″S), 20.viii.2022, R. L. Ferreira GoogleMaps ; ISLA 104837, municipality of Carinhanha , Gruna do Pedro Cassiano I cave (43°54′50.4″W, 13°47′52.8″S), 14.ix.2021, R. L. Ferreira. GoogleMaps
Etymology. The epithet “infernalis” is a Latin noun referring to those belonging to hell, or to the lower regions, thus being a reference to the subterranean habitat of the species.
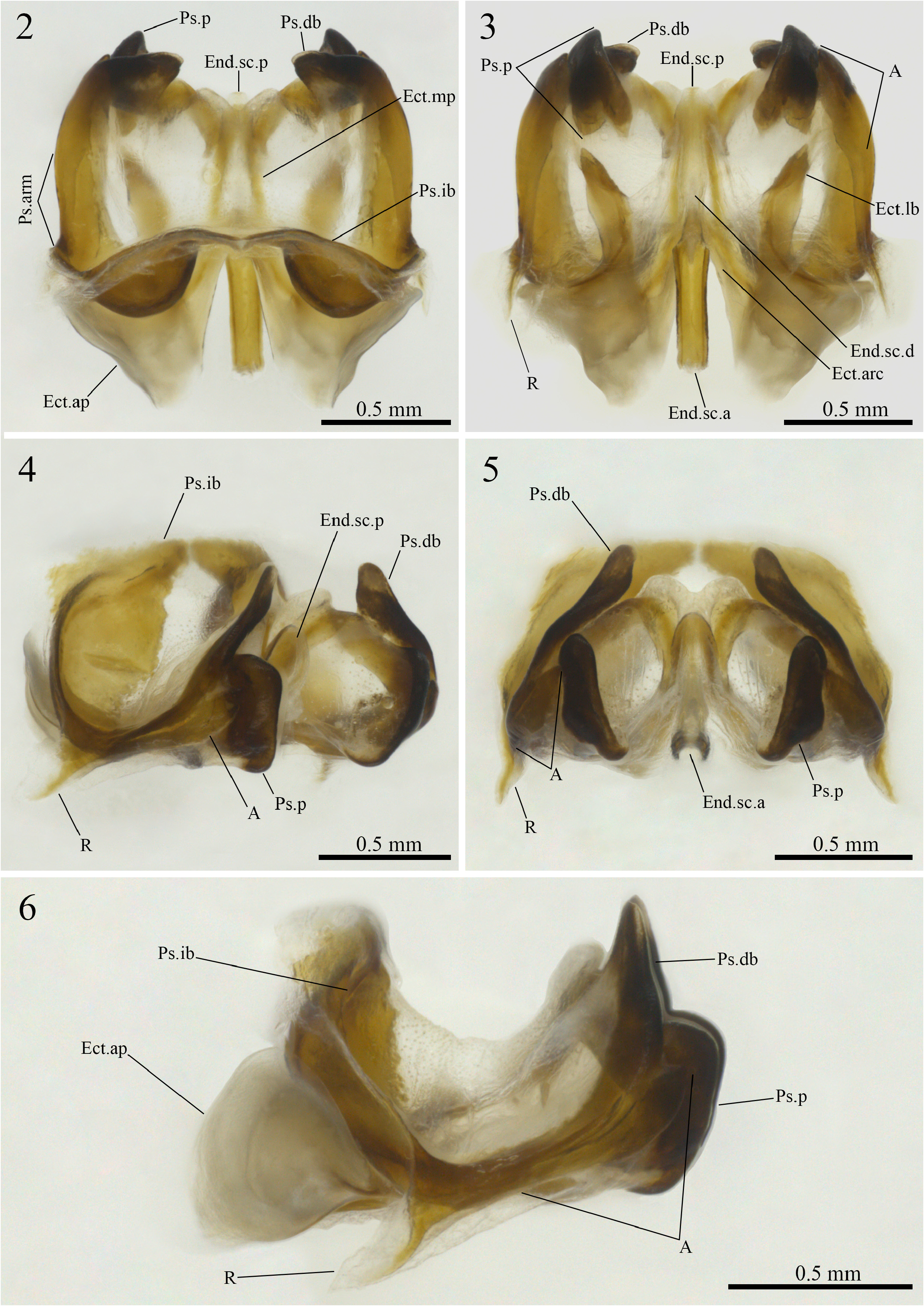
Combination of the following characteristics: pseudepiphallic dorsal branches (Ps.db) dorsally projected and inclined inwards, apex broad and subtriangular in shape, with a less sclerotized tip; pseudepiphallic ventral branches (A) elongated, almost fused to the pseudepiphallic dorsal branches, apex broad and triangular in shape; pseudepiphallic rami (R) underdeveloped and falciform; ectophallic arch (Ect.arc) narrow and conical; ectophallic lateral bars (Ect.lb) short and contorted on themselves, apex acute; endophallic sclerite anterior portion (End.sc.a) elongated and lacking a crest opposite to its central groove.
