Pholcus phalangioides (Fuesslin, 1775)
|
publication ID |
https://doi.org/ 10.5281/zenodo.8315895 |
|
publication LSID |
lsid:zoobank.org:pub:88030C1D-8A6E-47E3-AA13-92EB1EEF580D |
|
persistent identifier |
https://treatment.plazi.org/id/AE1D7161-FF97-FFEC-CB07-F9D27E5AFEBF |
|
treatment provided by |
Felipe |
|
scientific name |
Pholcus phalangioides |
| status |
|
2. Pholcus phalangioides View in CoL View at ENA
A. Blood circulation
The swaying and deformations of the abdomen are, in this species, more extensive and complex; and this, let's state it immediately, is due to the greater flexibility of the integument.
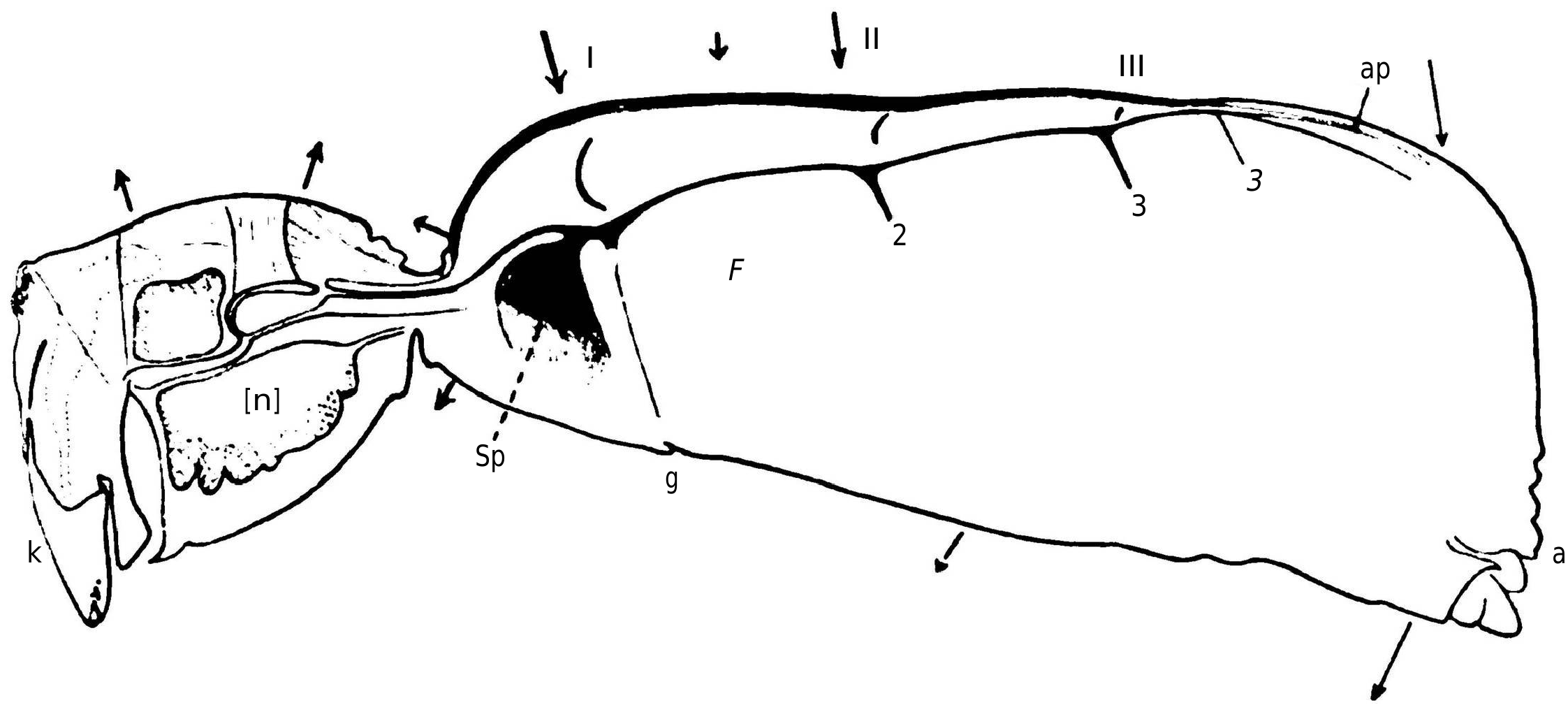
When the animal is fixed by the legs brought dorsally, the opisthosoma shows, with each cardiac systole (usually 134 times per minute), a marked lowering movement as shown in the attached diagram ( Figure 4 View Figure 4 ). However, unlike the case of Araneus , the pedicel is not immobile, and furthermore, the posterior dorsal region of the prosoma is also lifted by the internal pressure, causing the opisthosoma to undergo alternating movements of lowering and raising, which in most cases result in approximately a rotation around a fictitious axis projected at F. To further analyze the deformations of the abdomen, it is preferable to eliminate the general swaying and immobilize it, for example, by laying the spider on its side on the slide.
With Araneus , we were forced to prioritize the study of these deformations because they constituted the only practical means of investigating the movements of a concealed organ [My colleague, Dr. S. de Boer, first assistant in physical physiology, and I have obtained electrograms revealing the contractions of the heart using Einthoven's string galvanometer; this study is not yet complete.]; in the case of Pholcus , the integument is more transparent, the heart is not hidden under a layer of digestive gland lobules, and it can be observed directly; the body deformations will no longer be our exclusive focus and will primarily serve to study the changes in local pressure resulting from the heart contractions. First, it is necessary to examine the anatomy of the heart [The figure given by Schimkevitsch of the heart of Pholcus , which is used by German authors as a representation of the heart of arachnids, gives a false impression; the data provided by Causard are accurate but entirely insufficient for the needs of our research.].
