Onthophagus cyanellus Bates, 1887
|
publication ID |
https://doi.org/ 10.5281/zenodo.7887628 |
|
publication LSID |
lsid:zoobank.org:pub:E701D60F-A455-4048-8279-DA450930ACB3 |
|
persistent identifier |
https://treatment.plazi.org/id/B31B87B0-1F7C-1013-4FFC-B51CFE7FF86D |
|
treatment provided by |
Felipe |
|
scientific name |
Onthophagus cyanellus Bates |
| status |
|
Onthophagus cyanellus Bates View in CoL
Onthophagus cyanellus Bates 1887: 81 View in CoL .
Onthophagus mesoamericanus Zunino and Halffter 1988: 123 View in CoL (new synonymy by Kohlmann and Solís 2001: 196).
Onthophagus mesoamericanus Zunino and Halffter View in CoL (restoration of species status by Pulido-Herrera and Zunino 2007: 109).
Onthophagus mesoamericanus Zunino and Halffter View in CoL (synonymy reaffirmed by Solís and Kohlmann 2012: 12).
Onthophagus mesoamericanus Zunino and Halffter View in CoL (restoration of species status by Moctezuma 2021: 194).
Onthophagus mesoamericanus Zunino and Halffter , reaffirmed subjective synonymy.
Zunino and Halffter (1988) described O. mesoamericanus Zunino and Halffter, 1988 based on three specimens, two males and one female from Costa Rica. In 2001, Kohlmann and Solís synonymized this species with O. cyanellus . This decision was based on studying the two paratypes of O. mesoamericanus deposited in the Muséum National d’Histoire Naturelle, Paris, and studying a lectotype of O. cyanellus from the Natural History Museum, London (Kohlmann and Solís 2001), plus 490 specimens of O. cyanellus deposited in the INBio collections. The main characteristics differentiating O. mesoamericanus from O. cyanellus , according to Zunino and Halffter (1988), are the green color, a black clypeal margin, and pronotal slope, and an unarmed male frontal keel. These characteristics, plus characteristics of the male genitalia, were studied and compared with the ones presented by Costa Rican O. cyanellus populations, and the diagnostic characteristics were found to fall within observable variation of O. cyanellus . The synonymy was based on the study of blue, green, and copper morphs of O. cyanellus from Costa Rica. Zunino and Halffter (1988) also wrote that the type locality, Cedros, referred to Cerro Cedial in the Cordillera de Talamanca. However, Cedros is a district of San José, which has been now thoroughly urbanized and lies in the Cordillera Central. Likewise, the only two paratypes of O. mesoamericanus are from “Rancho Redondo” in Cartago, a locality located in the Cordillera Central.
In 2007 Pulido-Herrera and Zunino published an atlas of the American Onthophagini which considered both species valid. This situation prompted a reanalysis by Solís and Kohlmann (2012) of material from Costa Rica and reaffirmation of their initial conclusion of synonymizing O. mesoamericanus and O. cyanellus .
Subsequently, Moctezuma (2021) resurrected O. mesoamericanus based on the analysis of its holotype and the following numbers of studied O. cyanellus : 20 specimens from Costa Rica, ten from Panama, and 390 specimens from Mexico, plus a photograph of the O. cyanellus lectotype and two photos of O. mesoamericanus from the BOLD database. He indicated that the database specimens belonged to two females; actually, the one he cited as ASSCR1194-11 is a hypothelic male, not a female. Further, he indicated that these specimens are registered as O. mesoamericanus in the BOLD database, whereas they are labeled as O. cyanellus in that same database ( Fig. 8 View Figures 6–11. 6 ). So, Moctezuma (2021) revalidated O. mesoamericanus based on comparing one specimen, the holotype, with specimens of O. cyanellus . We disagree with his action for the reasons indicated in the sections below.
For the present study, we examined the Costa Rican O. cyanellus material stored in the Museo Nacional (National Museum) of Costa Rica, housing 617 specimens and Nicaraguan material from the Ángel Solís Collection, as well as the BOLD taxonomic platform. We also examined personally the two paratypes of O. mesoamericanus ( Fig. 34–37 View Figures 34–39 ). The following taxonomic analysis utilizes morphological (external and genitalia), genetic, and biogeographical characteristics.
