Pseudoanthidium nanum, (MOCSARY, 1880)
|
publication ID |
https://doi.org/ 10.1093/zoolinnean/zlab062 |
|
publication LSID |
lsid:zoobank.org:pub:CF1BB523-4E43-486B-9A4F-E510F1854B9B |
|
DOI |
https://doi.org/10.5281/zenodo.5823097 |
|
persistent identifier |
https://treatment.plazi.org/id/AE06D043-FFF1-FF93-FF67-9477FD8CF93F |
|
treatment provided by |
Plazi |
|
scientific name |
Pseudoanthidium nanum |
| status |
|
PSEUDOANTHIDIUM NANUM ( MOCSÁRY, 1880) View in CoL
( FIGS 2F View Figure 2 , 4 View Figure 4 , 5 View Figure 5 , 9A, C, E View Figure 9 , 10A View Figure 10 , 11A View Figure 11 , 12A View Figure 12 , 13A View Figure 13 )
Apis liturata Panzer, 1801: 21 , (♀). Type locality: ‘Legi primo vere in sylvaticis 1800’ [collected in early spring in forest (in Germany, based on the title of Panzer’s work, ‘ Faunae Insectorum Germanicae ’)]. Junior primary homonym of Apis liturata Gmelin, 1790: 2789 .
Anthidium reptans Eversmann, 1852: 85 View in CoL , ♀ ♂, nom. oblit., synon. nov. Type locality: ‘in promontoriis Uralensibus australibus’ [ Russia: Urals]. Lectotype, ♂, by present designation: ‘Spask Jun’ [ Russia: Orenburg Prov., Saraktash Distr., Spasskoe], ‘ Anthidium reptans View in CoL ♂. Evers.’, ‘ Lectotypus ♂ Anthidium reptans Eversmann, 1852 View in CoL design. Fateryga et Proshchalykin 2019’ [red label] (ISZP) ( Fig. 4 View Figure 4 ). Paralectotypes: ♀, ‘Spask Jun’, [golden disc], ‘ Paralectotypus ♀ Anthidium reptans Eversmann, 1852 View in CoL design. Fateryga et Proshchalykin 2019’ [red label]; ♀, ‘Spask Jun’, ‘ Paralectotypus ♀ Anthidium reptans Eversmann, 1852 View in CoL design. Fateryga et Proshchalykin 2019’ [red label]; ♀, ‘Spask Jun’, ‘ Anthidium reptans View in CoL ♀. Evers.’, ‘ Paralectotypus ♀ Anthidium reptans Eversmann, 1852 View in CoL design. Fateryga et Proshchalykin 2019’ [red label]; ♀, ‘Spask Jun’, [golden disc], ‘4’ [purple label], ‘ lituratum Panz. View in CoL ’, ‘ Anthidium reptans View in CoL ♀. Evers.’, ‘ Paralectotypus ♀ Anthidium reptans Eversmann, 1852 View in CoL design. Fateryga et Proshchalykin 2019’ [red label] (ISZP).
Anthidium nanum Mocsáry, 1880: 51–53 View in CoL , ♀ ♂, nom. protect. Type locality: in Latin ‘Hungaria centrali, meridionali et orientali‘ [central, southern and eastern Hungary] and in Hungarian ‘Hazánk központi, déli és keleti részében’ [in the central, southern and eastern part of our country]. Lectotype, ♂, by present designation: ‘ nanum View in CoL Mocs’, ‘ Anthid. lituratum ♂ det. Friese 1897’, ‘ Pécs 6/9 Biró’ [Pécs, Hungary; ‘Biró’ may refer to L. Biró, collections assistant to Sandor Mocsáry at the HMNH ( Bohart & French 1986)], ‘Hungarian Natural History Museum Hymenoptera Coll. Budapest’ [blue label], [red label] (blank), ‘ Lectotypus Anthidium nanum Mocsáry 1880 View in CoL design. J. Litman 2020’ [red label] (HNHM) ( Fig. 5 View Figure 5 ).
? Anthidium tenellum var. grandii Alfken, 1936: 111 View in CoL , ♀ ♂. Type locality: ‘ Molina di Quosa , Pisa’ [ Italy].
Material examined: 195 females, 117 males (see Supporting Information, Table S1 for specimen data).