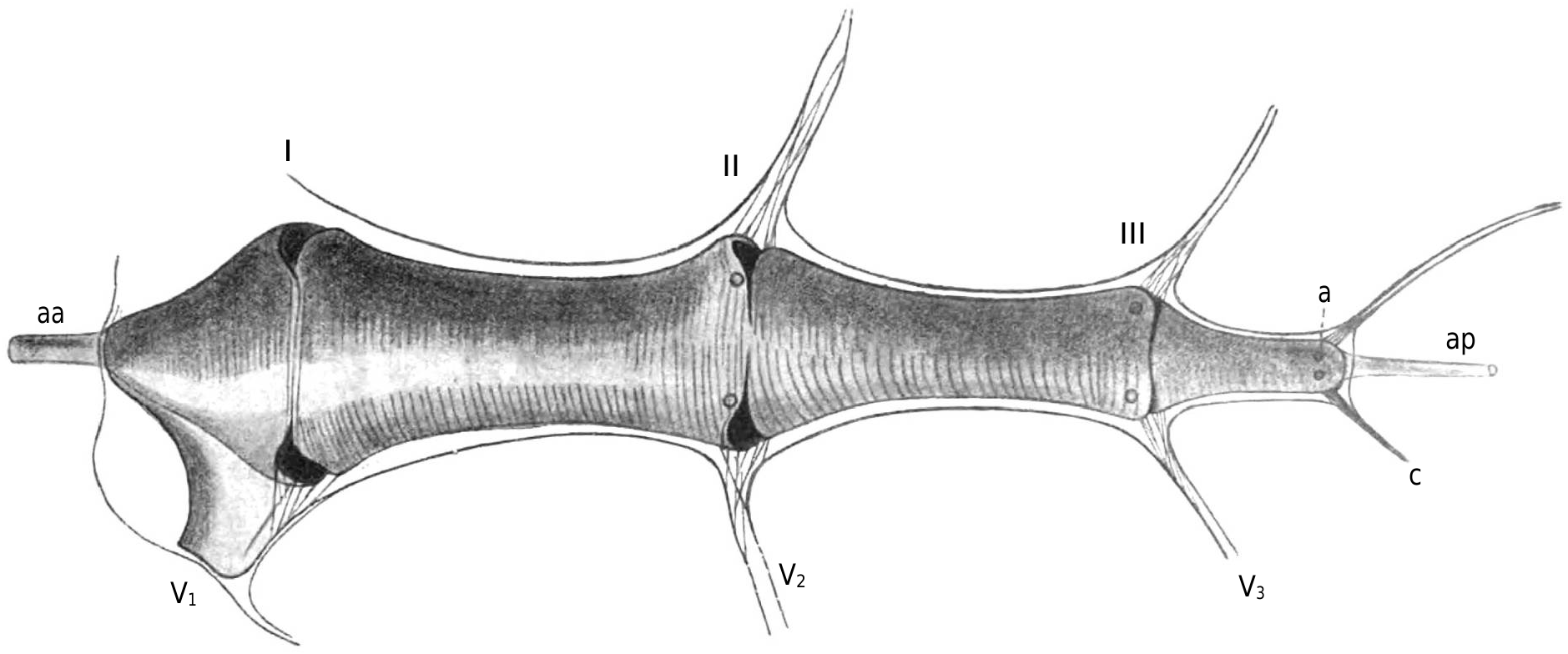
I have depicted in Figure 5 View Figure 5 the heart of Pholcus seen dorsally, along with the boundaries of the pericardial sinus and its lateral expansions; Figure 4 View Figure 4 shows the heart in place in a sagittal section of a male specimen. The muscular portion of the heart walls only consist of annular fibers, whose ends fuse together on the dorsal midline: a persistent index, perhaps, of an ontogenic peculiarity of the organ. Unlike Schimkevitsch's findings, there is no X-shaped crossing of muscle fibers at the level of the ostia; the dorsal midline suture of the fibers is not interrupted there.
To avoid lengthy descriptions, I refer the reader to Figures 5 View Figure 5 and 6 View Figure 6 and the accompanying legends. I must emphasize, as we will use this anatomical information specifically, the previously unnoticed fact that each lip of every ostium merges with the corresponding lip on the opposite side to form a sort of semi-lunar muscular veil, nearly vertical. Additionally, it should be noted that, in contrast to Araneus , each group of pteripyles and commissural ligaments corresponding to ostia II and III forms a roughly transverse vertical fibrillary blade that plunges into a narrow blood sinus, similar to a pulmonary vein, and functions as a pathway for blood return to the heart. This arrangement is less specialized, if I am not mistaken, than that of Araneus , as it corresponds to what is observed in newly hatched individuals of the latter species. I have only observed hypocardiac ligaments at the level of ostium I: Figure 6D View Figure 6 shows that the arrangement of lateral fibrillary blades allows for a downward traction of the heart and pericardium, which must be ensured elsewhere by hypocardiac ligaments.
I mentioned that heart contractions can be seen through the roof of the pericardium. During diastole, the heart, judging from the examination of the dorsal side, nearly fills the pericardial cavity; and examination of fixed organ sections confirms that the ventral region of the heart is closely applied to the floor of the pericardium, except at the level of the ostia.
It is the region comprising ostia II and III that contracts the most during systole, and it is precisely at the level of the ostia that the greatest decrease in transverse diameter is observed. However, the contraction of the heart is not very pronounced: behind pylocarde II, in a region that is particularly easy to observe and where the contraction is relatively strong, the lateral wall of the heart retracts towards the midline by only about 1 / 15 of the transverse diameter, so that there, usually, the diameter only decreases by about one-seventh during systole.
This slight contraction, combined with the rapid rhythm of cardiac beats (130 per minute), indicates that arterial blood pressure must be relatively considerable. This supposition is confirmed by other facts, such as:
1. The considerable distance over which cardiac pulsations are felt. Indeed, it is observed that the systole of the heart is accompanied by a noticeable lifting of the integument in the dorsal midregion of the prosoma, a phenomenon that proves that the pulsation is felt beyond the arteries, reaching into the sinuses of the general lacunar system. The pulsation propagates, as previously seen, even close to the tips of the legs, causing an extension jerk in them, particularly a swaying of the last segment that can reach an amplitude of 2 mm. Beating in the ventral region of the opisthosoma, immediately behind the pedicel, indicates that the cardiac pulsation is felt in the large opisthosomal sinus where the blood from the prosoma gathers before passing into the lungs. [This important fact can be observed using another method. By using intense direct illumination, it is possible to perceive through the integument, even with a relatively low magnification of 40-50 diameters, the globules of the circulatory fluid, which then appear as bright dots against a darker background. In the currently considered region, one can observe the flow advancing rhythmically into the ventral sinus and causing oscillations in the uncontracted longitudinal muscles, and one can perceive the jerky flow swirls in this irregularly shaped cavity.]; we will see later that it cannot propagate beyond this point.
2. The rapid and serious hemorrhage that accompanies an injury such as the sectioning of a leg. At the same time, the drop in blood pressure is evidenced by the slowing of the heart rate, the smaller amplitude of the pulsating beats we have mentioned, and by the collapse of the vault of the pericardial cavity.
Interestingly, in our spiders, it is the blood pressure that keeps the pericardial cavity extended [In crustaceans and insects, it is the rigidity of the more solid integuments that counterbalances the traction of the suspensory ligaments of the heart]. During a significant hemorrhage, one can observe a strong collapse of the dorsal wall of the opisthosoma: a longitudinal depression forms, which is more pronounced at the level of the pulmonary veins and other lateral pericardial expansions, and it is interrupted, between the deepest hollows, by prominent ridges formed by integument folds.
One must wonder what mechanism allows the integument to resist this internal pressure and what is its antagonist? Before Causard, the fibrous strips that line the inner side of the integument and form what Causard calls the opisthosomal connective sac were believed to be of a muscular nature [See figures of this abdominal sac in Causard's paper; a representation of connective strips at a larger scale can be found in fig. 4, pl. VII de E. Lamy, Recherches anatomiques sur les trache̕es des Araigne̕es (Ann. des. sc. nat. Zool., VIIIe se̕r., 15, 1902).]. Since these strips are connective and not contractile, it must be admitted that they are normally in a state of extension, and this extension replaces the muscular tone that one might have invoked in the past. Furthermore, one can experimentally confirm that other connective fibers, connected to the opisthosomal sac, are also in a similar state of permanent extension: in the case of significant hemorrhage, vertical depressions appear on the sides of the animal, just at the level of the insertions of the two pairs of connective strips representing the cardiac lateral ligaments, and there, the collapse of the walls is more significant than any preformed blood cavity, and it is accompanied by a displacement of digestive gland tissue; these deformations, therefore, result from the traction of the strips, and the phenomenon indicates that the strips are normally in a state of extension, even during cardiac diastole.
The heart contracts as a unit, similar to that of Limulus [A. J. Carlson, The nervous origin of the Heart-beat in Limulus . (American Journal of Physiology, Vol. XII, 1905)]. This observation is surprising, considering the length of the tetrameric cardiac tube of arachnids, and if one refers to the general descriptions given by anatomists of the hearts of arthropods and insects in particular. It is often said that the heart is formed of a series of ventriculites separated by constrictions (the ostial valves), and its contraction occurs in a wave that propagates from the back to the front, with a new wave possibly starting at the posterior end before the previous one has reached the aorta; that each ventriculite, thanks to the alternating movements of the valvular folds that separate it from the previous and the next one, ejects, during its systole, the blood into the one immediately ahead... [Recent authors, such as Popovici-Baznosanu (1905), Verson (1908), react against this view. It can also be observed that the long dorsal vessel of Periplaneta americana , a nevertheless archaic form, presents rapid pulsations similar to those of the heart of Pholcus , without any apparent contractile wave. Until further information is available, I consider the functional description often given of the hearts of arthropods as not very general]
There is nothing similar in Pholcus . Moreover, the structure of the ostial lamellae does not correspond to this double set of valves that, while opposing the reflux of blood towards the pericardial cavity during systole, would also close the transverse lumen of the heart. Figures 3, 6 View Figure 6 , and 12 show, if one considers the slight narrowing of the heart during its contraction, that the valves are not organized to reach the ventral surface of the organ's wall.
At the beginning of systole, as in Araneus , the region corresponding to the anterior end of the heart is projected forward, by about 10 μ [I have represented on Figure 4 View Figure 4 , using arrows, the direction and relative size (at a scale 25 times larger than the drawing itself!) of the displacements of some points in the middle region of the body. The three fine lines indicated for the posterior half of the abdomen correspond to the complex vertical oscillation of the abdomen; the thick lines correspond to local elementary deformations.]. This deformation shows that at this stage of cardiac contraction, a stream of blood is propelled forward, distending the anterior portion of the heart tube, which has a less thick muscular wall. A rise of about 5 μ in the chitinous piece of the pedicel reveals the distension of the aorta. We have already noted that the cardiac pulsation is noticeable in the sinuses of the prosoma and even in the ventral opisthosomal sinus.
Direct examination shows that in Pholcus , the systole of the heart is accompanied by a drop in pressure in the pericardial cavity: one can observe the lateral boundaries of it, made up of soft lobules of the digestive gland, jumping at each systole and to some extent following the contour of the heart.
This drop in pressure also leads to the deformation of the ceiling of the pericardial cavity. It is observed that the region above ostium I and the pulmonary veins collapses the most; next, it is the region covering ostium II and the second lateral veins. These two regions are separated by a much less mobile transverse band that presents a slight rocking movement because the two zones that border it, anteriorly and posteriorly, collapse differently.
The rhythmic deformation of the pericardial region varies in magnitude depending on the observed case, probably due to variations in the overall internal pressure. In a robust specimen, at the beginning of the observation, the movements are barely perceptible; a few hours later, probably when the opisthosomal muscle contraction caused by manipulation has subsided and the internal pressure has returned to a more normal value, it can be observed that the deformations become more pronounced. Moreover, they can be amplified at any moment by causing hemorrhage through the appropriate section of a leg segment.
The study of the rhythmic deformation of the dorsal region reveals another detail. If we compare the movement of the dorsal line with that of a cardiac contour or with that of the supra-peduncular region, which is easier to observe in the same microscope field, we note that the sinking of the dorsal line lags behind the moment of the cardiac pulsation and is less abrupt than the latter. These specific characteristics show that the collapse of the pericardial ceiling is not specifically due to a direct traction that the heart would exert through the epicardial ligaments, but rather results from the drop in pericardial pressure, which also follows the heart's systole only to the extent of the inertia of the blood returning to the central organ [The different development of the epicardial ligaments in Araneus and Pholcus , where they are represented only by insignificant fibrils, leads to differences in the mechanism of the deformations considered in the two forms; I will not address this secondary point at the moment.].