Color and mtDNA variation
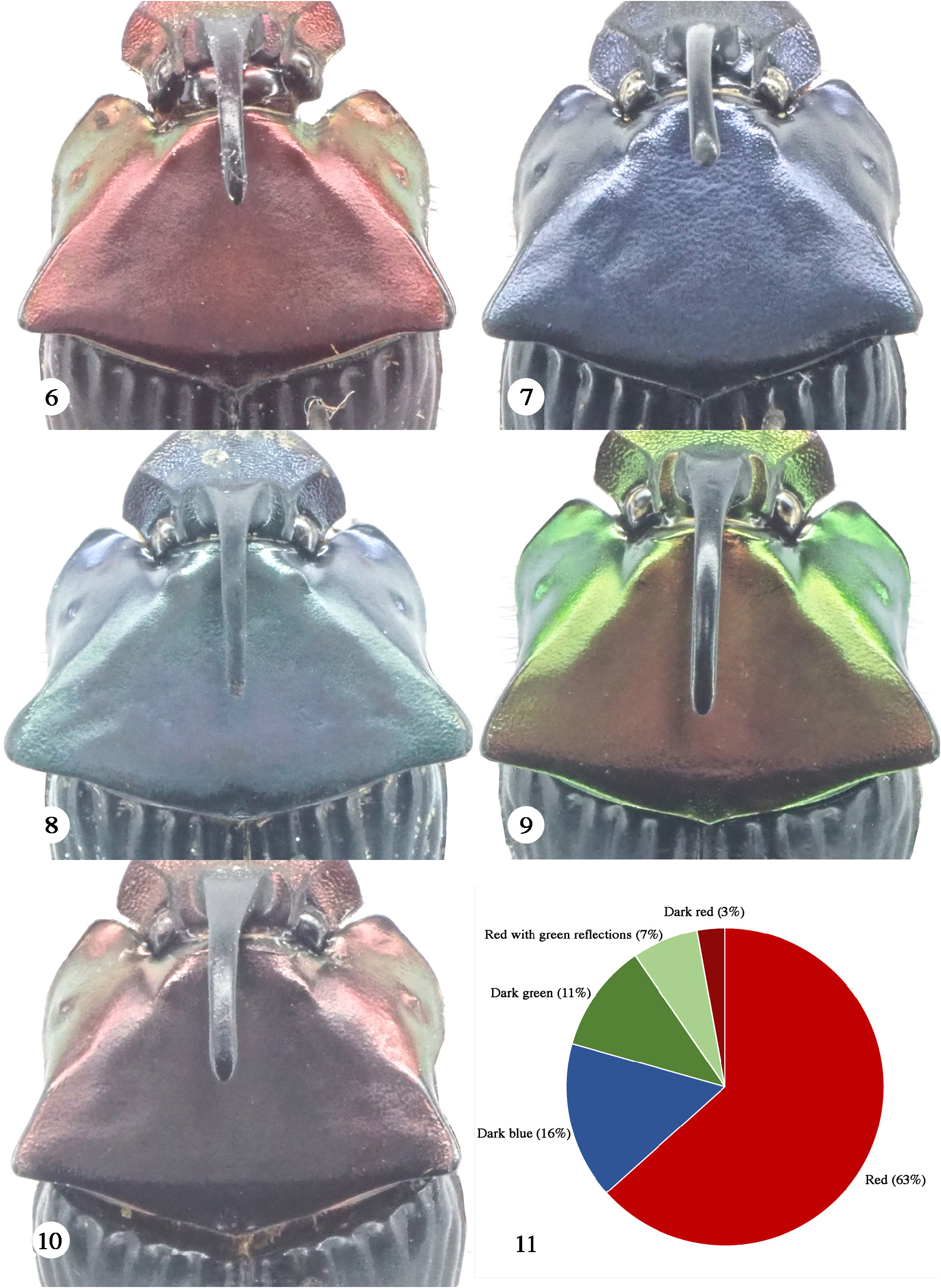
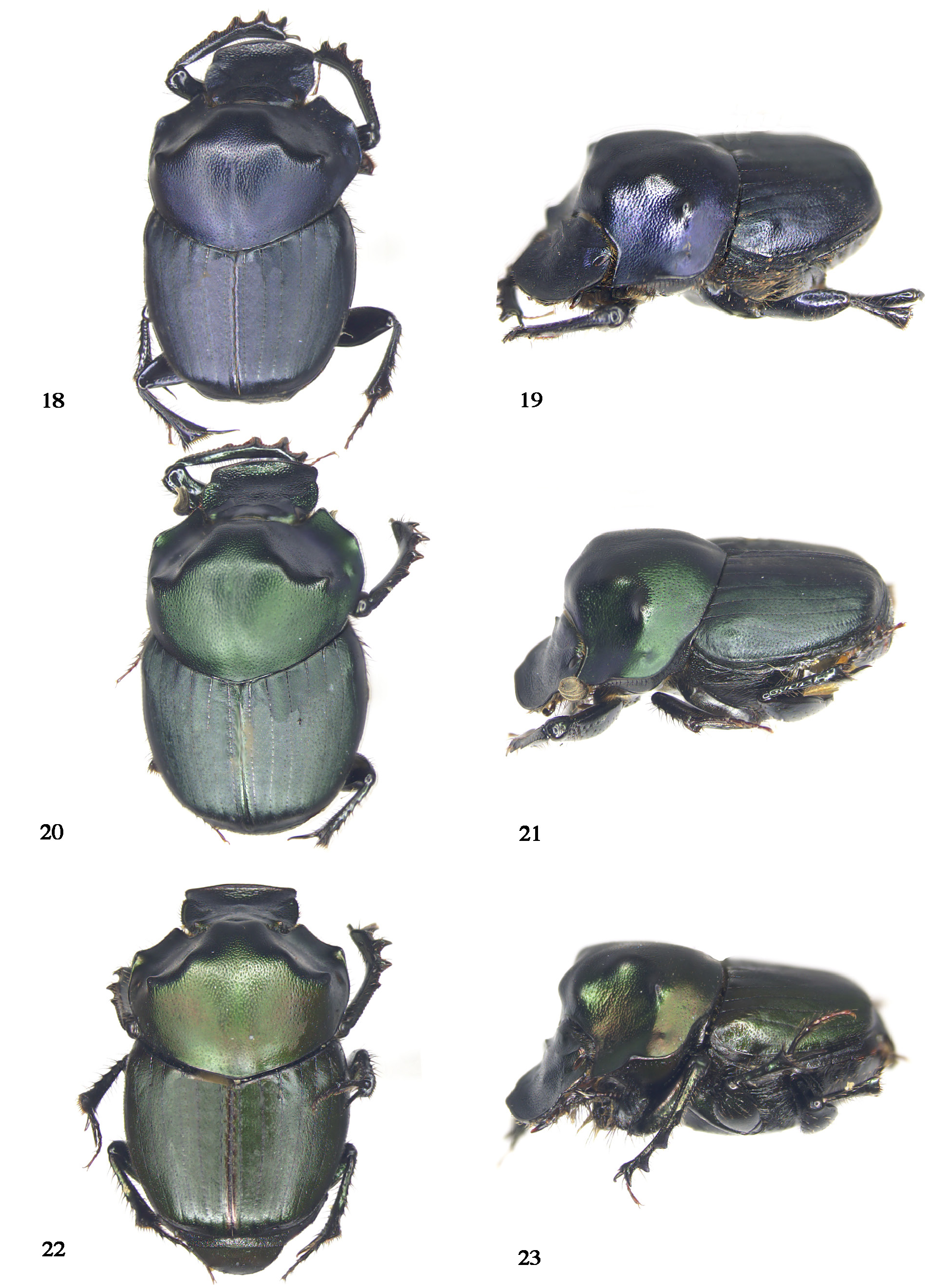
Many years of collecting by us reveal that many Costa Rican populations of O. cyanellus contain blue, copper, and green specimens in the same locality at the same time ( Fig. 18–23 View Figures 18–23 ) along the Cordillera Central and the Cordillera de Talamanca. In other words, polychromy is part of the population variation of this species in Costa Rica. A similar phenomenon has been observed and reported for Costa Rica in the case of Canthon cyanellus LeConte, 1859 ( Solís and Kohlmann 2002), populations of which can include up to five color morphs in one locality. Table 1 summarizes color morph frequencies for O. cyanellus in two Costa Rican locations.
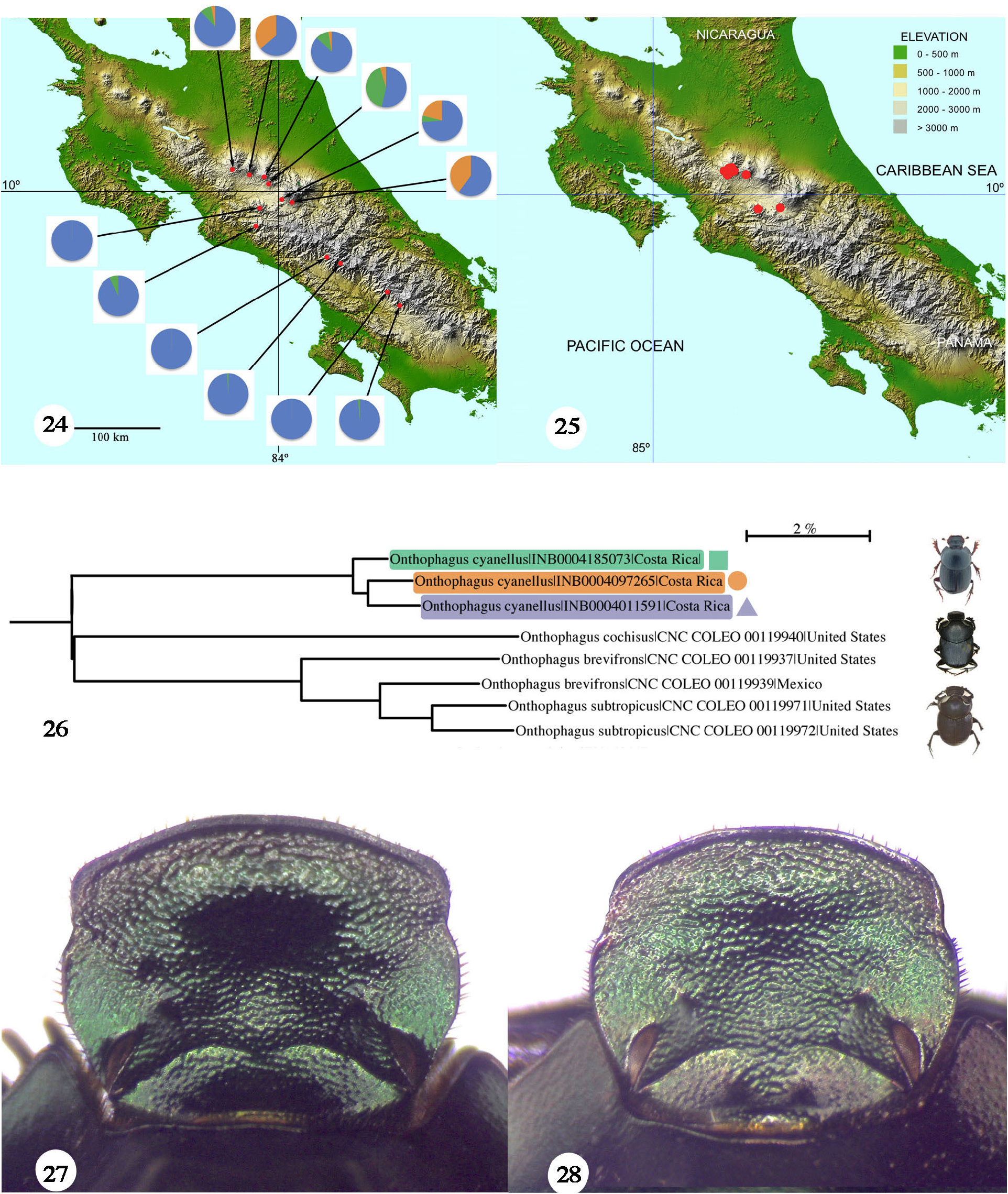
Of note is the fact that relative abundance (frequency) of blue morphs declines while frequencies of green and copper morphs increase in the Cordillera Central compared to the Cordillera de Talamanca ( Fig. 24 View Figures 24–28 ). The blue morph always dominates, with green and copper morphs coming in second or third place ( Table 1). Some rare color morphs are blue-green and copper-green individuals. One possible explanation for the presence of color morphs is the recent colonization of the Cordillera Central by populations originating from the Cordillera de Talamanca and the concomitant genetic drift caused by small founder populations. This recent spread could have been caused by the Last Glacial Maximum (≈20 ka, Jackson et