Cestocampa
|
publication ID |
https://doi.org/ 10.5281/zenodo.210286 |
|
DOI |
https://doi.org/10.5281/zenodo.5618828 |
|
persistent identifier |
https://treatment.plazi.org/id/B33BFF25-FFF7-CD34-FF09-2ED3FEA5E810 |
|
treatment provided by |
Plazi |
|
scientific name |
Cestocampa |
| status |
|
Problems delimiting Cestocampa
The only diagnostic character that defines Cestocampa is the telotarsal process. This character has been used as a taxonomic criterion to define genera, and occasionally species, in Diplura ( Silvestri 1902; Silvestri 1912; Silvestri 1933a; Silvestri 1933b). However, Condé (1959) reported two different forms of the telotarsal process in a single specimen of Podocampa spenceri ( Silvestri,1933c) from Texas ( USA), in which, during molting, the setiform telotarsal process of the exuvia became laminar and pubescent. Similarly, Condé and Geeraert (1962) found a population of the same species in Louisiana ( USA) that included specimens with setiform or laminar telotarsal processes, as well as intermediate forms. ( Sendra et al. 1986, Sendra et al. 2006) Similar variation in the telotarsal process has been demonstrated in other Podocampa spp. from the Iberian Peninsula and Libanocampa Condé 1955a from the Anatolian Peninsula. The laminar and pubescent telotarsal process allows walking on smooth surfaces (glass for instance) and are more frequently found on specimens in dry and warm habitats, as observed in Lepidocampinae . Nevertheless, the same process can also be found in species from humid, subterranean habitats, such as the Iberian cave-dwelling Paratachycampa ( Bareth and Condé 1981) or the monotypic genera Vandelicampa Condé (1955a) from Lebanon and Patrizicampa Condé (1956b) from Sardinia. In the case of V. tisserandi Condé (1955a) , these structures are very similar to those observed in Cestocampa spp. Interestingly, Vandelicampa and Cestocampa share additional features, such as the complex chaetotaxy on thoracic and abdominal tergites (with more than one pair of la, lp or post macrochaetae), urotergites with la and lp macrochaetae, femur III with one dorsal macrochaeta, claws with ridged lateral crests but without talons in cavedwelling species and small lateral crests in endogean species, urosternite VIII with 2+2 macrochaetae, first urosternite without glandular setae on the posterior margin males with a lateral-internal field of glandular a2-setae (male unknown in C. balcanica ).
Phylogeographic patterns and population structure. Little is known about phylogeographic patterns and population structure in Dipluran species. However, a comparative analysis of the complete mitogenomes of two Campodea diplurans has found a high degree of diversity among the species, which suggests either accelerated rates of nucleotide substitution or a remarkable morphological conservatism ( Podsiadlowski et al. 2006). Furthermore, studies conducted on other soil-dwelling arthropods, such as Collembola, have revealed striking levels of differentiation among localities, even over distances in the order of tens of kilometers ( Cicconardi et al. 2010; Garrick et al. 2007; Timmermans et al. 2005). Patterns of deep genetic divergence were also observed among Cestocampa iberica n. sp. samples. The largest divergence is between a specimen from Cova de l’Orao and the remaining populations. The divergence observed, however, was similar to that between Plusiocampa and Cestocampa , which may suggest that the Cova de l’Orao specimen may actually represent a different species misidentified as C. iberica n. sp.
Phylogenetic relationships of C. iberica n. sp. haplotypes showed a clear geographic pattern: one clade included haplotypes found in the northern populations and another clade of southern specimens. Northern haplotypes showed higher levels of genetic divergence, which may indicate a longer time of divergence. Therefore, a north-to-south colonization route could be hypothesized. The reciprocal monophyly of the two main lineages, however, was not strongly supported. A more thorough population sampling and additional molecular markers will be necessary to fully explore this hypothesis.