The drop in pericardial pressure determined by the cardiac systole is transmitted to the very wide pulmonary veins and to the sinus that encloses each lung: their flexible walls are seen to jump, at each systole, towards the interior of the blood cavity, with a sudden jolt similar to that of the digestive gland walls of the pericardium itself [The amplitude is of the order of one-hundredth of a millimeter.]. On the other hand, as in Araneus and more easily than in this species, the collapse of the pulmonary region of the integument can be observed at each systole. This brings us to the explanation of the mechanism of circulation and pulmonary ventilation.
B. Circulation and pulmonary ventilation
From the systolic depression in the pericardial cavity and in the pulmonary veins, there results an aspiration towards the heart of the blood contained in the pulmonary veins and, consequently, in the entire pulmonary lacunar system. Direct observation, with suitable lighting, allows one to witness the progression of the blood thus aspirated from the lungs and the abrupt exit of blood cells detaching from the edges of the pulmonary folds. [By using intense direct illumination, it is possible to perceive through the integument, even with a relatively low magnification of 40-50 diameters, the globules of the circulatory fluid, which then appear as bright dots against a darker background. In the currently considered region, one can observe the flow advancing rhythmically into the ventral sinus and causing oscillations in the uncontracted longitudinal muscles, and one can perceive the jerky flow swirls in this irregularly shaped cavity.]
But a very easy observation reveals a more interesting phenomenon: the reduction in thickness of the pulmonary lamellae, or rather, the compression of this lamellar system, with each contraction of the heart. As they then return to their original volume, one can see, through the integuments, the white mass of the pulmonary lamellae playing like a harmonica with one face fixed ventrally. And this movement remains constantly synchronous with the cardiac pulsation: I have seen it follow the rhythm of the heart in a case where the pulsations were in pairs; and its amplitude, on the other hand, varies with that of the pericardial depression, which can be deduced from the conditions previously studied.
Moreover, the movement of the lamellae and the beating of the pulmonary vein walls, although synchronous, have different appearances: the latter begins with a sudden jump inward into the canal, followed by a slower collapse; the former involves more gradual and equal movements back and forth. This is because the vein's wall almost immediately experiences the variations in pericardial pressure; for the lamellae, these variations are attenuated by the influx of blood flowing through these organs: the observed movements, which correspond to the inertia of the circulating fluid, measure the resistance opposed by the pulmonary sinuses to the passage of blood.
Similar concordances prove that the narrowing of the pulmonary lamellae is a purely passive phenomenon; it is not necessary to invoke, to understand the blood circulation in the lungs, an active contraction of the lamellar columns, as some biologists have done when studying the mechanism of this circulation without considering the conditions of the general blood circulation. Their hypothesis, which I mentioned in the introduction, requires attributing a contractile function to the columns that is not consistent with their histological structure; and this would not explain the constant synchrony of this hypothetical contraction with the cardiac pulsation.
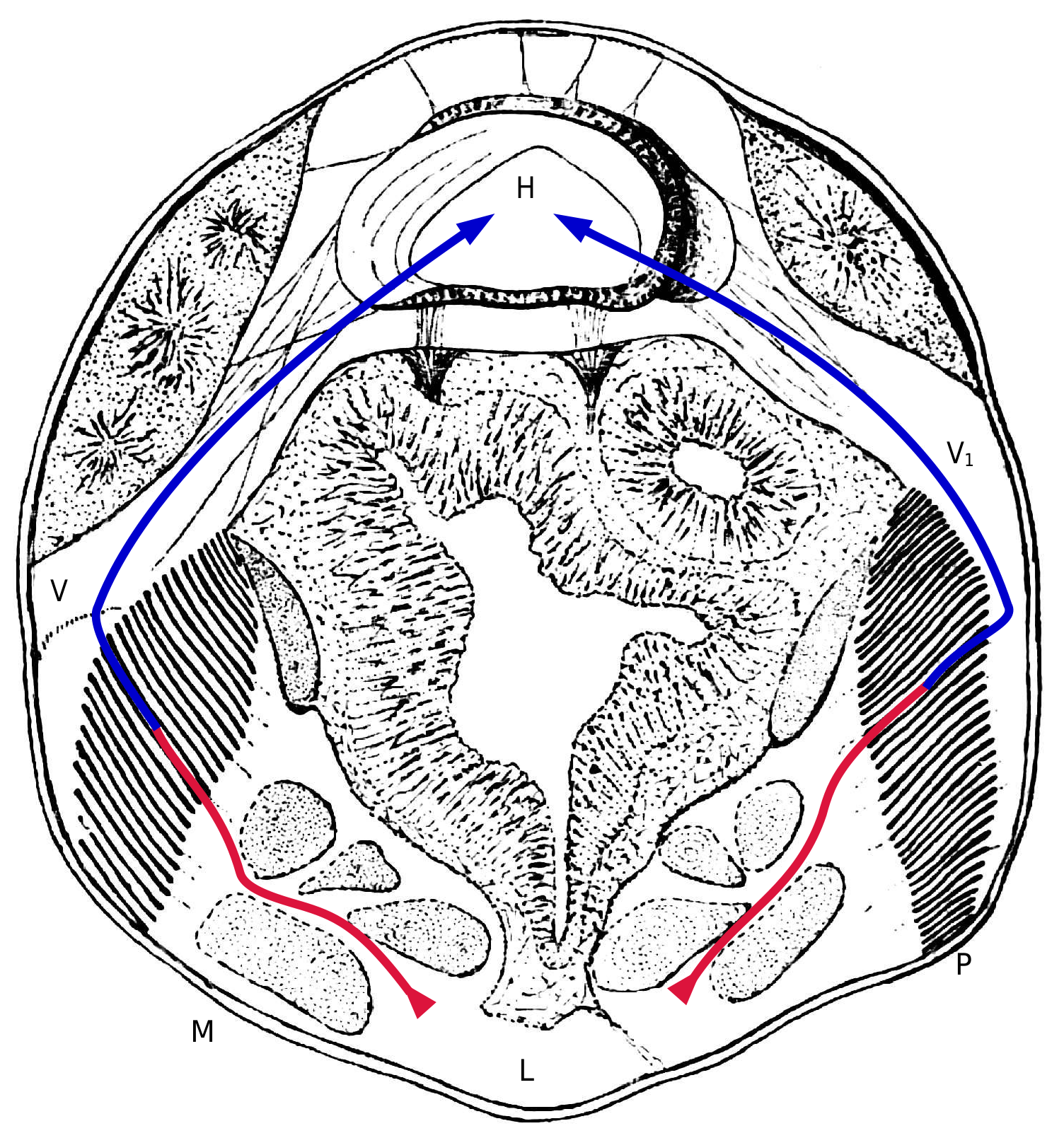
However, as we have seen previously, the blood from the large ventral sinus, which is going to enter the lungs, still undergoes the systolic thrust. Therefore, pulmonary circulation is ensured not only by the aspiration we have characterized but also by this thrust, both originating from the heart. The coexistence of two different pressures, one upstream and the other downstream of the lungs, reveals that there should be no direct communication between the ventral sinus and the pulmonary vein (sinus) other than the pulmonary system with its relatively high resistance.
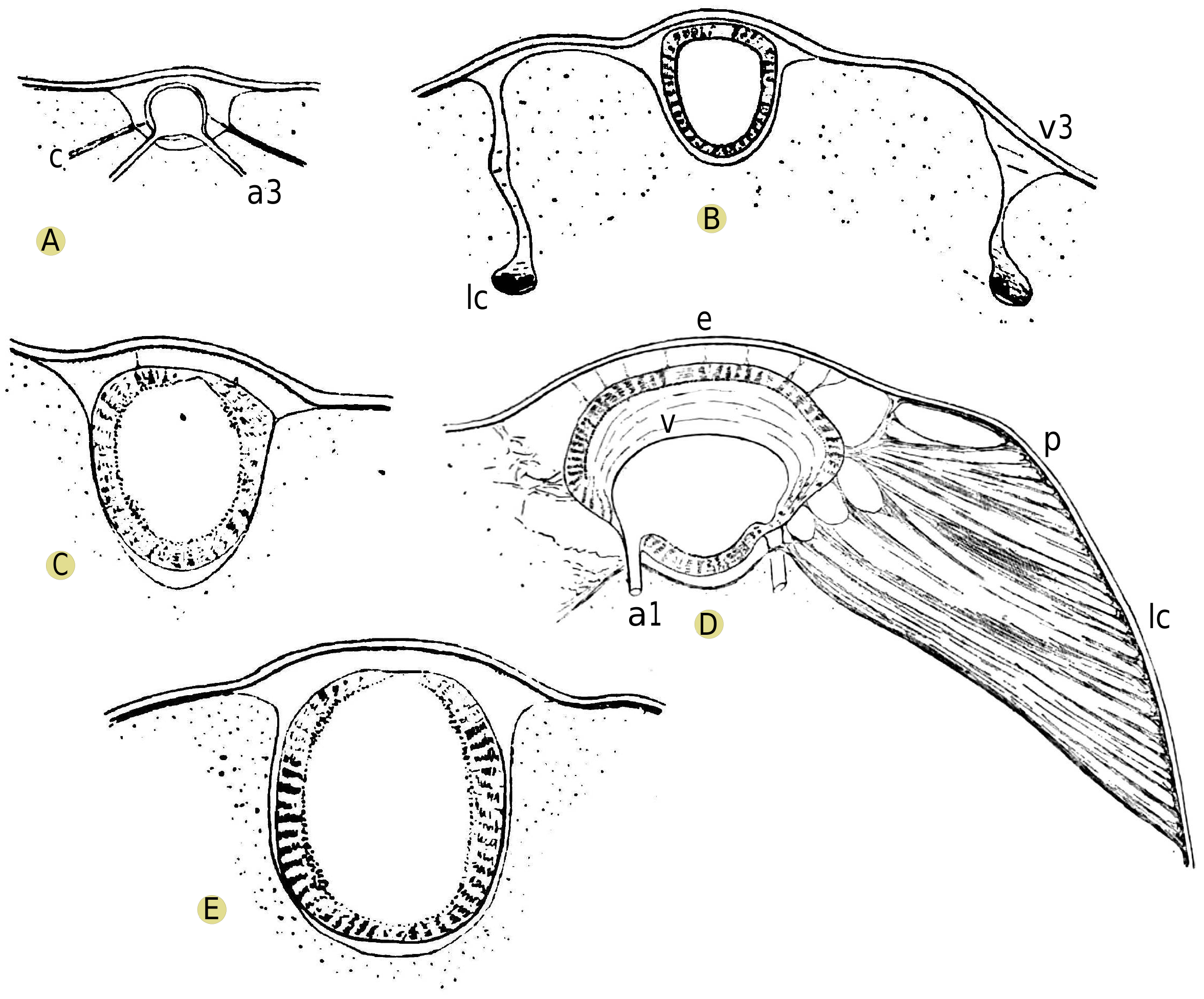
Figures 9, 10 View Figures 9-11. 9 , and 11, representing approximately horizontal sections at levels increasingly closer to the ventral surface, demonstrate this. The pulmonary vein (V in Figure 5 View Figure 5 ) gradually changes shape as it embraces the outer edges of the pulmonary folds; from being cylindrical, it transforms into a flattened system of sinuses interposed between the integuments and the outer edges of the pulmonary folds; these sinuses almost disappear at the level of the most ventral folds, where they continue to merge with lamellar sinuses. On the other hand, the large ventral sinus only appears at the level of the abdominal extensions of the tergal saddle of the pedicel (lv in Fig. 10 View Figures 9-11. 9 ).
Nowhere, in the serial sections, is a direct communication observed between the two systems: to pass from the ventral sinus, which thins upward, to the extra-pulmonary sinus that is the origin of the vein, the blood must pass through the system of the lung folds. Figure 12 View Figure 12 , representing an almost transverse section of Pholcus , shows these relationships and also allows us, in conjunction with Figure 4 View Figure 4 , to understand the main facts of the blood circulation.
This is an opportunity to point out that in Pholcus , we do not find the muscle that C. Börner illustrates, in Araneidae [Loco citato, fig. 50, page 101.], attaching to the anterior end of the pulmonary folds and whose contraction, through a mechanism otherwise not well understood, would determine the flattening of the lamellae.
Let us now consider the consequences for the air cavities of the lungs of the facts we have observed: relatively high blood pressure, even in the return sinuses surrounding the lungs, and rhythmic oscillations of this pressure.