al. 2019; Kohlmann et al. 2019) or facilitated by the establishment of cattle ranching in the area during the last 470 years. Another possibility is that O. cyanellus populations in the Costa Rica – Panama area represent a limit to the distribution range for this species. O. cyanellus populations in the Sierra Madre Oriental in Mexico represent the other distributional (possibly initial) range limit. Due to different climatic and ecological conditions, allele frequency may differ from the northern to this extreme southern boundary. Zunino and Halffter (1988: 135) considered that O. cyanellus represents a successful old distribution, having crossed the two North American tropical portals, the Tehuantepec Isthmus, and the Nicaraguan Lake region, into the mountains of Costa Rica and Panama.
Further study of color morph variation figures in the comprehensive mtDNA barcode analysis (COI) that is being done for the Scarabaeinae fauna of Costa Rica ( Solís and Kohlmann 2012; Kohlmann et al. 2019. An mtDNA barcode tree is presented here ( Fig. 26 View Figures 24–28 ), encompassing several species of the O. chevrolati species group ( Zunino and Halffter 1988), of which O. cyanellus is a member. Three color morphs from two Costa Rican localities, one green from Grecia (Alajuela) and a blue and a copper morph from La Unión (Cartago), were analyzed, forming the O. cyanellus species-complex separate from the other complexes derived from the analysis. The average mtDNA genetic distance separating the three different O. cyanellus color morph