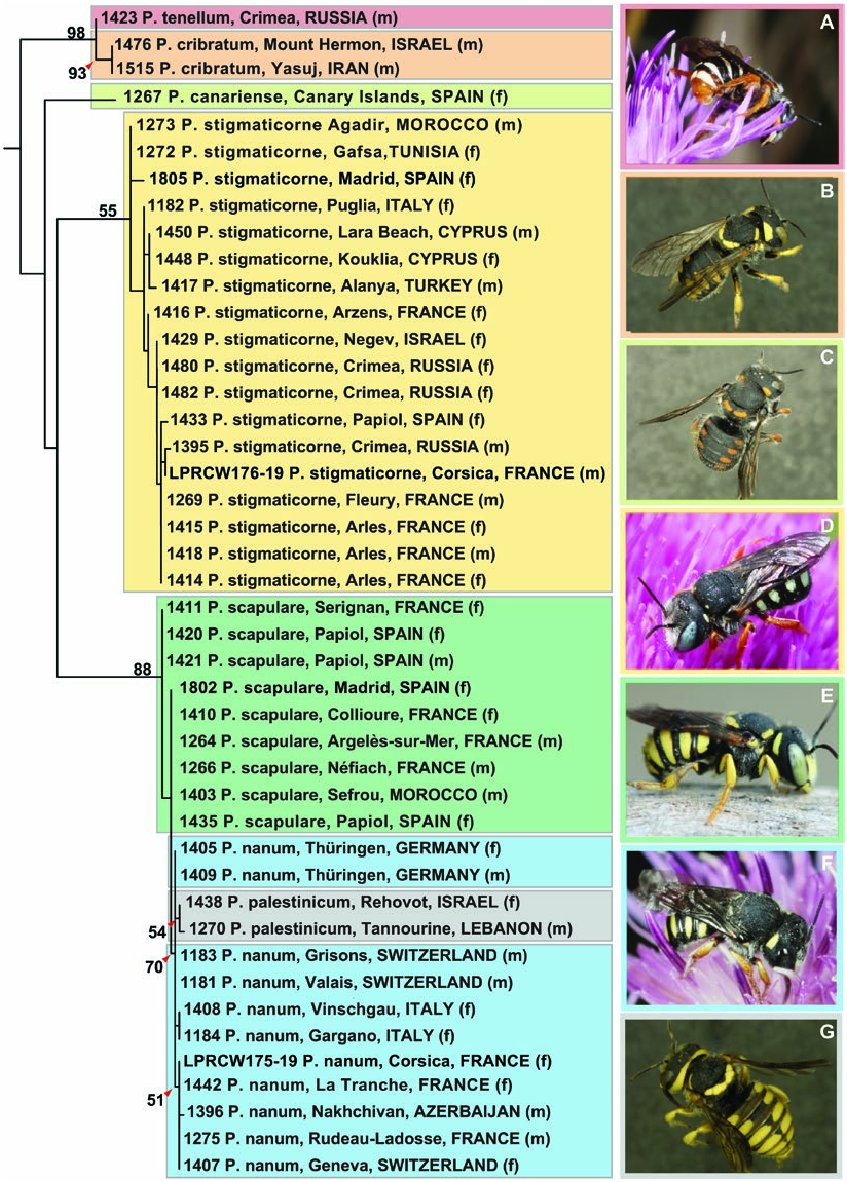
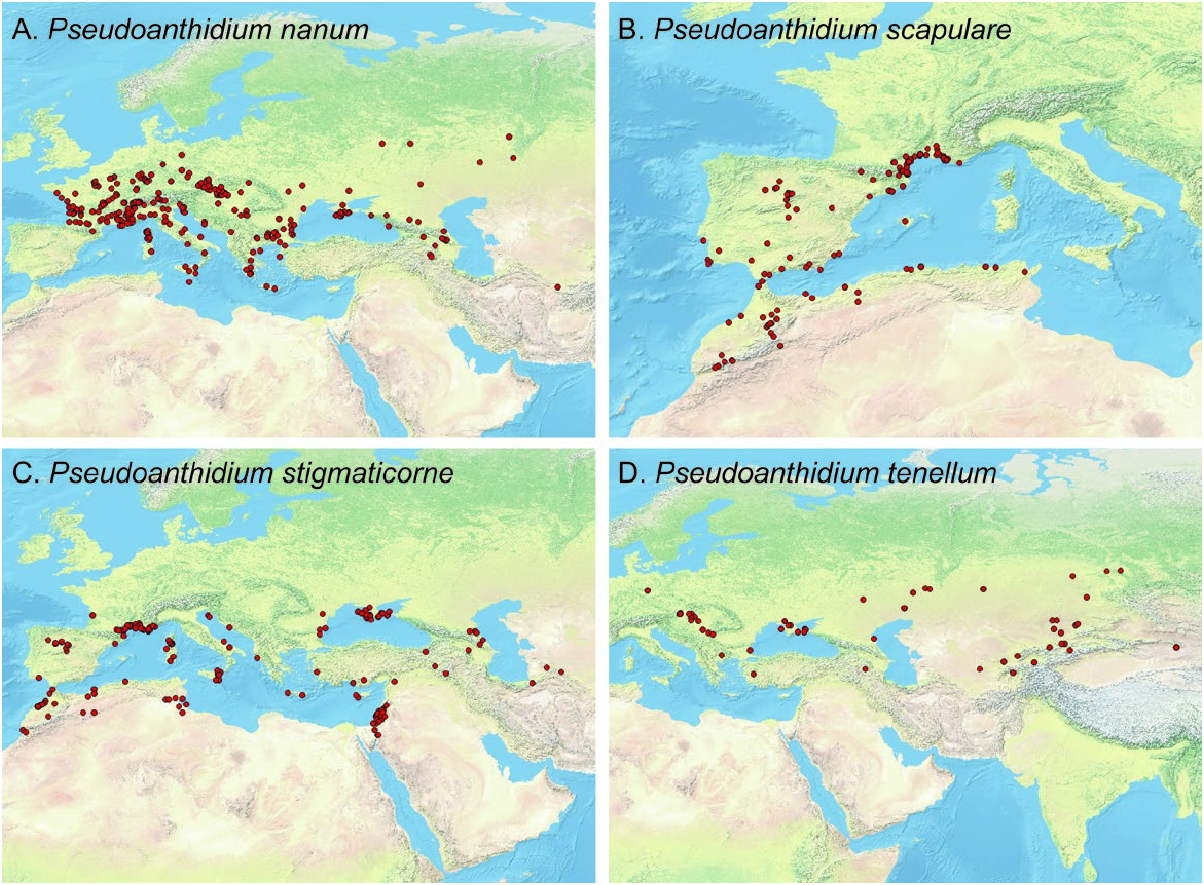
Distribution: Abkhazia, Albania, Armenia, Austria, Azerbaijan, Belgium, Bulgaria, Crimea, Croatia, Czech Republic, France (including Corsica), Georgia, Germany, Greece, Hungary, Italy (including Sardinia and Sicily), Kazakhstan, Malta, Moldova, the Netherlands, Romania, Russia (European part, Urals), Serbia, Slovakia, Slovenia, Switzerland, Turkey (European part), Turkmenistan and Ukraine ( Fig. 6A View Figure 6 ). The single female specimen mentioned from Portugal in Baldock et al. (2018) was examined and determined to be a male P. stigmaticorne . Introduced in the USA.
Host-plant associations: Asteraceae south-western Germany Cirsium vulgare (Savi) Ten. , Centaurea paniculata L., C. jacea L., C. scabiosa L., Onopordum acanthium L. ( Westrich, 1990); Hungary Centaurea biebersteinii DC. ( Mocsáry, 1880) ; Crimea Arctium sp. (female visit), Centaurea jacea subsp. substituta (Czerep.) Mikheev (female visit), Grindelia squarrosa (Pursh) Dunal (male visit), Inula aspera Poir. (female visit), Helianthus sp. (male visits, personal observations); Fabaceae Crimea Lotus corniculatus L. (female visit) (personal observation, A. V. Fateryga).
Remarks: The name of this taxon has been the subject of much discussion. The name Apis liturata Panzer, 1801 , long considered valid, was eventually recognized as a junior homonym of Apis liturata Gmelin, 1790 ( Warncke, 1980) . Nevertheless, Warncke (1980) assigned priority to the name Anthidium lituratum ( Panzer, 1801) , justifying this decision by referring to Article 59b(ii) from an amendment to the Code published in 1973 ( ICZN, 1973), regarding the assignment of priority in cases of secondary homonymy. Yet Apis liturata Panzer, 1801 and Apis liturata Gmelin, 1790 , representing identical species names described in the same genus are, in fact, primary homonyms and not secondary homonyms. This was noticed by Schwarz et al. (1996), who subsequently designated Apis liturata Panzer, 1801 as a junior primary homonym of Apis liturata Gmelin, 1790 , permanently preventing further use of Panzer’s name.
While Articles 23.9.1.1 and 23.9.1.2 of the present version of the code ( ICZN, 1999) allow priority to be assigned to a junior primary homonym in ‘prevailing usage’ under certain circumstances, the version of the code available to Schwarz et al. (1996) did not include these articles. The recommendation at the time concerning primary homonyms was simply that junior primary homonyms were to be considered invalid. Therefore, regardless of the fact that certain authors have continued to use the name liturata Panzer, 1801 after the publication of Schwarz et al. (1996), this name was permanently invalidated by Schwarz et al. (1996) in accordance with the Code available at the time. The fourth edition of the code is clear regarding the stability of taxonomic decisions made using previous editions of the code, especially that ‘new names must not upset actions taken by past generations operating under different, and less restrictive, nomenclatural rules or conventions’ ( ICZN, 1999).
We thus uphold Schwarz’ decision and consider Apis liturata Panzer, 1801 as a junior primary homonym of Apis liturata Gmelin, 1790 and thus invalid. Finally, we consider the decision regarding the validity of a name as a separate issue from the decision regarding the taxon to which that name may be applied. When Schwarz et al. (1996) invalidated the name Apis liturata Panzer, 1801 , they assigned priority to what they considered to be the next available name, P. scapulare (as Anthidium scapulare ). While we maintain the decision to invalidate the name Apis liturata Panzer, 1801 , we do not consider P. scapulare as a synonym of Apis liturata Panzer, 1801 , but instead as a distinct taxon (see section below on P. scapulare for discussion).
Chronologically speaking, P. reptans ( Eversmann, 1852) is the next available name for this taxon and an examination of the type material confirms that the name P. reptans indeed refers to this taxon. Yet to the best of our knowledge, P. reptans has not been used as a valid name since 1899. Anthidium nanum Mocsáry, 1880 , on the other hand, has been used as the presumed valid name for this particular taxon in at least 25 works, published by at least ten authors in the immediately preceding 50 years and encompassing a span of not less than ten years (Supporting Information, Appendix S1). Given that the conditions outlined in both Articles 23.9.1.1 and 23.9.1.2 of the Code are fulfilled, and thus in accordance with Article 23.9.1, Pseudoanthidium nanum ( Mocsáry, 1880) is the valid name for this taxon and is considered a nomen protectum, while Pseudoanthidium reptans ( Eversmann, 1852) is assigned the status of nomen oblitum.
No specimens explicitly labelled as belonging to the type series of Anthidium nanum were located, despite extensive inquiries made at different collections, but a male specimen was located at the HNHM bearing labels written in Mocsáry’s handwriting, as well as a simple dark red label often found on type specimens designated by Mocsáry, and collected at a locality compatible with the somewhat vague type locality described in Mocsáry (1880): ‘In Hungaria centrali, meridionali et orientali’ [in central, southern and eastern Hungary] ( Fig. 5 View Figure 5 ). We thus consider it likely that this specimen belonged to the type series of P. nanum and designate it as a lectotype.
A final note regarding the name Pseudoanthidium nanum: Mocsáry published the name P. nanum (as Anthidium nanum ) in Vol. 16, No. 1 of Mathematikai es Termeszettudomdnyi Közlemenyek. A footnote on the first page of this publication suggests a citation date of 1879 but the printing of Volume 16 was not officially closed until 1881 ( Baker, 1996). As a result, the name P. nanum appears in most works as either P. nanum (Mocsáry, 1879) or P. nanum (Mocsáry, 1881) . However, in a note published in 1996, Baker clearly stated that Vol.16 No. 1 was itself published in April 1880 and proposes this publication year for all taxa described in Vol. 16 No. 1 of Mathematikai es Termeszettudomdnyi Közlemenyek. Few authors, if any, have followed Baker’s recommendation but at the present moment we see no reason not to do so. We thus propose that in the future, this taxon be referred to as P. nanum ( Mocsáry, 1880) .
Anthidium sinuatum Lepeletier de Saint Fargeau, 1841 View in CoL has also been proposed as a possible synonym for this taxon ( Warncke, 1980; Schwarz et al., 1996; Přidal, 2004; Aguib et al., 2010; Kuhlmann et al., 2021). The lectotype and a paralectotype of Anthidium sinuatum View in CoL , designated as such by Donald B. Baker in 2003 (unpublished), are deposited in the Oxford University Museum of Natural History. Photographs of these specimens were examined and were determined to be the taxon commonly referred to as Anthidium loti Perris, 1852 View in CoL ( Fig. 7 View Figure 7 ). We hereby recognize this unpublished designation as detailed below and remove A. sinuatum View in CoL from synonymy with P. nanum View in CoL . We treat A. sinuatum View in CoL as a nomen oblitum because to the best of our knowledge, it has never been used as a valid name after 1899. We consider A. loti View in CoL a nomen protectum, since this name has been used as the presumed valid name for this particular taxon in at least 25 works, published by at least ten authors in the immediately preceding 50 years and encompassing a span of not less than ten years (Supporting Information, Appendix S2), thus fulfilling both Articles 23.9.1.1 and 23.9.1.2 of the Code.
Anthidium sinuatum Lepeletier de Saint Fargeau, 1841: 374–375 View in CoL , ♀ ♂. Type locality: ‘Espagne. Musées de France et du général Dejean’ [ Spain. Museums of France and of the General Dejean] . Lectotype ♀, designated by D. B. Baker 2003, published here: ‘[unreadable]’, ‘sinuatum ♀ ’, ‘ Lectotype Anthidium sinuatum Lep., 1841 D.B. Baker View in CoL des. 2003’ [red label] ( OUMNH) ( Fig. 7 View Figure 7 ) ; Paralectotype: ♀, ‘ Paralectotype Anthidium sinuatum Lep., 1841 D.B. Baker View in CoL des. 2003’ [yellow label] ( OUMNH) .
Warncke (1980) also proposed Anthidium floripetum Eversmann, 1852 as a synonym for this taxon but an examination of the type material of A. floripetum in the ISZP collection revealed that the latter in fact belongs to the genus Icteranthidium Michener, 1948 View in CoL and is thus not applicable to this taxon. We hereby transfer the epithet and designate a female lectotype and a male paralectotype for Icteranthidium floripetum . To the best of our knowledge, this species epithet has never been used in combination with the genus Icteranthidium View in CoL ; whether I. floripetum represents a distinct species or a junior or senior synonym of an existing species must still be investigated. We call attention to the fact that while we designate a lectotype for I. floripetum , we are not using this name as the presumed valid name for any particular taxon, but are simply proposing a new combination. The validity of this name must be properly investigated, ideally within the framework of a revision of the genus Icteranthidium View in CoL .
Icteranthidium floripetum ( Eversmann, 1852) , comb. nov. Anthidium floripetum Eversmann, 1852: 83–84 , ♀ ♂. Type locality: ‘in prov. Orenburgensi’ [ Russia: Orenburg Prov.]. Lectotype, ♀, by present designation: ‘ Spask Aug’ , [golden disc], ‘ floripetum ♀ Evers .’, ‘ Lectotypus ♀ Anthidium floripetum Eversmann, 1852 design. Fateryga et Proshchalykin 2019’ [red label] ( ISZP) ( Fig. 8 View Figure 8 ) . Paralectotype: ♂, ‘ Indersk’ [currently Inder Distr. in Atyrau Prov. of Kazakhstan], ‘ floripetum ♂. Evers .’, ‘ Paralectotypus ♂ Anthidium floripetum Eversmann, 1852 design. Fateryga et Proshchalykin 2019’ [red label] ( ISZP) .
Anthidium peregrinum Costa, 1885 View in CoL , described from Sardinia, has alternately been placed in synonymy with P. nanum View in CoL (as P. lituratum View in CoL in Warncke, 1980; Přidal, 2004) and with P. stigmaticorne View in CoL (as P. leucostoma in Nobile, 1995); other authors have treated this taxon as a subspecies of P. nanum View in CoL (as P. lituratum Rasmont et al., 1995 View in CoL ; Ornosa et al., 2008). Costa’s original description does not provide enough detail to clarify to which taxon this name applies. Only an examination of the type material will clarify the status of P. peregrinum View in CoL . For the moment we include it as a synonym of P. stigmaticorne View in CoL (see below).
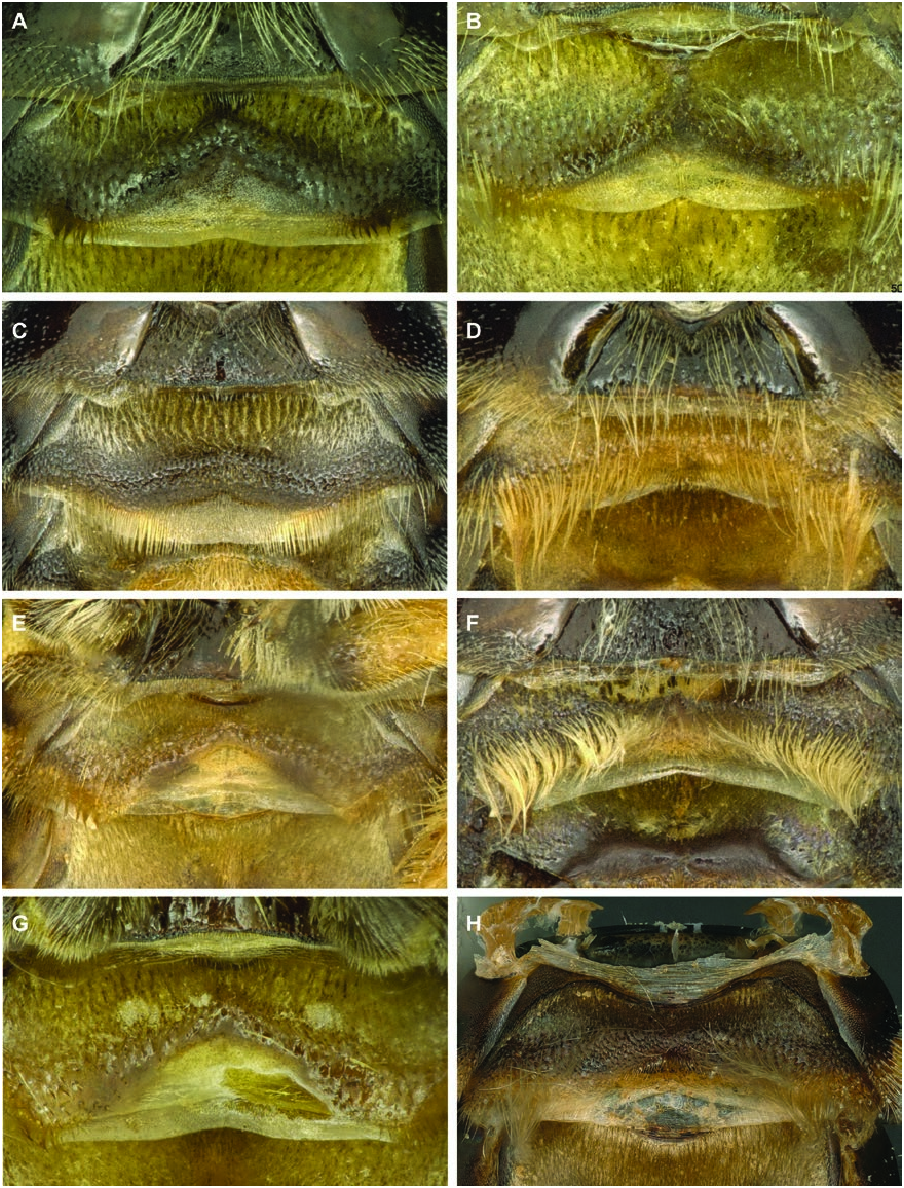
Diagnosis female: The female of P. nanum may be distinguished from other members of this complex by the following combination of characters: overall impression of cuticle shiny; punctation on terga, namely on T1–T2, narrow and dense with shiny interspaces between puncture; posterior margin of T1– T4 shiny ( Fig. 9A, C, E View Figure 9 ). Nevertheless, in their zone of overlap, differentiating females of P. nanum from those of P. scapulare and, in some cases, from those of P. stigmaticorne may be challenging.