There must be an opposing mechanism to the external compression, capable of preventing it from crushing the air cavities (interlamellar spaces and vestibule) by expelling the air present under atmospheric pressure. However, there are no resistant muscles or ligaments that, attached to the lungs, radiate towards fixed external points and whose traction would keep these hollow organs open, with their generally delicate walls. On the other hand, there are unique cuticular structures on the internal walls of the air cavities: small rods that stand perpendicularly to the dorsal surface of the lamellae, and more or less branched trunks whose branches anastomose into a spongy network on the free edges of the lamellae and on the walls of the vestibule [See especially the cited paper by L. Berteaux]. Histologists, by attributing to the small rods the apparent function of keeping the very delicate pulmonary lamellae apart, have partially suspected the role of these more or less rigid structures; but they have not seen, due to artificial shrinkage in preserved organs, that all these chitinous structures come into contact with each other everywhere, even in the vestibule.
From the viewpoint we are considering, the entirety of the air spaces can be compared to a cavity filled with an elastic felt-like material: oscillations of the external pressure necessarily lead to alternating reductions and increases in volume, the successive elements of pulmonary ventilation.
It would be somewhat presumptuous to expect to experimentally verify this explanation of pulmonary ventilation by measuring the changes in the gaps between the pulmonary lamellae, which are deep organs, separated by a few micrometers and oscillating twice per second. But one can think of a simpler verification: demonstrating inspirations and expirations of air through the spiracle, by using a water droplet placed on this orifice to close it with a movable plug. The experiment is not difficult to perform under the microscope. At least in Pholcus , it reveals new facts.
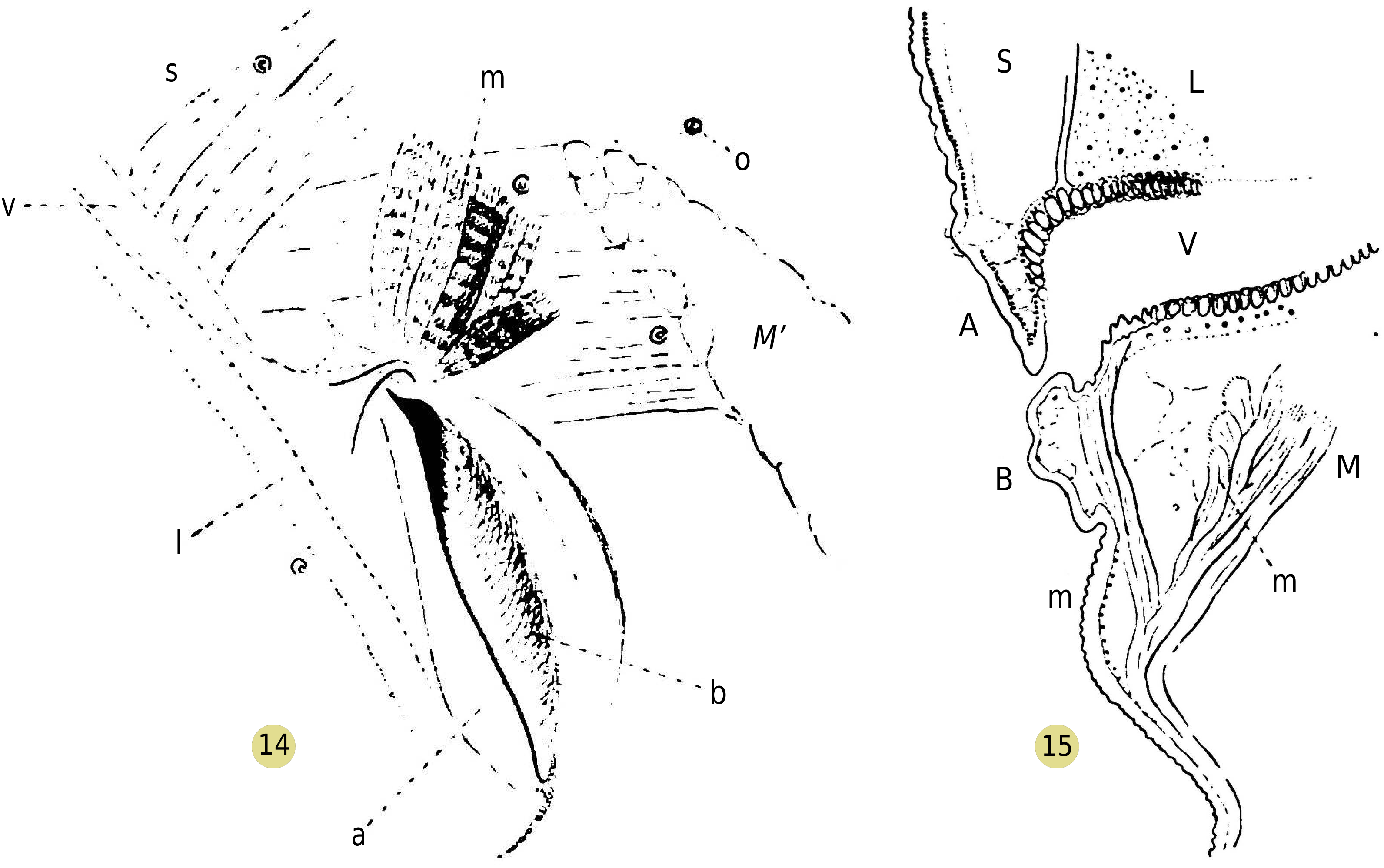
First, contrary to what observers of Araneids say, in specimens kept under the microscope, the spiracle is rarely found wide open, and even then, it remains open only for an instant; the posterior face of the orifice swells into a rounded bulge, which fits closely against the bevel of the anterior edge ( Figure 14 View Figures 14-15. 14 ). After some time, however, a collapse of the bulge occurs at the upper part, releasing the dorsal angle of the spiracle and restoring limited communication between the vestibule and the exterior ( Figure 14 View Figures 14-15. 14 ); but this collapsed part of the bulge exhibits a sort of continuous trembling, and the orifice closes again at the slightest stimulation of the animal.