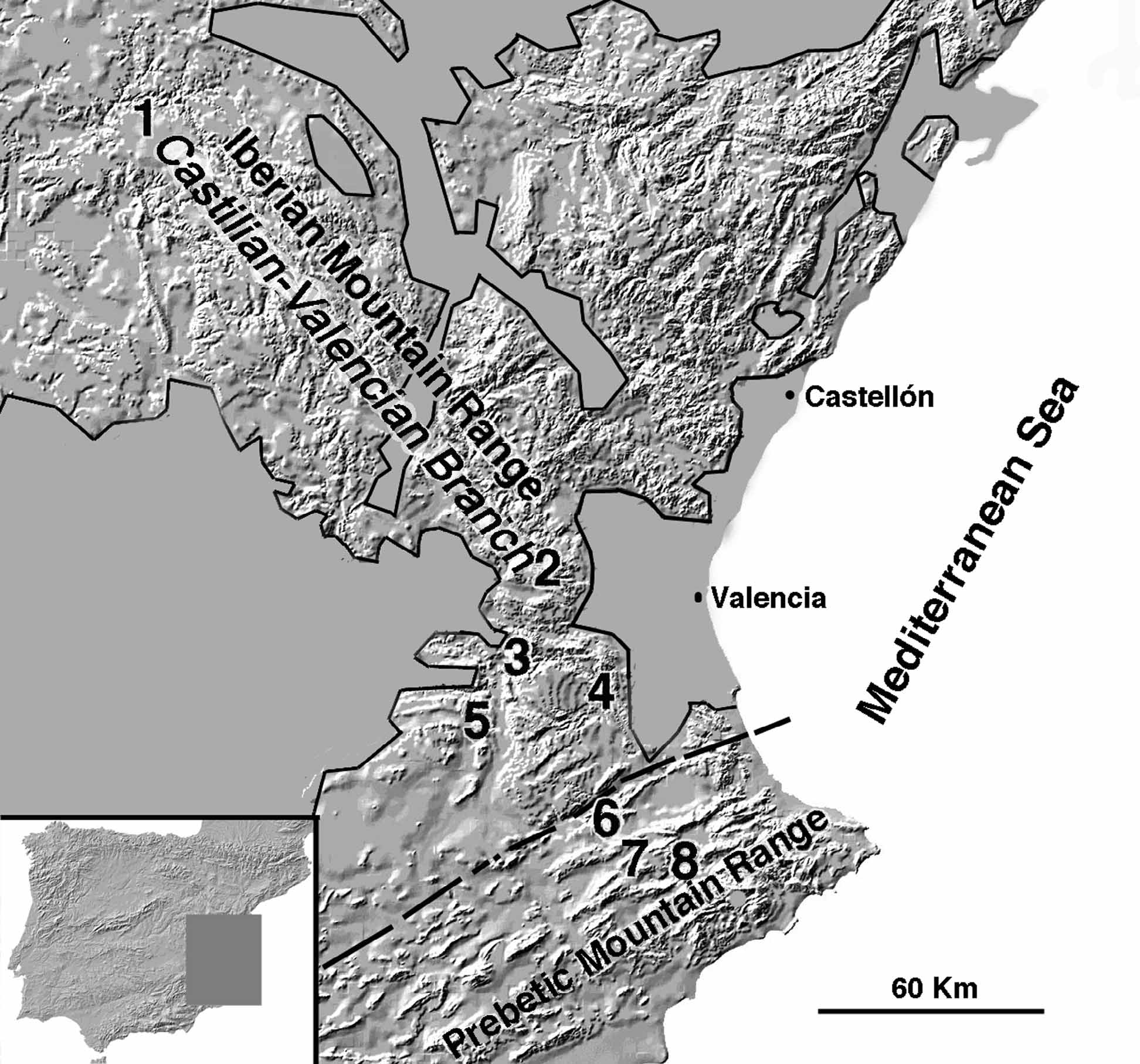
Obligate cave-dwellers have restricted dispersal abilities as a result of extreme adaptations and narrow ecological preferences ( Gibert & Deharveng 2002). Accordingly, many troglobites have extremely small ranges and show strong population structure (e.g., Crouau-Roy & Bakalowicz 1993; Snowman et al. 2010). Non-obligate cave-dwellers, on the other hand, usually have larger ranges and higher levels of gene flow because of greater continuity between habitats ( Caccone 1985). Our analyses revealed that most C. iberica n. sp. haplotypes were restricted to single caves, which supports their limited dispersal capabilities. The distribution range of the species, however, spans more than 200 km, covering large areas that are not suitable for sustaining an underground means of dispersal ( Fig. 5 View FIGURE 5 ).
Therefore, either the ancestor of C. iberica n. sp. was a widespread soil-dweller or C. iberica n. sp. is not an obligate cave-dweller, able to disperse over the surface, at least during certain climatic conditions. The latter alternative may explain the presence of haplotypes from two divergent lineages in Cueva Hermosa , which suggests genetic admixture through gene flow from different sources. In the case of Cova de la Sarsa, where some haplotypes are more similar to Cova de les Meravelles and others are closer to Avenc Vinalopó, a better explanation is the possible existence of underground connections between these caves, since they all belong to the same karst system.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |