Sparsorythus multilabeculatus Sroka & Soldán, 2008
|
publication ID |
https://doi.org/10.11646/zootaxa.4695.6.1 |
|
publication LSID |
lsid:zoobank.org:pub:95507FE2-F562-4227-91F8-96995EC41FA5 |
|
persistent identifier |
https://treatment.plazi.org/id/AC265165-FFAA-7A1B-FF5B-EE226353759A |
|
treatment provided by |
Plazi |
|
scientific name |
Sparsorythus multilabeculatus Sroka & Soldán, 2008 |
| status |
|
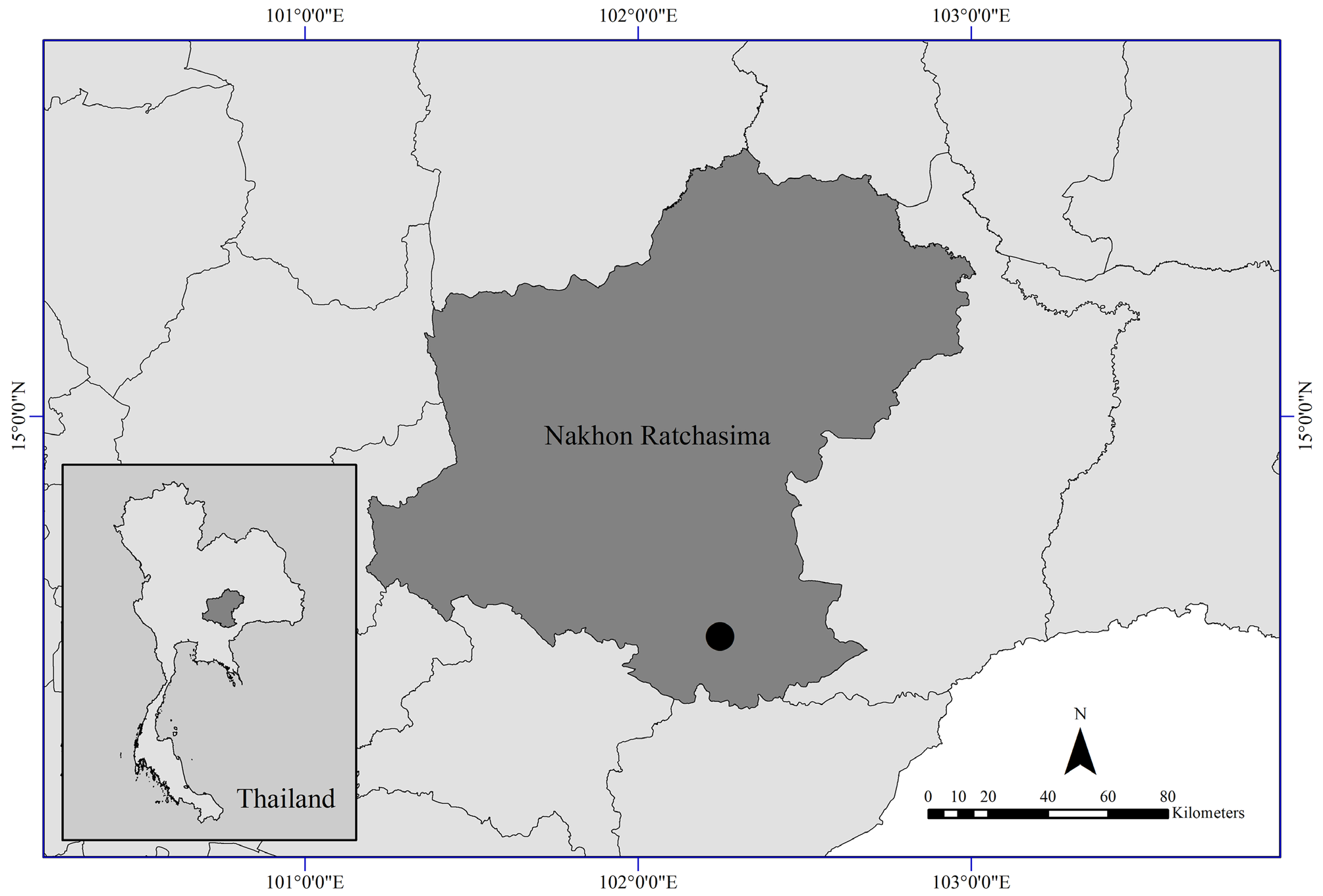
Sparsorythus multilabeculatus Sroka & Soldán, 2008 View in CoL , were collected from the stream of Wang Tao waterfall, Thap Lan National Park, Khon Buri District, Nakhon Ratchasima Province , Thailand ( Figs. 1–2 View FIGURE 1 View FIGURE 2 ). It is located at 14 o 20’13.64’’N, 102 o 14’48.00’’E and elevation 223 m a.s.l. The collections were made between 2013 to 2018 GoogleMaps , five to ten years after the male imago of the species from Vietnam was originally described by Sroka and Soldán in 2008. Nymphs were collected by using an aquatic net with 450 µm mesh size and by hand picking. Adults were collected by light trap overnight using an ultraviolet lamp (15-W fluorescent tube). All specimens were preserved in 95% ethanol. Photographs were taken using a Canon 550D digital camera and Nikon AZ100 Multizoom microscope. Eggs from female nymphs and female adults, and other materials for SEM study were dehydrated in a graded ethanol series, dried by the critical point method, sputter coated with gold and observed with an LEO 1450VP scanning electron microscope.