The bulge in question ( Figures 11 View Figures 9-11. 9 , 15 View Figures 14-15. 14 , horizontal section) is a simple hollow fold, inside which only some connective tissue and blood fluid can be observed: its swelling is thus a passive phenomenon, due to an increase in blood pressure in the abdomen, under the influence of the contraction of muscles radiating from the pedicle to the abdominal walls.
The retraction of the upper region of the bulge remains to be explained. Guided by the previous observation, I succeeded in identifying the presence of a small muscle ( Figures 14, 15 View Figures 14-15. 14 ), consisting of a few short muscle fibers, detaching from the large lateral muscle to insert, some (2 or 3) at the very top of the angle of the spiracle, and others (m') a little more numerous, on the inner region of the posterior wall of the vestibule. This small muscle may be the homologue of those that C. Börner has identified in tarantulids.
Therefore, the orifice of the pulmonary vestibule is organized in such a way that it closes during the contraction of flexor muscles of the opisthosoma on the prosoma. However, under these circumstances, the intervention of a small dilator muscle, which contracts in a jerky manner, keeps a restricted communication between the vestibule and the open air. When a drop of water of suitable size for observation is placed on this orifice, one can witness the trembling of the bulge, and a small amount of water enters the vestibule, drawn in by the recoil of the wall. The general phenomenon of inspiration and expiration that one expects is masked by the intervention of the dilator muscle.
It is worth mentioning that this investigation does not require the use of a water droplet. It is noticed that the external surface of the exoskeleton of Pholcus is coated with a greasy secretion that, sometimes, accumulates by capillarity in the angle of the spiracle. Observing the deformations and movements of this tiny index is sufficient to study the movements of the orifice. After many attempts, where I strived to avoid any excitation of the spider by a shock to the microscope slide or by breathing on it, I succeeded, with a specimen that had lost all its legs due to autotomy [This legless specimen, kept in a humid chamber, is still very much alive three months after autotomy.], in glimpsing for a few moments independent oscillations of the meniscus of the index, which I believe are unrelated to the trembling of the dilator muscle, and they follow a rhythm that is in agreement with that of the heart.
Therefore, we observe that other factors can come into play in pulmonary ventilation, besides the rhythmic oscillations of blood pressure: a small dilator muscle of the vestibule can act as an inspiratory muscle [I mention here, in passing, that bands of the conjunctive sac lining the posterior wall of the vestibule (I have depicted them in Figs. 6 View Figure 6 , 10 View Figures 9-11. 9 , 14 View Figures 14-15. 14 ) should be considered, due to their elasticity, as passive antagonists to external pressure; they act in the same direction as the internal cuticular structures.]. On the other hand, the contraction of the flexor muscles of the abdomen, especially that of the lateral muscle, may lead to forced expiration. One can verify this by immersing a Pholcus in alcohol: the general contraction of muscles is accompanied by the expulsion of a drop of air through the spiracle; then, after the death of the animal and the relaxation of its muscles, it is found that liquid occupies the cavity of the vestibule and a part of the interlamellar spaces, indicating the difference between a maximal expiration and the post-mortem capacity.
Do these occasional factors occur only exceptionally, or does it normally happen at more or less regular intervals that inspiratory and expiratory muscular movements take place to renew the air in the vestibule more efficiently, mixing it with the air in the interlamellar spaces driven by oscillations of cardiac origin? This is a question that I have not been able to elucidate, given the microscopic dimensions of the spiracle and the difficulties mentioned earlier.
Pholcus phalangioides does not possess tracheae. The season has not yet allowed me to study tracheal ventilation in appropriate species. It is provisionally permissible to assume that the ventilation mechanism is analogous to that of the lungs.
General considerations
Upon the first examination of the circulatory system of Araneus and Pholcus , one is struck by the significance of the anterior artery, whose caliber is remarkably larger than that of the lateral and posterior arteries [The dimensions shown in my drawings are measured from sections; they are probably more accurate than the data provided by injections.]. The majority of the blood propelled during each cardiac systole flows to the prosoma, and the main portion of the circulatory system is represented by the cycle: heart, prosoma, lungs, pulmonary veins, ostium I. Hence, the pulmonary veins are highly developed, and the anterior ostia are the most important of the three pairs of cardiac ostia, causing the dorsal vessel to shift forward in the abdomen. All this indicates, physiologically, that oxygenated blood from the lungs is distributed almost exclusively to the regions of the body, prosoma, and anterior part of the opisthosoma [In fact, apart from a few insignificant muscles in the anal region, all the striated muscles of Pholcus are located in front of the lungs and the genital orifice.], which include the nerve centers and locomotor muscles: this phenomenon is analogous to circulation in other animals in the animal kingdom.
The blood that the heart sends through the opisthosomal veins can return to it without passing through the lungs. According to Causard, in the forms he injected, there is an extensive cavity surrounding the fibers of the opisthosomal sac, extending below and even above the pericardium when, as in the case of Araneus , it is covered by the digestive gland. This large peripheral cavity receives blood coming from the digestive gland. According to Causard, this provides a pathway for venous blood to re-enter the pericardium without passing through the lungs. There is no doubt about this for very young spiders, where blood cells can be observed entering the pericardium following the depressions on the surface of the digestive gland (corresponding to the exocardial ligaments) in front of the middle and posterior ostia. Injection results in spiders suggest that this may still occur in adults; the injected material can infiltrate the subcutaneous cavity between the fibrils of the exocardiac ligaments, indicating a possible infiltration pathway to the pericardium. However, there is no wide-open canal communication, and the amount of blood following these return paths is certainly low, according to Causard. My sections of Pholcus show that the subcutaneous cavity may well be, in many regions, a virtual cavity distended by injections; on the other hand, as seen in my drawings, the two pairs of posterior lateral veins described by Causard in young individuals of other species persist in adults. These veins constitute serious return pathways to the heart for blood coming from the opisthosomal arteries. Therefore, this blood completes a particular cycle, but it is evident that it partially mixes with the pulmonary blood, both in the heart and in the pericardial cavity.
This semi-independence of the two circulatory cycles and the functional relationship between the lungs and the organs in the anterior region of the spider's body explain the constant position of the lungs at the very anterior part of the abdomen.
The interdependence of the respiratory organs and the heart is further highlighted when comparing these systems in arachnids with those of another group in the same phylum, the insects, which have evolved in a different direction.