specimens (1.15%) in Figure 26 View Figures 24–28 is below a 2% bp sequence divergence, considered the conventional threshold for separating species ( Jones et al. 2011), so the specimens can be considered as assignable to the same species (see discussion below).
Zunino and Halffter (1988: 30) illustrate the chevrolati species group using a phylogenetic diagram. They define and further divide the 58 species comprising the group into several species’ complexes ( Zunino and Halffter 1988: 30; see also Halffter et al. 2019) This species group is perhaps the most common one on the American continent. Several species complexes of the O. chevrolati species group are recovered at distances greater than a 2% COI divergence, supporting the proposed species complexes proposed originally by Zunino and Halffter (1988). Three species complexes are evident: cyanellus , chevrolati ( O. cochisus Brown, 1927 ), and brevifrons ( O. brevifrons Horn, 1881 and O. subtropicus Howden and Cartwright, 1963 ). Zunino and Halffter (1988) proposed the cyanellus species complex within this species group, which is cleanly recovered in the species tree. The cytochrome c oxidase I (COI) results show a clear mitochondrial DNA distance difference between the cyanellus species complex and the branch with the other members of the chevrolati species group ( Fig. 26 View Figures 24–28 ). Although the present analysis does not include all species, the tree recovers three of the ten species complexes proposed by Zunino and Halffter (1988) and Halffter et al. (2019).