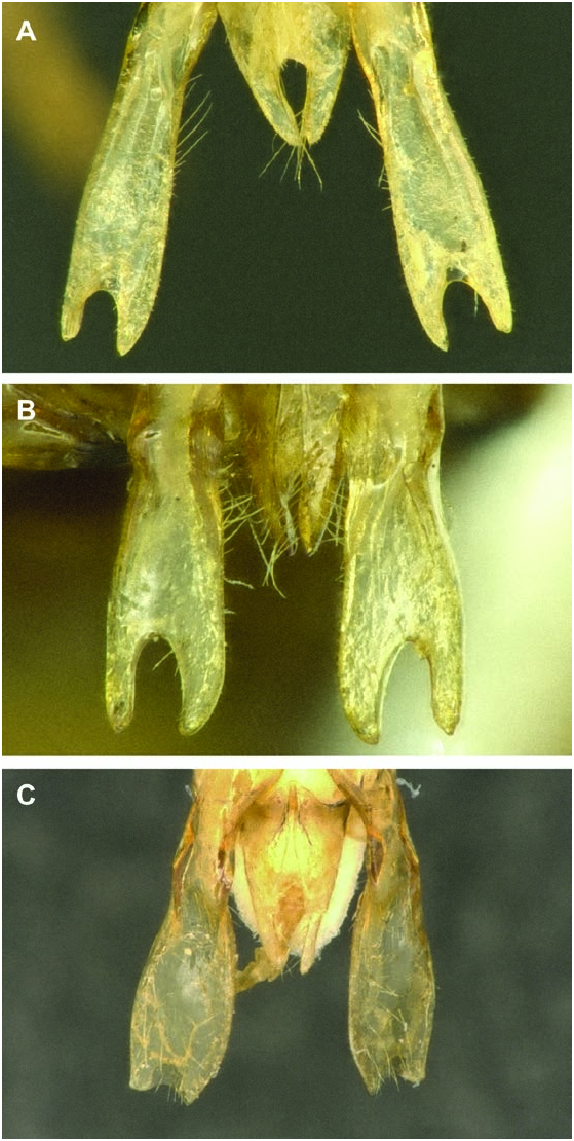
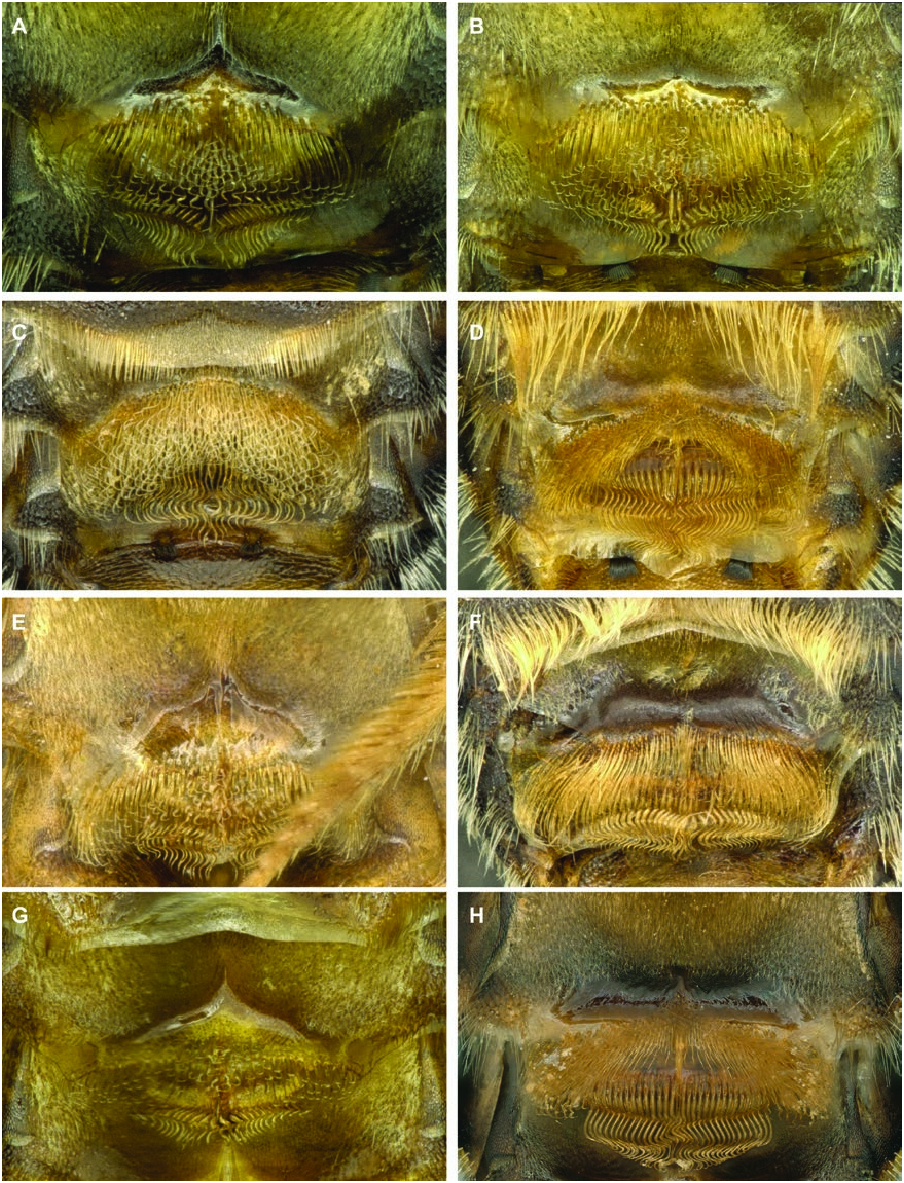
Diagnosis male: The male of P. nanum may be distinguished from other members of this complex by the following combination of characters: gonostylus nearly parallel-sided, only slightly wider at apex than at base (approximately 1.25 times wider at apex than at base in P. scapulare and considerably more so in other species) ( Fig. 10A View Figure 10 ); notch at apex of gonostylus at least as deep as notch is wide, nearly centred at apex and V-shaped (notch either deeper or less deep in other species or broadly rounded) ( Fig. 10A View Figure 10 ); lateral comb on S5 small, with longest teeth shorter than maximal width of hind basitarsus (either larger in other species, as in P. scapulare , P. tenellum and P. palestinicum , or even smaller in other species, such as P. stigmaticorne and P. cribratum ) ( Fig. 11A View Figure 11 ); comb of S5 wider than arm preceding it; posterior, premarginal brush on S3 with hairs hooked at tips (hairs unhooked in P. tenellum and P.cribratum ) ( Fig. 12A View Figure 12 ); shiny, hairless zone on S3 between posterior premarginal brush of hairs and anterior zone of dense, velvety pilosity chevronshaped, with the tip of chevron extending anteriorly as a carina along the midline of sternum ( Fig. 12A View Figure 12 ) (no shiny hairless zone in P. stigmaticorne ; extending nearly straight across sternum in P. scapulare , with similar medial extension; raised hairless zone matte in P. tenellum , extending across width of sternum but without medial extension; raised hairless zone in P. cribratum trapezoidal, without medial extension); raised zone on S2 shiny, dark and in shape of chevron ( Fig. 13A View Figure 13 ); hairs on ventral surface of trochanter 3 short and of even length but not velvety, tuft of longer hairs present at base of trochanter.