In insects, the respiratory organs, the tracheae, are not confined to a restricted space; they branch out extensively, and the air, as often described, seeks the blood in the farthest corners of the organism. However, if one observes the heart pulsations in an Insect like the cockroach or the water beetle, it is striking how comparatively insignificant these contractions are compared to the incessant movements of the intestinal convolutions. This observation convinces us that the mixing of abdominal blood is much more a result of the peristaltic movements of the intestine rather than the activity of cardiac systole. On the other hand, the respiratory movements, which are ample and seemingly powerful, regularly compress the abdominal contents, suggesting that they play a more significant role than the heart in facilitating blood exchange between the abdomen and the thorax. It gives the impression that the dorsal vessel is, in large insects at least, a regressive organ, serving mainly to draw blood from the abdomen and then discharge it through its single artery into the cephalic region, which is less affected by respiratory movements due to the distance and limited development of air sacs.
I am inclined to believe, as a guiding hypothesis for future research, that the development of tracheae has led to the regression of the dorsal vessel in insects. On the other hand, in arachnids, no such influence has been observed, and the dorsal vessel must have retained its role as a propulsive organ of the circulatory fluid. Moreover, the reduction of respiratory organs in arachnids has turned them into a highly resistant spongy body, increasing the workload of the heart, which has maintained, or acquired, the volume that we observe in it.
ORIGINAL FRENCH VERSION
OBSERVATIONS SUR LA CIRCULATION SANGUINE ET LA RESPIRATION PULMONAIRE CHEZ LES ARAIGNÉES. PAR VICTOR WILLEM. TRAVAIL DU LABORATOIRE DE PHYSIOLOGIE DE L'UNIVERSITÉ D'AMSTERDAM.
Nous ne connaissons rien de pre̕cis sur les mouvements respiratoires chez les Araigne̕es. Le seul observateur moderne qui les ait cherche̕s expe̕rimentalement est F. PLATEAU: après avoir, en 1884, publie̕ un me̕moire fondamental sur les mouvements respiratoires chez les Insectes [F. PLATEAU. Recherches expe̕rimentales sur les mouvements respiratoires des Insectes. Mém. de l'Acad. roy, de Belgique, t. XLV, 1884.], il voulut poursuivre ses recherches sur d'autres Arthropodes ae̕riens et appliquer aux Arachnides les me̕thodes inge̕nieuses qui l'avaient servi avec les Insectes. Ce fut sans succès, aussi bien avec les Arane̕ides qu'avec les Scorpionides et les Phalangides. ,,Aucune des me̕thodes d'investigation connues", conclut-il [F. PLATEAU. De l'absence de mouvements respiratoires perceptibles chez les Arachnides. Archives de biologie, t. VII, 1887 (p. 344).], „ne permet de de̕terminer en quoi consistent re̕ellement les mouvements respiratoires des Araigne̕es. ”
L'e̕chec d'un expe̕rimentateur aussi adroit et aussi minutieux que Plateau semble avoir de̕tourne̕les naturalistes de toute nouvelle tentative; il n'est pas àma connaissance qu'on ait, depuis 1887, publie̕ d'expe̕rience sur la fonctionnement des organes respiratoires des Araigne̕es. On admet avec Plateau, semble-t-il, qu' „il est inutile de chercher àvoir dans les parois abdominales des changements de diamètre" [Loco citato, p. 346.]. Mais on se rabat sur des hypothèses, dont la principale remonte àJ.
MacLeod [MacLeod. Recherches sur la structure et la signification de l'appareil respiratoire des Arachnides. Archives de biologie, t. V, 1884.]: les changements de capacite̕des poumons seraient duŝ aux changements d'e̕paisseur des feuillets pulmonaires, gonfle̕s de sang, dont les deux lamelles se rapprocheraient et s'e̕loigneraient l'une de l'autre, par la contraction des colonnettes qui les re̕unissent: le volume des poumons restant constant, l'espace occupe̕par l'air varie en sens inverse de celui des feuillets. MacLeod voyait dans les colonnettes une portion musculaire, mais son interpre̕tation a e̕te̕ reconnue inexacte par les histologistes plus re̕cents. Et ne̕anmoins, faute d'une hypothèse d'apparence plus vraisemblable, on a voulu conserver aux colonnettes une contraction propre: L. Berteaux, à l'occasion de recherches sur la structure des poumons, se croit oblige̕d'attribuer la fonction contractile aux cellules e̕pithe̕liales qui constituent les colonnettes [L. Berteaux. Le poumon des Arachnides. La Cellule, t. V, 1889.]. D'autre part, C. Börner, se se̕parant de cette opinion pour ainsi dire classique, de̕crit, dans son me̕moire sur les Pe̕dipalpes [C. Börner. Beiträge zur Morphologie des Arachnides. 1. Ein Beitrag zur Kenntnis der Pedipalpen. Zoologica, Heft 42, 1904 (fig., 50, p. 101).], chez certains d'entre eux, des fibres musculaires s'inse̕rant sur l'extre̕mite̕ante̕rieure des feuillets pulmonaires, fibres dont la contraction et le relaĉhement de̕termineraient un re̕tre̕cissement, puis un e̕largissement et des espaces ae̕riens inter laminaires et des sinus sanguins des lames. Chez les Tarantulides, d'autres muscles, inse̕re̕s sur la paroi poste̕rieure du vestibule du poumon, pourraient être des dilatateurs de cet espace ae̕rien.
J'ai voulu reprendre expe̕rimentalement cette question inte̕ressante et si peu claire encore; je suis arrive̕, je crois, àl'e̕lucider graĉe àdes recherches que j'ai pu faire au Laboratoire de physiologie de l'Universite̕ d'Amsterdam; je tiens àremercier ici son directeur, le Prof. G. van Rijnberk, de la cordialite̕avec laquelle il m'a accueilli, dans des circonstances oùla sympathie qu'on rencontre est doublement appre̕cie̕e. Mon travail a porte̕principalement, jusqu'ici, sur Epeira ( diadema et sclopetaria ), puis sur Pholcus phalangioides , une espèce pre̕cieuse pour semblables recherches, en raison d'une organisation plus simple et de la translucidite̕des te̕guments. [Je dois àl'obligeance de M. H. Boschma, assistant de zoologie, la plupart des exemplaires de cette espèce, relativement rare, que j'ai utilise̕s.]
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.