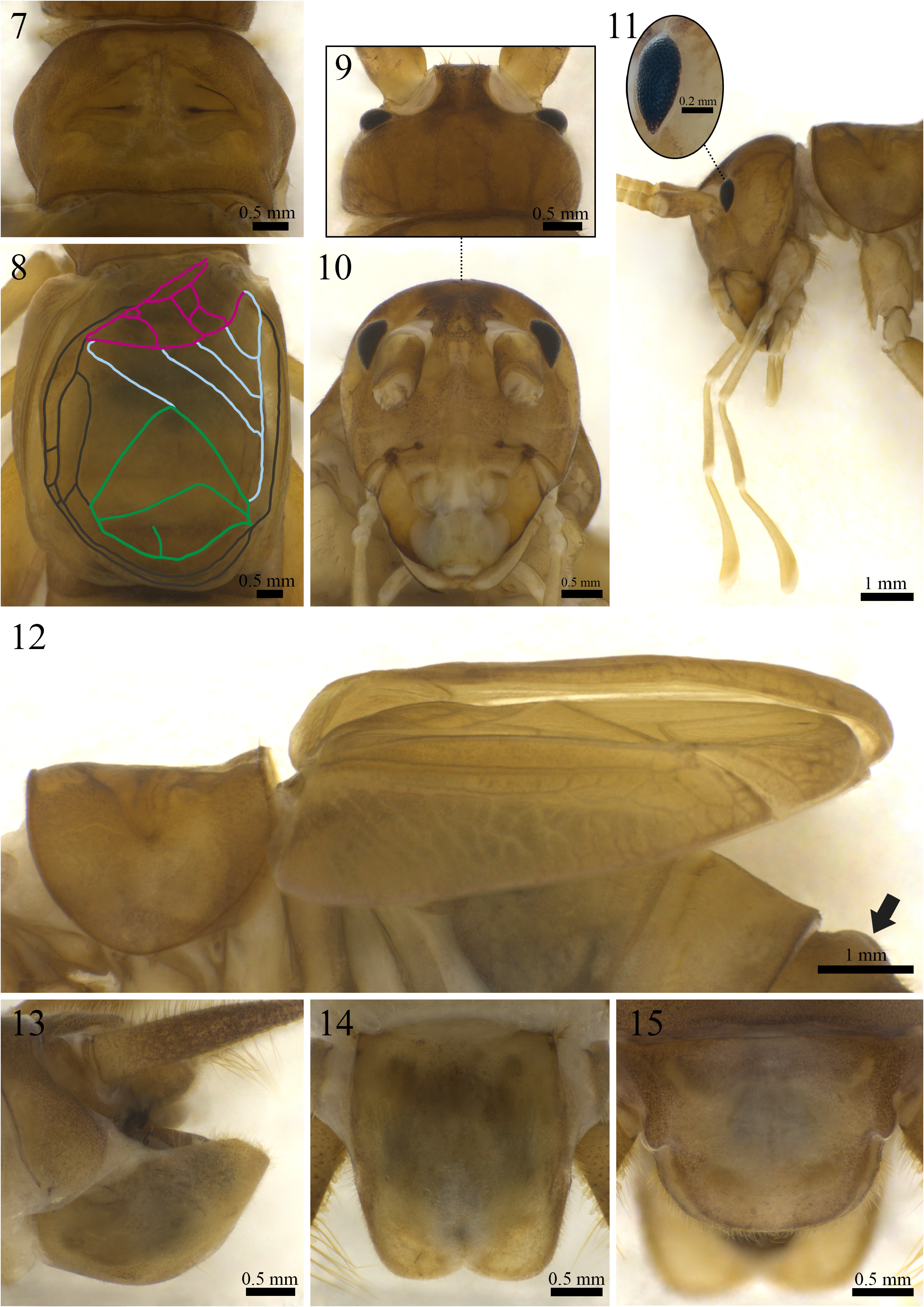
Morphology (paratype ISLA 104837, Figs. 7–15 View FIGURE 7–15 ). Body color: dorsal head yellowish brown, with irregular brown patches reaching the occiput; pronotum yellowish brown; abdomen yellowish brown dorsally and light yellowish brown ventrally ( Figs. 7, 9–15 View FIGURE 7–15 ); entire legs yellowish brown, whitish at their proximal portion ( Figs. 18–21 View FIGURES 18–21 ), cerci yellowish brown at the base ( Fig. 13 View FIGURE 7–15 ) and brownish towards the apex. Head: slightly pubescent, elongated in frontal view; front and gena yellowish brown; fastigium with long bristles, extending the vertex in an inclined plane pointing downwards; clypeus and labrum whitish with yellowish spots; mandibles yellowish brown and more sclerotized at the apex and margins; maxiles whitish and more sclerotized at the apex ( Fig. 10 View FIGURE 7–15 ); all maxillary palpomeres light yellowish brown and pubescent, the first two short and same-sized, the other three are longer, the palpomere V (2.21 mm) is claviform and whitish at the apex ( Fig. 11 View FIGURE 7–15 ); all labial palpomeres light yellowish brown, pubescent and increasing in size, the third one dilated from base to apex, which is whitish ( Fig. 11 View FIGURE 7–15 ); scape, pedicel and flagellomeres yellowish brown, distal portion whitish; compound eyes reduced, ommatidia black, ocelli absent ( Figs. 9–11 View FIGURE 7–15 ). Thorax: pronotum slightly pubescent, dorsal disk broader than long (2.38 and 3.86 mm in length and width respectively), lateral lobes subtriangular and leaning towards the anterior portion of the body, anterior and posterior margins arched and uneven, posterior margin with two laterally oriented small humps ( Fig. 7 View FIGURE 7–15 ). Legs: femur, tibia and tarsus pubescent. Leg I ( Figs. 20 and 21 View FIGURES 18–21 ): tibia slightly longer than the femur, with an oval tympanum on its inner side and two same-sized ventral apical spurs; first tarsomere ventrally serrated and longer than the second and third together. Leg II ( Figs. 20 and 21 View FIGURES 18–21 ): tibia slightly longer than the femur, with two same-sized ventral apical spurs; first tarsomere ventrally serrated and longer than the second and third