Botryllus schlosseri ( Pallas, 1766 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4353.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:ADA0A151-DB0D-4363-B395-E274EF7DEC12 |
|
DOI |
https://doi.org/10.5281/zenodo.6031719 |
|
persistent identifier |
https://treatment.plazi.org/id/AE61D712-FF88-FF97-FF3E-F909FCEA0A52 |
|
treatment provided by |
Plazi |
|
scientific name |
Botryllus schlosseri ( Pallas, 1766 ) |
| status |
|
Botryllus schlosseri ( Pallas, 1766)
Alcyonium schlosseri Pallas, 1766: 355 .
Type data: neotype MSNVE catalog No : MSNVE-24197 Type locality: Lagoon of Venice.
Botryllus schlosseri: Michaelsen, 1921: 108 .? Botryllus schlosseri: Savigny, 1816: 200 . Not Botryllus schlosseri: Van Name, 1945: 220 . Not Botryllus schlosseri: Brewin, 1946: 112 . Not Botryllus schlosseri: Berrill, 1950: 216 .? Botryllus schlosseri: Millar, 1966: 88 .
? Botryllus schlosseri: Millar, 1970: 70 .
Not Botryllus schlosseri: Kott, 1985: 267 .
? Botryllus schlosseri: Boyd et al. 1990: 239 -250.
History. B. schlosseri was initially figured by Rondeletius (1555) as a mass with star-like structures at its surface, and included among the Zoophyta as Uva marina (i.e., marine grapes). Then, it was at the same time described by Schlosser (Schlosser & Ellis 1755), who discovered that “…every one of those stars to be a true animal… ” (page 450), and by Ellis (Schlosser & Ellis 1755), who noticed the presence of “eggs” between the radii of the stars (“ The smallest eggs are globular, and as they advance in size, change to an oval figure; from thence they assume the shape of the one of the radii of the stars ” page 452). Only in 1766 this species was formally described by Pallas.