The male of P. nanum is morphologically similar to both P. palestinicum and P. kaspareki ; for more information concerning the differentiation of these three species, see the section entitled ‘Diagnosis male’ for P. kaspareki .
Geographical variation: Males of P. nanum are mostly black, with yellow markings that are restricted to the clypeus (typically entirely pale yellow) and present on the paraocular area laterally to inner margin of the eye and posteriorly as far as the basal margin of the clypeus, as well as a single small yellow marking on the preoccipital margin just posterior to each eye, and paired yellow markings laterally on T1–T4, T1–T5 or T1–T6. In some populations (e.g. in Switzerland, Crimea, Dagestan and Azerbaijan), tarsi, tibiae and apex of femora are dark orange-yellow; in other populations (e.g. those in France), these parts are bright lemon-yellow. While most individuals have lemonyellow markings on the terga, in some populations (e.g. Azerbaijan, Corsica and Germany), these markings are pale yellow. In females, the colour of the clypeus is variable and may range from entirely black, to black with lateral yellow spots, to yellow with black markings, to entirely yellow. It should also be noted that the colour of the mandibles, used as a diagnostic feature in other keys [e.g. that of Aguib et al. (2010)], is variable and can range from nearly black in certain specimens to yellow in others. Paired, lateral yellow markings on the terga are present from T1–T4 or T1–T5.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Pseudoanthidium nanum
| Litman, Jessica R., Fateryga, Alexander V., Griswold, Terry L., Aubert, Matthieu, Proshchalykin, Maxim Yu., Divelec, Romain Le, Burrows, Skyler & Praz, Christophe J. 2022 |
Anthidium tenellum var. grandii
| Alfken JD 1936: 111 |
Anthidium nanum Mocsáry, 1880: 51–53
| Mocsary S 1880: 53 |
Anthidium reptans
| Eversmann E 1852: 85 |
Anthidium sinuatum
| Lepeletier de Saint Fargeau ALM 1841: 375 |
Apis liturata
| Panzer GWF 1801: 21 |
| Gmelin JF 1790: 2789 |