together. Leg III ( Figs. 18 and 19 View FIGURES 18–21 ): femur developed; tibia slightly longer than the femur (11.44 and 11.11 mm, respectively); tibia armed with four subapical spurs on the outer side, the distal being the shortest, and three on the inner side (four subapical spurs on the inner side of the right leg), the proximal being the shortest, three apical spurs on the outer side ( Fig. 19 View FIGURES 18–21 ; a, b, c), spur “a” being the longest and “c” the shortest, and four on the inner side ( Fig. 18 View FIGURES 18–21 ; d, e, f, g), spurs “e” and “f” longer the “d” and “g” (three apical spurs on the inner side of the left leg, “d” probably lost during capture); first tarsomere longer than the second and third together, with two apical spurs, the inner being the longest. Right tegmen: slightly sclerotized; covering the first two urotergites (6.31 and 4.80 mm in length and width, respectively); harp with three well-marked crossveins and four cells; mirror subtriangular, with one well-marked crossvein and two cells, distal cell with a short and incomplete vertical vein; basal field with three secondary veins connecting Cu2 to 2A, secondary veins 2 and 3 connected by a crossvein, secondary vein 1 anteriorly bifurcated, 2A well-marked and proximally bifurcated, 1A absent; lateral field with two longitudinal veins and many irregular weakly marked secondary veins ( Fig. 8 View FIGURE 7–15 ); stridulatory file with 115 teeth. Abdomen: tergite III with an anterior semi-halved protuberance ( Fig. 12 View FIGURE 7–15 ), tergites IV, V, VI and VII with small and less conspicuous anterior humps; cerci short in comparison to body lenght, pubescent, with globose setae at the base, mainly on the inner side, and long bristles throughout all their extension, proximal portion dilated; sub-genital plate light yellowish brown, longer than wide, lateral margins slightly curved towards the outer side, distal margin rounded, with a small V-shaped dent in the center ( Fig. 14 View FIGURE 7–15 ); supra-anal plate whitish brown, distal margin rounded, shorter than the sub-genital plate, lateral projections rounded and pointing outwards, paraprocts as long as the supra-anal plate and barely visible in dorsal view ( Fig. 15 View FIGURE 7–15 ).
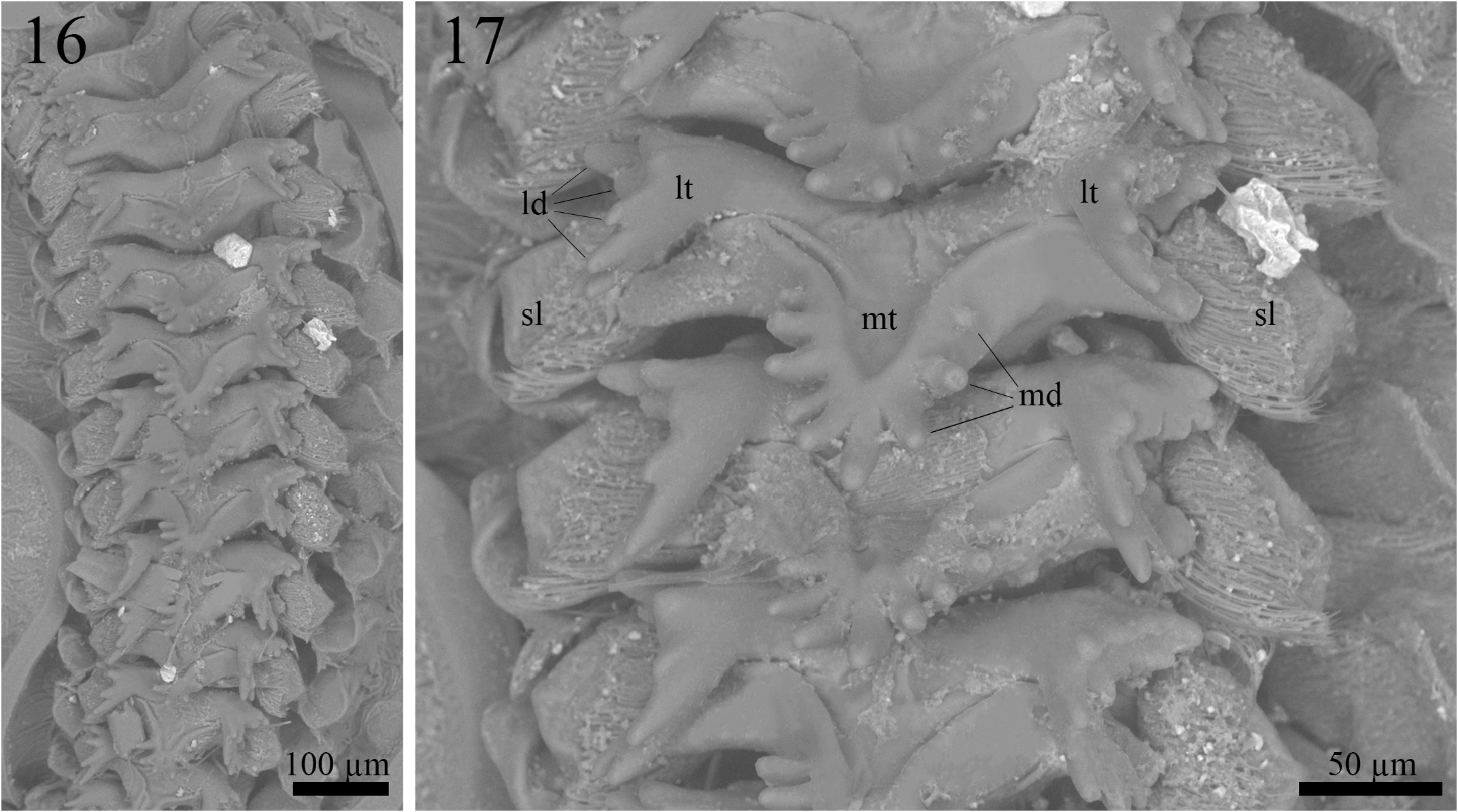
Proventriculus (paratype ISLA 104843, Figs. 16–17 View FIGURES 16–17 ). Proventriculus internally organized in six rows of 12 overlaid sclerotized appendices (sa); sclerotized lobes (sl) bearing a cluster of bristles are visible on each side of the sclerotized appendices; sclerotized appendices formed by a median tooth (mt), with at least four, but up to nine median denticles (md), and two lateral teeth (lt), with several lateral denticles (ld); median denticles elongated and rounded at the tip; lateral denticles also elongated and rounded at the tip; the most posterior sclerotized appendix does not have a median tooth.
Male phallic sclerites (paratype ISLA 104837, Figs.2–6 View FIGURES 2–6 ). Phallic complex uniformly broadened, of intermediate length and almost quadrangular contour in dorsal and ventral views. Pseudepiphallus: arms short and slightly inclined outwards in dorsal view ( Fig. 2 View FIGURES 2–6 , Ps.arm); dorsal branches dorsally projected, apexes broad, subtriangular in shape, with an undeveloped and less sclerotized tip, and inclined towards the interior of the phallic complex in frontal view ( Figs. 2 and 5 View FIGURES 2–6 , Ps.db); “A” sclerite well-developed, elongated, almost fused to the dorsal branch, apex broad and triangular shaped, reaching the dorsolateral central region of the parameres in lateral view ( Fig. 4 and 6 View FIGURES 2–6 , A); paramere 1 and 2 fused in a single circular and concave structure, slightly projected past dorsal branches in dorsal view, ventral portion bifurcated, distal region highly sclerotized, dorsal portion sclerotized, forming a scythe-like edge that partially covers the posterior portion of the ectophallic median projections in dorsal view ( Fig. 2 View FIGURES 2–6 , Ps.p); inner bars broad in frontal view, central region deeply concave, forming a basket-like structure, dorsal portion arranged in arc in dorsal view ( Fig. 2 View FIGURES 2–6 , Ps.ib); rami underdeveloped, reduced to a short, scythe-shaped ventral projection in lateral view ( Fig. 6 View FIGURES 2–6 , R). Ectophallic invagination: arc well-developed, expanding itself longitudinally rather than laterally, anterior and posterior central parts conical in dorsal view, posterior margin more sclerotized than the anterior margin ( Fig. 3 View FIGURES 2–6 , Ect.arc); apodeme developed, ventrally positioned and inclined towards the interior of the sclerite in dorsal view ( Fig. 2 View FIGURES 2–6 , Ect.ap); lateral bar developed, broad, shorter than the ectophallic median projections, slightly curved towards the exterior of the sclerite, apex acute and contorted on itself in ventral view ( Fig. 3 View FIGURES 2–6 , Ect.lb); median projection long, slender and inclined inwards, reaching the parameres and fusing at the apex ( Fig. 2 View FIGURES 2–6 , Ect.mp). Endophallus: anterior portion well-developed and sclerotized, elongated, rectangular in shape, with a central groove, but no crest opposite to the groove ( Fig. 3 View FIGURES 2–6 , End.sc.a); duct short and mostly membranous, reaching the distal portion of the ectophallic median projections in ventral view ( Fig. 3 View FIGURES 2–6 , End.sc.d); posterior portion conical, slightly sclerotized at the apex and barely exceeding the length of the ectophallic median projections in dorsal view ( Fig. 2 View FIGURES 2–6 , End.sc.p).