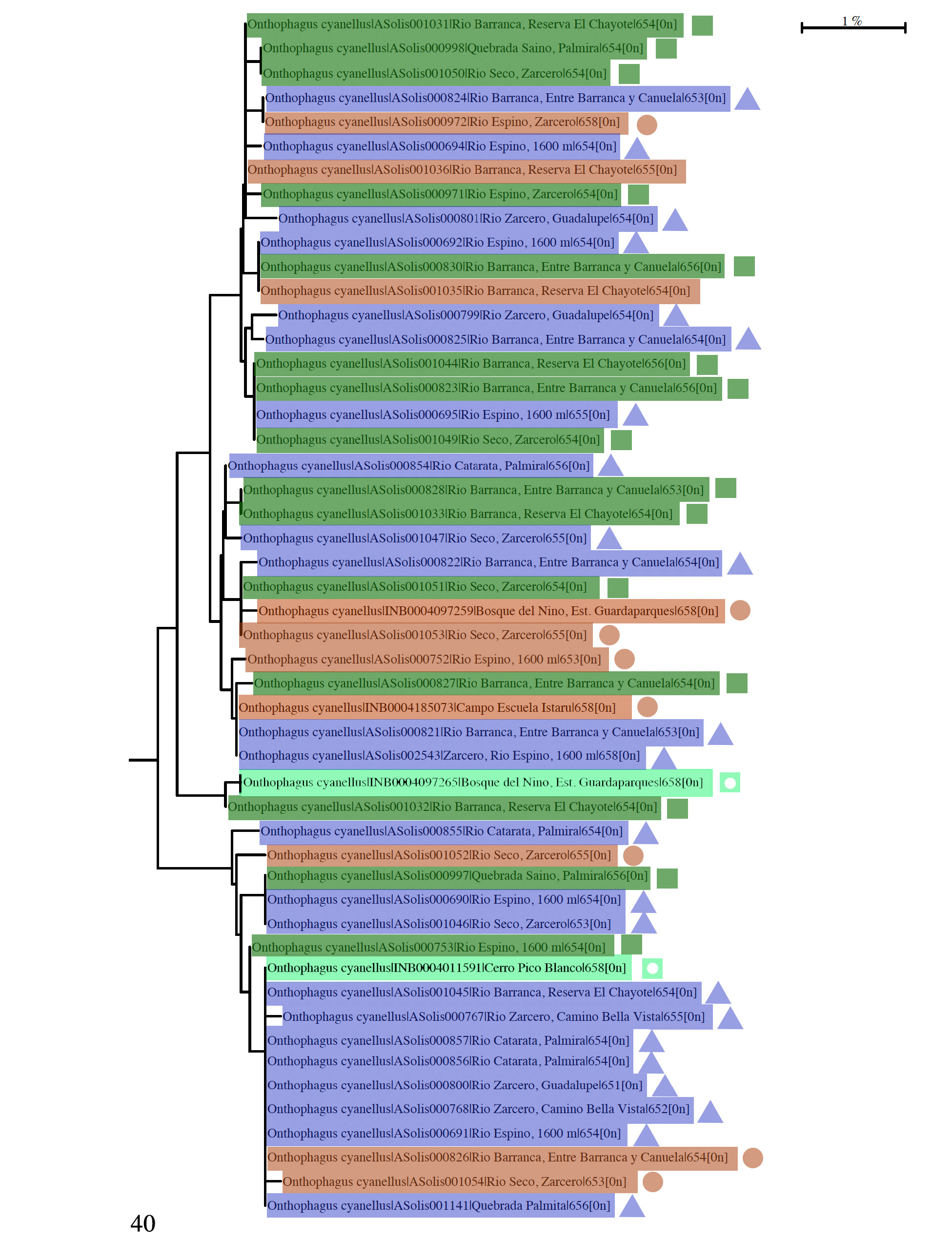
A more detailed mtDNA barcode tree ( Fig. 40 View Figure 40 ), mixing all three-color morphs and several localities, shows that the O. cyanellus populations in Costa Rica form a single species, including those from localities in the Cordillera Central and the Cordillera de Talamanca ( Fig. 25 View Figures 24–28 ). The intraspecific cytochrome c oxidase I (COI) results for O. cyanellus show a lower mitochondrial DNA average genetic distance difference (average Kimura-2-parameter [K2P] =1.15%, max = 2.3%, min = 0%) than found for a Phanaeus sister-species pair, P. beltianus Bates, 1887 and P. changdiazi Kohlmann and Solís, 2001 (average Kimura-2-parameter [K2P] = 3.0%) ( Solís and Kohlmann 2012). These average values are below those that Johns and Avise (1998) found (K2P difference)—3.5% in 47 pairs of bird sister species—and well below the value of 12.56% that measures the average Kimura-2-parameter [K2P] representing the average genetic distance between O. cyanellus and the O. brevifrons + cochisus + subtropicus branch. O. cyanellus shows a 12.26% average genetic distance difference with O. brevifrons , a 12.75% average difference with O. cochisus , and a 12.76% average difference with O. subtropicus . These results support the proposal that the three-color morphs of O. cyanellus are assignable to a single species.