Morphology. Colony. Colonies are polychromatic, flat, investing on hard substrate or epibionthic, usually 1–2 mm thick, sometime up to 3–4 mm according the morphology of the substratum (Cima et al. 2015 for review). Their surface is smooth. Very large colonies may produce flat lobes in which zooids are arranged on the two sides (a possible explication of this phenomenon is given in Brunetti (1974).
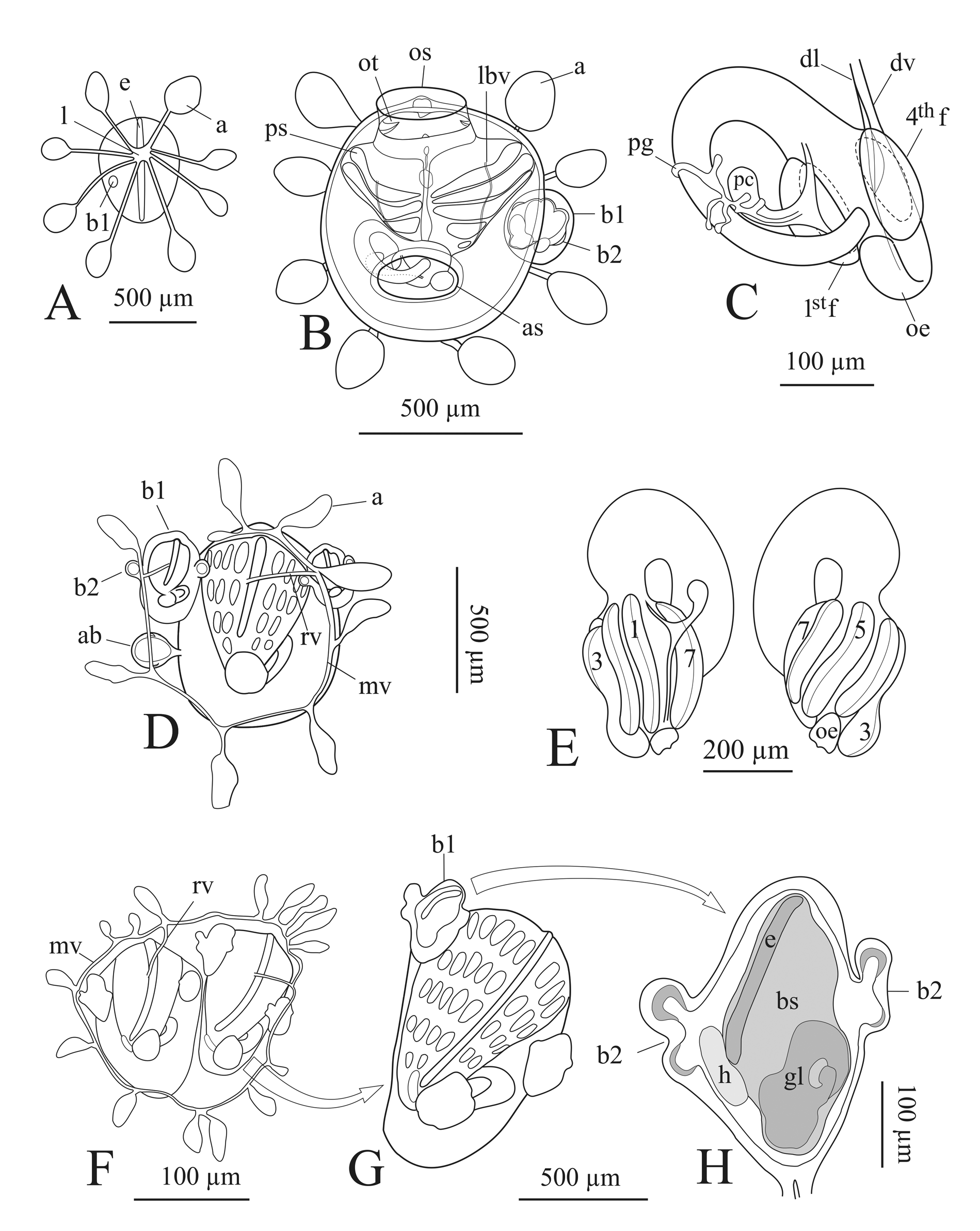
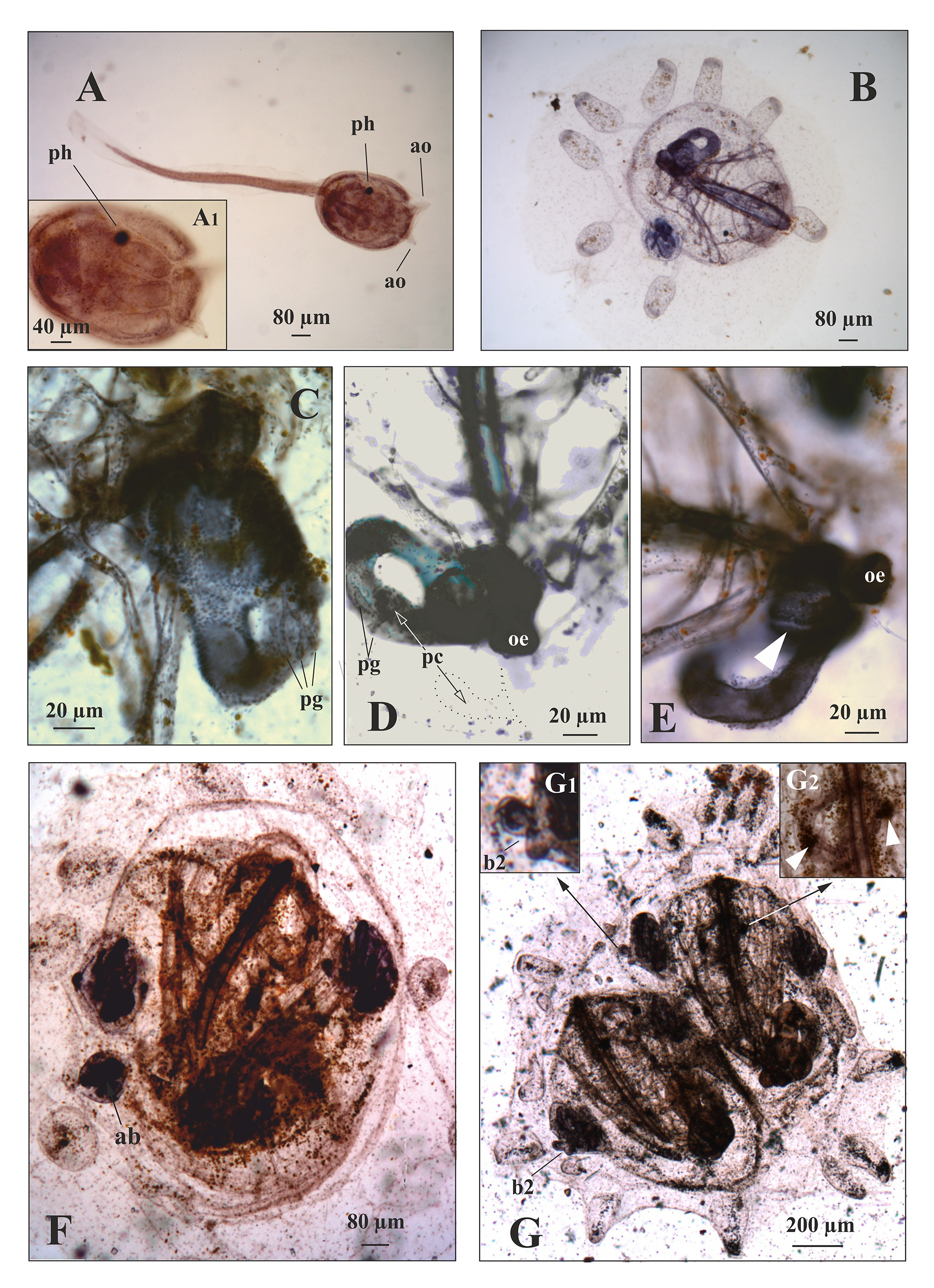
Zooid ( Figure 1 View FIGURE 1 and Figure 2 View FIGURE 2 ). They are usually up to about 1.5 mm long and inclined as regard the colony surface ( Figure 1 View FIGURE 1 , A–B; Figure 2 View FIGURE 2 , A). There have generally eight tentacles of two size orders. Tentacles are spoon shaped with the concavity towards the outside ( Figure 1 View FIGURE 1 , C; Figure 2 View FIGURE 2 , C–D); the two laterals of the first orders are most developed, with a mass of blood cells in a vascular lacuna at their bases ( Figure 2 View FIGURE 2 , C; Figure 4 View FIGURE 4 , G 2 View FIGURE 2 ). These masses are often visible at the surface of the colony as two pigmented spots. The body wall has a net of fine muscle fibres. Singularly or in pairs, they run in part as circular fibres mainly concentrated around the oral syphon, and extending to the remaining body wall, in part as longitudinal fibres departing from the external wall of the oral syphon to the lateral region of the zooid. The branchial sac has 8 rows of stigmata: the