Variations in phallic sclerites (holotype and paratypes, n = 7, ISLA 104838, 104839, 104840, 104841, 104842, 104843, 104844). Phallic complexes vary slightly in size and degree of sclerotization; pseudepiphallic dorsal branches (Ps.db) vary subtly in degree of inclination towards the center of the sclerite; furthermore, its external contour and curvature also vary, ranging from less acute to more acute, with or without evident vertices; pseudepiphallic ventral branch (A) may be more or less fused to the dorsal branch; bifurcated ventral portion of the pseudepiphallic parameres (Ps.p) may be evident or slightly evident; dorsal portion of the pseudepiphallic parameres in the form of a short or long scythe, covering only the apexes of the ectophallic median projections (Ect. mp) or, at most, half of their length; pseudepiphallic inner bars (Ps.ib) perpendicular or not to the longitudinal axis of the sclerite; ectophallic lateral bar (Ect.lb) contorted on itself only at the apex, throughout all of its extension or, at least, half of its length; ectophallic median projections sinuous or almost linear, but always merging at the apex; endophallic sclerite anterior portion (End.sc.a) rectangular or narrowed at the apex; endophallic sclerite posterior portion (End.sc.p) of conical or trapezoidal shape.
Variations in male right tegmen ( Figs. 22–27 View FIGURES 22–27 ). Stridulatory file with 122 ± 7.31 teeth (n° = 8, holotype and paratypes). Harp outline conserved; two or three diagonal crossveins; three or four cells; a short and incomplete diagonal crossvein may be present ( Fig. 23 View FIGURES 22–27 ); one of the main crossveins may have a few knob-like projections throughout its extension ( Fig. 26 View FIGURES 22–27 ). Mirror outline always subtriangular; one or two crossveins, the proximal one being V-shaped; a short vertical or horizontal incomplete crossvein may be observed ( Figs. 22 and 27 View FIGURES 22–27 ); the distal crossvein may branch out into two crossveins, one short and one long, yet both reach the distal margin of the mirror ( Fig. 25 View FIGURES 22–27 ). Basal field outline conserved; 1A may be absent ( Fig. 22 View FIGURES 22–27 ); secondary veins may branch out from 1A, reaching the Cu2 or the 2A, and forming small cells ( Figs. 23, 25 and 26 View FIGURES 22–27 ); secondary crossveins may connect Cu2 to 2A directly ( Fig. 22 View FIGURES 22–27 ). Lateral field with two longitudinal veins that may or may not converge, but are usually connected through secondary veins and rarely completely parallel and isolated from one another throughout their extension.
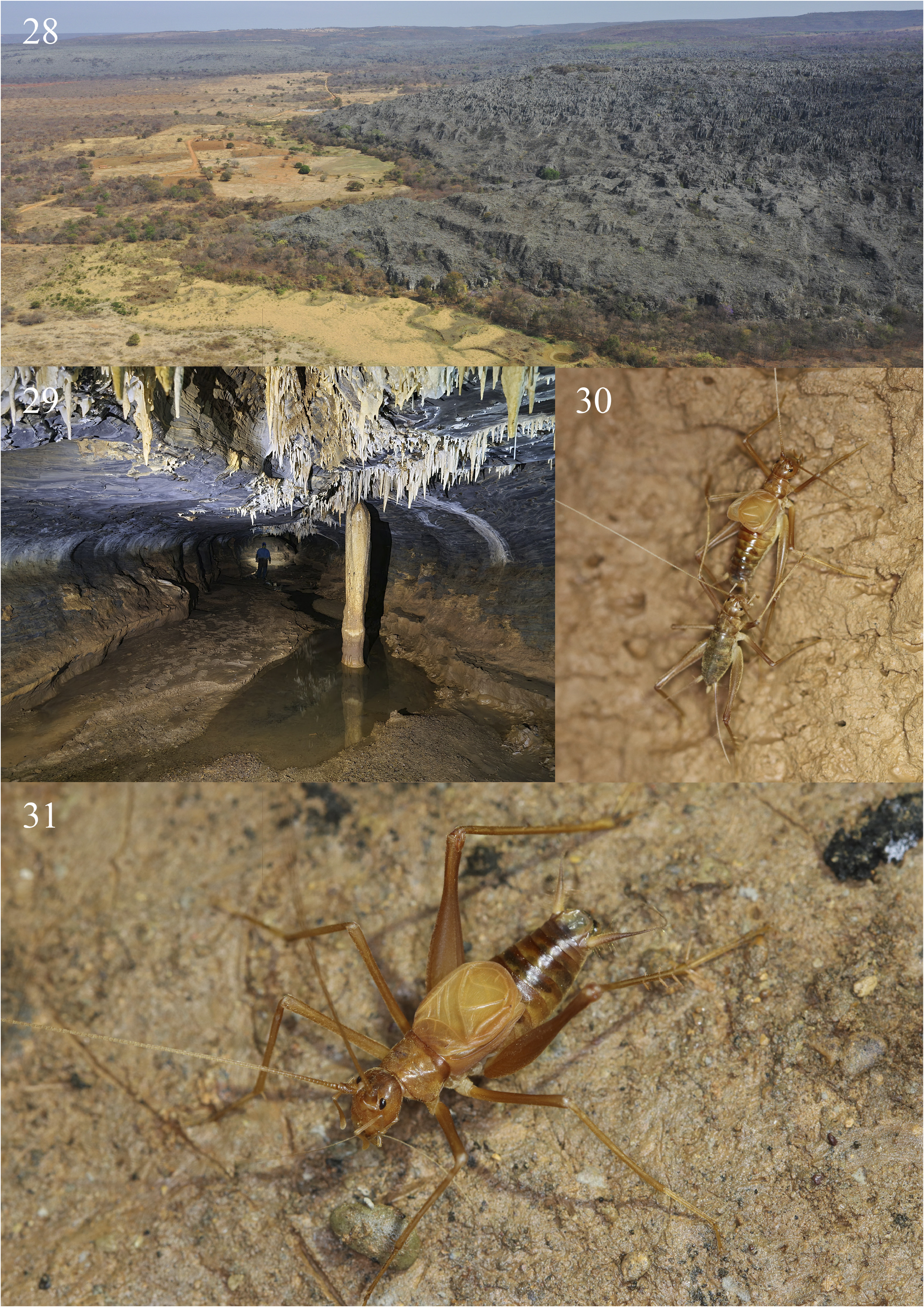