The maximum genetic distance difference (2.3%) between O. cyanellus specimens in Figure 40 View Figure 40 is the one shown between a blue specimen from Río Zarcero (ASolís000801) and a copper specimen of Río Seco (ASolís001054). The two localities are only 4.5 km apart and apparently do not support a genetic difference caused by widely separated localities. As can be seen from the previous values, the maximum genetic distance difference (2.3%) is much below average interspecific mtDNA genetic differences (12.56%) within the O. chevrolati species group. Moreover, the genetic variation of O. cyanellus (0.0%–2.3%) does not overlap with the interspecific genetic variation (11.70%–13.75%) of the O. chevrolati species group ( Fig. 26 View Figures 24–28 ) forming a barcode gap, thus suggesting again that the three-color morphs of O. cyanellus represent a single species.
It is essential to consider the significance of intraspecific COI I variability in more detail, and in this context, it is useful to consider the work of Zhang and Bu (2022). Following an analysis of 64,414 insects, they found that 44.43% of the species studied showed a maximum intraspecific genetic distance ranging from 0 to 1% and 62.87% of the species varied from 0 to 2%. By contrast, the numbers of species whose maximum intraspecific genetic distances exceeded 2% and 3% were 37.13% and 26.62%, respectively. The maximum intraspecific genetic distances over 3% for Coleoptera (8968 studied species) were observed in 23.1% of the species studied. Approximately one-quarter of the species of Insecta studied by them showed high intraspecific genetic variation (>3%), and a conservative estimate of this value ranges from 12.05% to 22.58% In a similar analysis of 1004 European species of Lepidoptera, Huemer et al. (2014) reported that for two localities separated by 1,600 km in Finland and Austria, maximum intraspecific genetic distances for the COI pooled data were less than 2% for 880 species (87.6%), while wider divergence was detected in 124 species. For the present study we found maximum intraspecific genetic variation of 2.3% for the Costa Rican populations of O. cyanellus , a value well within the limit for comparing variations among conspecific populations.
Clypeal margin, pronotal slope, frontal keel, and polymorphism
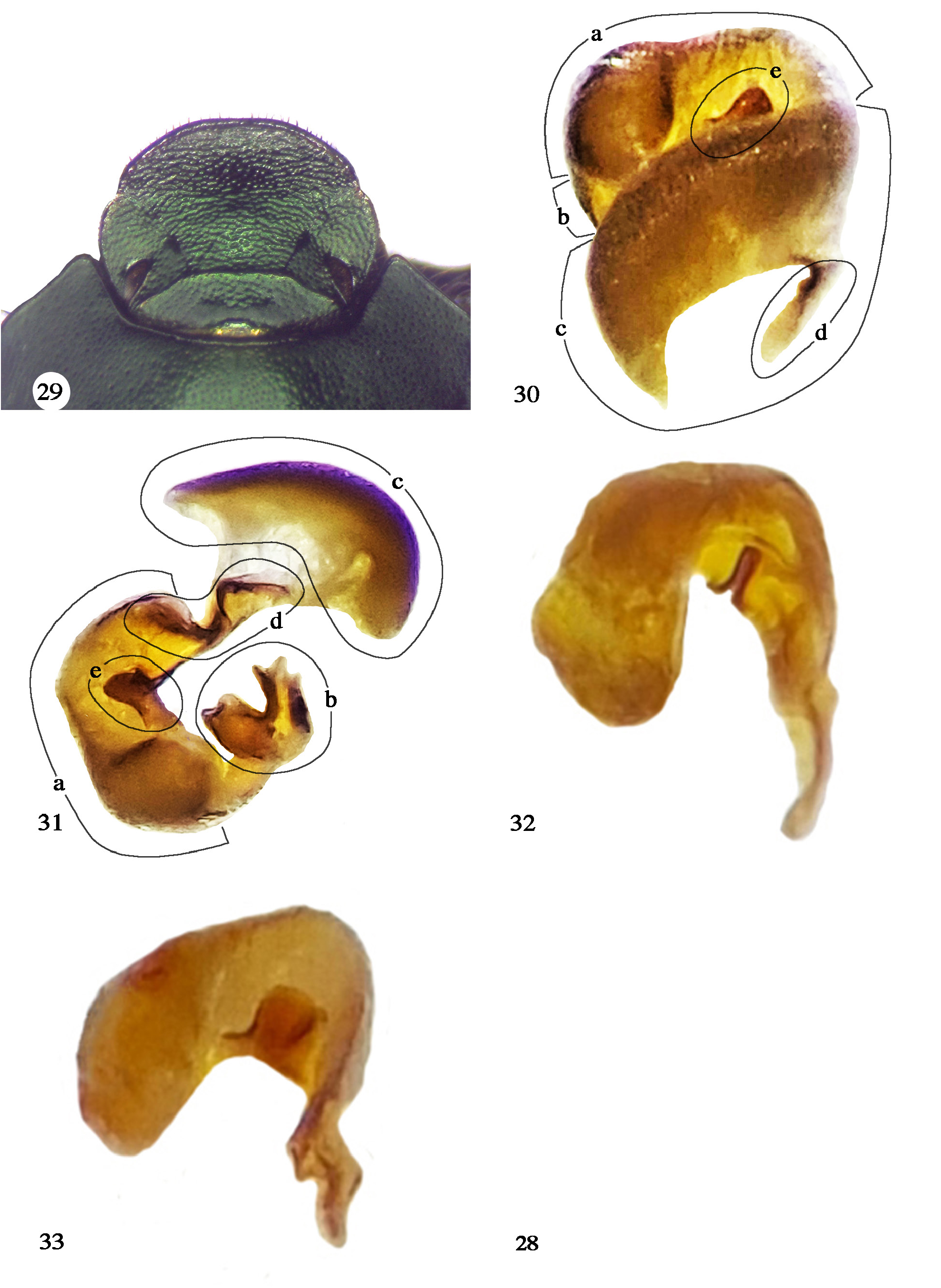
Zunino and Halffter (1988) specified a black clypeal margin and pronotal slope, and a simple male frontal keel as diagnostic characters of Onthophagus cyanellus . The black clypeal margin and pronotal slope are a matter of color contrast. In blue color morphs of O. cyanellus , because the morph is dark blue, neither the clypeal margin nor the pronotum stands out. However, in the green and copper color morphs, because they are paler, the color contrasts strongly with black areas. Regarding the unarmed male frontal keel, the holotype of O. mesoamericanus is a euthelic specimen. However, hyperthelic specimens of the green morph have an armed frontal keel ( Fig. 27 View Figures 24–28 ). Moctezuma (2021: 195) warns against using characters derived