second row is, on both sides, dorsally incomplete ( Figure 1 View FIGURE 1 , A; Figure 2 View FIGURE 2 , B) and the 8th often shows irregular stigmata. Sometime also the third row may be incomplete. The branchial sac has a conical shape having about 17 stigmata in the 1 st half row and about 8 in the 7th. The dorsal and ventral branchial sectors are broader than the two laterals ( Figure 2 View FIGURE 2 , E) and at the middle of the branchial sac the stigmata are usually arranged according the formula E 5,3,3,5 DL. We were not able to verify the presence of muscles fibres along the transversal branchial vessels as found in some species of the same subfamily ( Brunetti 2010, fig. 5; Brunetti & Mastrototaro 2012, fig. 5). The dorsal lamina rises from the dorsal vessel as a simple membrane at the level of the 4–5th row of stigmata, becomes a double membrane at the level of 5–6th, and then ends on the left side of the oesophageal opening at the level of the last row ( Figure 1 View FIGURE 1 , D; Figure 2 View FIGURE 2 , E 1 View FIGURE 1 ). Masses of haematic cells were observed in correspondence of the rows of stigmata, on both sides of the endostyle. These masses, named ventral cell islands ( Manni et al. 2014), consist of different kinds of blood cells (at least macrophages and stem cells) here migrated from the ventral cell islands of regressed parental zooids. They are visible in zooids when they open the syphons and then are completely dispersed by blood circulation before the new change of generation (usually at the colonial stage 9/8/5–6— Sabbadin 1955a; Manni et al. 2014).
The anterior edge of the first curve of the intestinal loop is at level of the 5–6th row of stigmata ( Figure 1 View FIGURE 1 , A), the second curve is open, sometime almost absent. This latter condition is present when there are very developed testis and/or embryos, which compress posteriorly the intestinal curve ( Figure 1 View FIGURE 1 , B). Between the two loops, the medium intestine is covered by the pyloric gland and runs parallel to the longitudinal axis of the stomach, along its ventral side or slightly by its parietal side. Under the ampullae of the pyloric gland, the wall of the medium intestine is longitudinally run by a band ( Figure 1 View FIGURE 1 , D) which might correspond to the internal band of low mucous cell described by Burighel & Milanesi (1977, fig 3). The rectum does not go forward beside the straight dorsal edge of the branchial sac but moves towards the atrial aperture. It is connected, shortly before the anus, to the branchial sac and to the body wall by two trabeculae ( Figure 1 View FIGURE 1 , B, D; Figure 2 View FIGURE 2 , F). As the oesophageal aperture, a smoothedged anus opens at the level of the last row of stigmata ( Figure 1 View FIGURE 1 , D; Figure 2 View FIGURE 2 , F). The stomach is horizontally arranged under the branchial sac ( Figure 1 View FIGURE 1 , A; Figure 2 View FIGURE 2 , A). It has an oval or cylindrical outline with 8 slightly spiralled folds run by a longitudinal rut ( Figure 1 View FIGURE 1 , E 2–E View FIGURE 2 3 View FIGURE 3 ; Figure 2 View FIGURE 2 , G) (for a histological description of the stomach folds see Burighel & Milanesi, 1973). The folds are closed at both ends and are almost equal in size and length; only the fold number 8 is shorter and has a characteristic narrowing at the middle of its length ( Figure 1 View FIGURE 1 , A– B and D–E). The pyloric caecum rises at two-thirds from the anterior end of the typhlosole. This last structure has about half of the stomach length ( Figure 1 View FIGURE 1 , A–B and D–E; Figure 2 View FIGURE 2 , G) and is straight or slightly curved and sloped back arrangement, and with a rounded tip turned toward the parietal side ( Figure 1 View FIGURE 1 , E). The pyloric gland duct debouches at the base of the pyloric caecum ( Figure 1 View FIGURE 1 , D; Figure 2 View FIGURE 2 , G). The part of typhlosole that is anterior to the pyloric caecum is a few wider than the posterior one; the typhlosole does not extend in the first tract of intestinal loop.
Gonads. The testis, with follicles arranged in a morula or fan-shaped cluster ( Figure 1 View FIGURE 1 , A–B), opens dorsally with a single vas deferens in the atrial cavity. On right side, the testis is situated immediately anterior to the stomach, on the left anterior to the pyloric intestinal curve ( Figure 1 View FIGURE 1 , A). In primary bud, usually there are up to 5 eggs arranged, in an arc dorsally to testis ( Figure 2 View FIGURE 2 , H, I). Embryos develop in the atrial cavity of the zooid attached by a placental cup ( Ärnbäck-Christie-Linde 1923; Zaniolo et al. 1987) to the body wall but this structure usually is not easy to see.
Larva. It is about 1.5 mm long (without fin), trunk 0.5 mm. It has, as usual in Stolidobranchia, three adhesive organs arranged in a triangle, 8 ectodermal ampullae and a black photolith ( Figure 4 View FIGURE 4 , A).
Oozooid and morphogenesis of the zooid ( Figure 3 View FIGURE 3 and Figure 4 View FIGURE 4 ). The following observations refer to colonies obtained from gametes of the neotype colony. The oozooids developed at ~18°C, since the quoted time of development is function of temperature ( Sabbadin, 1955a). After settlement, the tail regresses and is reabsorbed while the metamorphosing larva completes its development. The first 8 ampullae are each at the end of a vessel elongating radially on the substrate from a central lacuna ( Figure 3 View FIGURE 3 , A). About 1.5 day after settlement, the oozooid opens syphons, but its morphogenesis is not completed. At this time, only the two lateral tentacles are visible; two more tentacles develop later on. The masses of pigmented blood cells at the bases of the two lateral tentacles of the zooid (see above) are already present. The oozooid has 5 protostigmata and a single internal longitudinal branchial vessel on each side. There is a single bud on the right side ( Figure 3 View FIGURE 3 , B; Figure 4 View FIGURE 4 , B). The stomach is globular, with 4 folds and a typhlosole from which a large pyloric caecum rises. Both the pyloric gland and the caecum are larger than those belonging to the blastozooid, when compared to the stomach size ( Figure 3 View FIGURE 3 , C; Figure 4 View FIGURE 4 , C, D). The stomach fold shown in Figure 3 View FIGURE 3 , C corresponds to an oozooid which has just opened the syphons; in the following days, they become run by a longitudinal rut as typically occurs in the stomach of blastozooids ( Figure 4 View FIGURE 4 , E arrowheads). The oozooid reaches a length of about 800 µm (average on 16 measured samples); moreover, the first 8 tunic vessels, derived by the 8 larval ampullae, change their course and begin to form the marginal vessels of the future colonial vascular systems ( Figure 3 View FIGURE 3 , D) (for a more careful description of the development of this vascular system see Brunetti & Burighel 1969).
After about 7 days from settlement, the oozooid regresses and is substituted by its bud which represent the zooid of the first blastogenic generation. It reaches a length of about 900 µm (average on 15 measured samples). This zooid presents 4 complete rows of vertical stigmata and a stomach with 7 folds ( Figure 3 View FIGURE 3 , D, E). It has usually a single bud on each side. Sometime a second bud is present on the right side, but this usually does not develop ( Figure 3 View FIGURE 3 , D; Figure 4 View FIGURE 4 , F).
When this zooids regresses, it is substituted by its buds that represent the zooids of second blastogenic generation and constitute the first system of the colony ( Figure 3 View FIGURE 3 , F; Figure 4 View FIGURE 4 , G). These zooids reach about 1400 µm in length (average on 9 measured samples). They have five (at least at the right side) all complete rows of stigmata ( Figure 3 View FIGURE 3 , G). At the left side the gut hinders a clear view of the fifth row ( Figure 3 View FIGURE 3 , G). These zooids have one bud (the 3th blastogenetic generation) on each side. These zooids have one bud (the 3th blastogenetic generation) on each side. The last buds show a very strong blastogenic power, splitting the appearing bud primordium per side into two or three primordia ( Sabbadin, 1969). However, usually not all these buds complete their development.
In one or two succeeding generations (which occur every 7 days at ~18°C) zooids assume the definitive morphology for the species (see above): the stigmata rows and stomach fold rises until they reach their typical number and the second row of stigmata become dorsally incomplete; furthermore, the gut loop progressively changes. Initially, in the oozooid, the stomach has its longitudinal axis only slightly tilted to the left, and its oesophageal end is close to the aperture of the posterior end of branchial sac, so that an oesophageal tract is practically absent ( Figure 4 View FIGURE 4 , C, D, E). In few succeeding generations, the longitudinal axis of the stomach becomes more and more angled until to become orthogonal to that of the zooid (compare Figure 4 View FIGURE 4 , C, D, E with Figure 1 View FIGURE 1 , A, B, D).
Symbols: a, ampulla; ab, atrophic bud; an, anus; ao, adhesive organs; as, atrial syphon; b1, first order bud; b2, second order bud; bs, branchial sac; bv, blood vessel; ddl, double dorsal lamina; dl, dorsal lamina; dt, dorsal tubercle; dv, dorsal vessel; e, endostyle; em, embryos; gl, gastric loop; h, heart; i, intestine; ilb, intestinal longitudinal band; l, lacuna; lbv, longitudinal branchial vessel; lr, longitudinal rut; lt, left testis; mv, marginal vessel; ng, neural gland; oe, oesophagus; os, oral syphon; ot, oral tentacles; ov, ovary; pbg, prebranchial groove; pc, pyloric caecum; pg, pyloric gland; pgd, pyloric gland duct; ph, photolith; ps, protostigmata; r, rectum; rbs, right branchial sectors; rt, right testis; rv, radial vessel; st, stomach; sdl, simple dorsal lamina; sf, stomach fold; t, testis; tbv, transversal branchial vessel; tr, trabecula; typ, typhlosole, 1st f, first stomach fold; 1st r, first row of stigmata; 4th f, fourth stomach fold; 1- 8, stomach folds.
Ecology. B. schlosseri is largely distributed in shallow waters along all European coasts, on stones, algae, other ascidians, etc. It is particularly abundant in harbours. It survives and growths at low temperatures and reproduces above 10°C ( Brunetti, 1974; Brunetti & Copello, 1978; Brunetti et al. 1980; 1984). A review on sexual and asexual reproduction of this species is given by Gasparini et al. (2015). It is also easy to rear in laboratory ( Sabbadin 1960; Boyd et al. 1986; Rinkevich & Shapira, 1998).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Botryllus schlosseri ( Pallas, 1766 )
| Brunetti, Riccardo, Manni, Lucia, Mastrototaro, Francesco, Gissi, Carmela & Gasparini, Fabio 2017 |
Botryllus schlosseri: Boyd et al. 1990 : 239
| Boyd 1990: 239 |
Botryllus schlosseri:
| Kott 1985: 267 |
Botryllus schlosseri:
| Millar 1970: 70 |
Botryllus schlosseri:
| Millar 1966: 88 |
| Berrill 1950: 216 |
| Brewin 1946: 112 |
| Van 1945: 220 |
| Michaelsen 1921: 108 |
| Savigny 1816: 200 |
Alcyonium schlosseri
| Pallas 1766: 355 |