Ecological remarks. Specimens of Endecous infernalis n. sp. were found in Baix„o da Canoa cave, located in the municipality of Coribe, and in other two cave systems: the Gruna da Água Clara cave system (ACCS) and Gruna do Pedro Cassiano I cave ( Fig. 29 View FIGURES28–31 ), both located in the municipality of Carinhanha, southwestern Bahia state, Brazil. The Água Clara cave system represents a set of functionally interconnected caves of approximately 24 km of extension. It is composed of four limestone caves trespassed by an intermittent stream, active during the austral summer (October until March) ( Souza-Silva et al. 2021). The Gruna do Pedro Cassiano I cave ( Fig. 29 View FIGURES28–31 ), in turn, is a single 2.660-meter-long cave that does not belong to the ACCS, but it is located near that system (approximately 4 km in a straight line). The Baix„o da Canoa cave was only recently discovered, so it was not yet properly explored. It is safe to say, however, that the surveyed conduits have at least 1.5 km of extension. This cave has a single known entrance, which leads to a crawling conduit that connects to a wide main chamber, representing the main cave conduit, which has an intermittent drainage active during rainy periods.
Differently from what is usually observed for most of the Endecous species occurring in Brazilian caves, the caves aforementioned had a very low population density of Endecous infernalis n. sp. Hence, it was relatively hard to find specimens (especially adults) in all the caves in which it occurs. Although practically the entire ACCS has been inventoried for cave invertebrates in a study carried out in 2017 ( Souza-Silva et al. 2021), only few specimens were found, mainly in deep and moistened areas within the system. It is noteworthy that the two caves located at the intermediate portion of the ACCS (Gruna dos Índios and Lapa dos Peixes caves) have a quite dry main conduit due to the airflow that comes from the entrances in both sides of their main conduits and trespasses both caves. Because of that, individuals of E. infernalis n. sp. were seldom observed in this intermediate portion of the ACCS, probably due to the unstable climatic conditions of the caves. A second population was observed in the Gruna do Pedro Cassiano I cave in deeper areas within the cave, far from the entrance. Most specimens found in both cave systems were observed freely walking on the cave floor or in the caves’ walls ( Figs. 30 and 31 View FIGURES28–31 ). Finally, a third population was recently discovered in the Baix„o da Canoa cave. Again, specimens were only observed in deeper and moistened areas, and, as a result of the low-density population, the individuals were thinly dispersed. A single adult male was collected, despite the sampling efforts employed along the cave.