from O. cyanellus hypothelic males for establishing diagnostic characters, without realizing that the same applies to euthelic and hyperthelic males, as is the case of the O. mesoamericanus holotype. Moctezuma (2021: 195) proposed more diagnostic characters based on a single euthelic holotype, including less developed pronotal secondary tubercles; an almost straight or convex pronotal projection of frontal border; a more robust pronotal projection; and a more strongly medially sinuated frontal carina. When a green O. mesoamericanus hyperthelic male is compared to the other hyperthelic color morphs, they all show the same characteristics. Thus, the preferable comparison is among specimens of the same degree of development. Figures 27-29 View Figures 24–28 View Figures 29–33 compare the male head in a hyperthelic ( Fig. 27 View Figures 24–28 ), euthelic ( Fig. 28 View Figures 24–28 ), and hypothelic ( Fig. 29 View Figures 29–33 ) green color-morph specimen and it is evident that the hyperthelic specimen has an armed frontal keel, the euthelic specimen shows a keel with a hint of horns, and the hypothelic specimen has an unarmed keel. Significant differences can also be observed in the shape of the clypeus. As can be seen from the photographs ( Fig. 27–29 View Figures 24–28 View Figures 29–33 ), as horn size decreases, the clypeus varies from subquadrate to sub-circular to a female-like circular one.
As Taborsky and Brockmann (2010) reported, the differences in head and pronotal armament that the Onthophagus manifest as male polymorphism represent a mechanism where the expression of alternative phenotypes is related to alternative reproductive tactics aimed at reproductive success. In the case of O. cyanellus , a clear male trimorphism occurs, hyper-, eu-, and hypothelic males ( Fig. 18–23 View Figures 18–23 , 27–29, 32–34 View Figures 24–28 View Figures 29–33 View Figures 34–39 ). Rowland et al. (2017) have studied male trimorphism in phanaeine dung beetles ( Phanaeus triangularis ( Say, 1823) and Oxysternon conspicillatum Weber, 1801 ) and propose the rock-paper-scissors (RPS) model of game theory and the environmental threshold (ET) model of quantitative genetics to model trimorphisms that are environmentally induced and result from the expression of two ontogenetic thresholds that produce three reproductive tactics: dominant alpha males (hyperthelic) defend mating arenas with long horns; subdominant beta males (euthelic) compete less successfully with short horns and female-like and hornless gamma males (hypothelic) sneak access to mating arenas. We believe this model can be applied to O. cyanellus . Although this species does not have as strongly developed head horns as the above phanaeines, pronotal projections vary considerably from significantly developed hyperthelic projections ( Fig. 18–23 View Figures 18–23 ) to almost female-like projections ( Fig. 34 and 36 View Figures 34–39 ). Head shape varies as well ( Fig. 27–29 View Figures 24–28 View Figures 29–33 ). We believe that these pronotal projections function analogously to head horns in other species.
Male genitalia, allometry, and shape variation
Halffter et al. (2019: 18) indicated that: “Kohlmann and Solís (2001) synonymized O. mesoamericanus with O. cyanellus , a synonymy that we maintain for this work. The lamella copulatrix of the holotype was broken during the initial preparation, preventing its study from confirming synonymy. Consequently, the remaining type material needs to be revised, and additional male specimens of O. mesoamericanus need to be collected and studied.” Notwithstanding their advice, Moctezuma (2021) referred to the broken lamella copulatrix in his treatment of O. mesoamericanus . He mentions in his figure caption (Moctezuma 2021: 220, fig. 27) that the right lobe of the lamella copulatrix of O. mesoamericanus is damaged, but he did not mention or notice that part of the keel (fold) of the basal margin of the lamella copulatrix was missing. The original drawing of the holotype of the lamella copulatrix of O. mesoamericanus by Zunino and Halffter (1988: 125, fig. 76) clearly shows the existence of two keel folds, not one, at the basal margin of the lamella copulatrix. Withal Moctezuma (2021: 195) indicated in his text that the lamella copulatrix of O. mesoamericanus was damaged, he nevertheless used the presence of a single keel fold as a characteristic differentiating O. mesoamericanus from the double keel of O. cyanellus .
We agree with the detailed morphological description of the lamellae copulatrices of the O. chevrolati species group presented by Zunino and Halffter (1988: 17–19) in all but a single detail: While they describe a spiny lamellar band, a membranous fold covered by small sclerified spines, our impression is that this lamellar band is not a spiny membranous fold but is instead a chitinous shell ( Fig. 30c, 31c View Figures 29–33 ) like the rest of the lamella copulatrix, which we refer to as lamellar band. We present here for the first-time depictions of the assembled lamella copulatrix ( Fig. 30 View Figures 29–33 ) and the lamellar band ( Fig. 30c, 31c View Figures 29–33 ), which were not illustrated by Zunino and Halffter (1988).
We examined personally the two paratypes ( Fig. 34–37 View Figures 34–39 ) of O. mesoamericanus deposited in the Museum National d’Histoire Narurelle (Paris). Both paratypes fall within the color and morphology variation patterns of O. cyanellus proposed here and do not show specific differences as reported previously by Kohlmann and Solís (2001) and Solís and Kohlmann (2012). The genitalia preparations could not be studied as they were poorly prepared. An attempt to dissolve the hardened Canada balsam of the male slide to study the lamella copulatrix was not successful. No further attempts were made for fear of further damaging the preparations. The paratypes mtDNA could not be studied, old specimens show degraded genetic material.
We undertook a comparative analysis of the male genitalia of hyper-, eu-, and hypothelic males ( Fig. 30–33 View Figures 29–33 ) of O. cyanellus . The keel of the basal margin of the lamella copulatrix varies in shape but not size. The hypothelic specimen ( Fig. 32 View Figures 29–33 ) shows the keel of the basal margin as a very narrow cleft; that of the euthelic specimen ( Fig. 31e View Figures 29–33 ) shows a more open cleft; and that of the hyperthelic specimen ( Fig. 33 View Figures 29–33 ) has a wide cleft. However, the size of the basal margin is about the same among the three morphs, which we interpret as a case of negative static allometry where the genitalia of small males of a species are disproportionally large, and those of large males are disproportionally small ( Eberhard et al. 2008). As in the case of the male frontal keel, clypeus, and pronotum we observe that specimens show an allometric variation in specific structures of the lamellae copulatrices. Eberhard et al. (2008) seem to have been the first to report that size and shape are independent traits of genitalia; that rapid divergence in the shape of genitalia is thus not inconsistent with the reduced variation in their sizes. Parzer et al. (2018) studied genital shape and size in Onthophagus and found significant intraspecific variation in genital shape and size variation. They also found that genital shapes evolved faster than relative genital size, whereas shapes of all structures evolved faster than their relative size. The keel of the basal margin of O. cyanellus seems consistent with the idea of Eberhard et al. (2008) and Parzer et al. (2018), that keel shape changes faster than its relative size ( Fig. 30–33 View Figures 29–33 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Onthophagus cyanellus Bates
| Solís, Ángel & Kohlmann, Bert 2023 |
Onthophagus mesoamericanus
| Solis A & Kohlmann B. 2012: 12 |
Onthophagus mesoamericanus
| Pulido-Herrera LA & Zunino M. 2007: 109 |
Onthophagus mesoamericanus
| Zunino M & Halffter G. 1988: 123 |
Onthophagus cyanellus
| Bates HW 1887: 81 |