It is important to mention the distance among the Gruna da Água Clara cave system (ACCS), Gruna do Pedro Cassiano I and Baix„o da Canoa cave. While ACCS and Gruna do Pedro Cassiano I cave comprise close systems (entrances located approximately 4 km in a straight line from each other), the Baix„o da Canoa cave is located far from the first two cave systems (22 km in a straight line). All these caves are associated with the main massif of the Serra do Ramalho mountain range, which comprises a large continuous carbonate outcrop that runs from southwest to northeast. Furthermore, the Baix„o da Canoa cave is located at a higher altitude, being associated with a hydrological recharge zone in the landscape, while the ACCS and the Gruna de Pedro Cassiano caves are located at lower altitudes, being associated with the base of the outcrops in hydrological discharge zone. Thus, it is plausible to assume the existence of subterranean connectivity between those systems.
An interesting observation was made in a deeper area of the Gruna da Água Clara cave (Part of the ACCS) during an expedition in 2017: an adult male was stridulating while a juvenile female was interacting with its cerci ( Fig. 30 View FIGURES28–31 ). Although it was not possible to observe in detail, it seemed that the female was touching the basal region of the male’s cerci, where modified setae (globose setae) can be found, with her mouthparts. In both Grylloidea and Blattodea, club-shaped and globose setae are hypothesized to act as gravity receptors ( Nicklaus 1969; Bischof 1975; Horn 1985; Hartman et al. 1987), but, as pointed out by Desutter-Grandcolas (1998a), further neuroethological studies of these insects are necessary to confirm or refute this function. Given the observed behavior, one can hypothesize that such setae may also attract females for mating, functioning eventually as a nuptial gift. Although the SEM images of such setae usually show them flattened, they are, in their natural state, swollen, due to being filled with fluid. Considering that the observed female was immature (the ovipositor was still short and not sclerotized), this behavior could represent an advantage in the case of oligotrophic environments and low-density populations, in which the encounter among specimens may be more infrequent, especially in extensive cave systems such as the ACCS. Nevertheless, such hypotheses deserve further studies in order to confirm or refute it.
It is important to mention the troglomorphic traits observed in this species, which will be discussed further on. When compared to other Endecous species (most of which are troglophilic), E. infernalis n. sp. presents reduced eyes and longer legs. Other Endecous species previously considered troglobitic, as E. peruassuensis and E. apterus , presents much less regressive morphological traits than those observed in the species herein described. Bearing in mind the species distribution within the caves it inhabits (always in deeper moist regions) and the quite severe external dry habitat surrounding the caves, it is plausible to assume it is, in fact, a troglobitic (cave-restricted) species.
Although Gruna do Pedro Cassiano cave receives local visitors sporadically, such visitors do not reach the cave deepest regions, where the specimens occur. The Gruna da Água Clara cave system, on the other hand, is only visited by speleologists during technical/scientific expeditions, which seldom occur. Such activities apparently do not represent a threat for the species. The Baix„o da Canoa cave was only visited once by the researchers who sampled the cave, thus it is pristinely preserved. The external environment surrounding the caves, however, is severely altered ( Fig. 28 View FIGURES28–31 ). Most of the original forests were replaced by pastures or other monocultures. The forests remain only in the top and border of the limestone outcrops, in randomly dispersed patches of vegetation or alongside intermittent drainages. Such impacts may reduce the organic input to the cave ecosystems and increase the silting in all those caves, thus altering several microhabitats of this species.
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Grylloidea |
|
Family |
|
|
SubFamily |
Luzarinae |
|
Genus |
|
|
SubGenus |
Endecous |