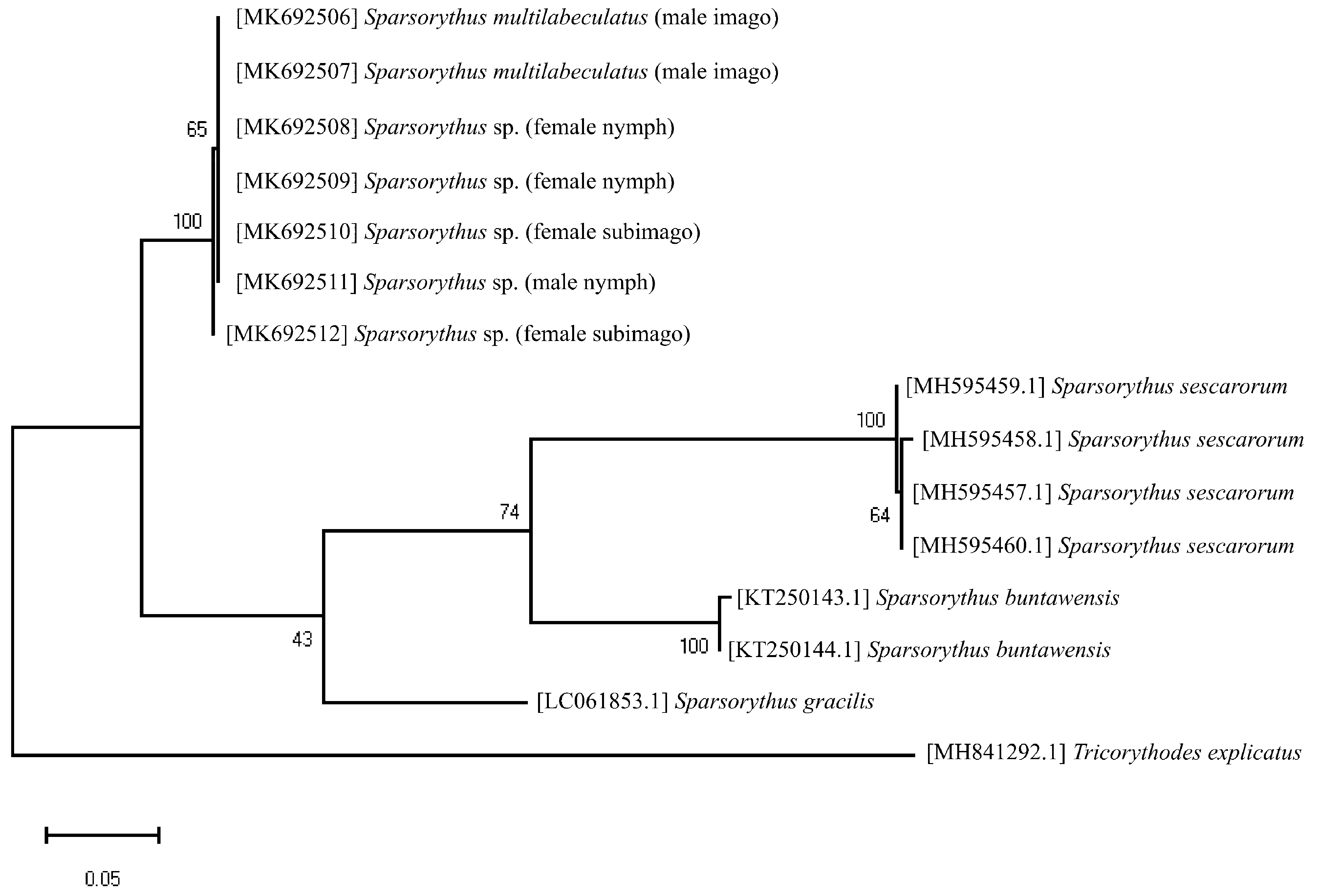
A piece of tissue from the legs of nymphs and adults was preserved in 95% ethanol. Total DNA extraction was performed using the PureLink Genomic DNA Mini Kit (Invitrogen, Carlsbad, CA, USA) extraction protocol and the DNA was stored at -20 °C. Each 50 μL polymerase chain reaction (PCR) contained (1) ~ 10–60 ng of template DNA; 0.4 U of Taq DNA polymerase with 1X ViBuffer, which was composed of 500 mM KCl, 100 mM Tris-HCl (pH 9.1 at 20°C) and 0.1% Triton™ X-100 and (2) 2 mM MgCl 2, 200 μM total dNTP and each primer at 1 μM. The fragment of COI gene was amplified with universal primers LCO1490 + HCO2198 ( Folmer et al. 1994), but we found that it did not work for the male nymph. Therefore, the modified primers LC01490_mod + HC02198_mod ( Garces et al. 2018) was used for the male nymph. Thermal cycling started with incubation at 94 °C for one min, followed by 35 cycles at 94 °C for one min, annealing at 48, 50 °C for 30 s and extension at 72 °C for one min, with a final extension step at 72 °C for five min. All PCR products were visualized on 1.5% agarose gels using Omnipur Agarose ( United States of America). PCR products were purified and then sequenced in both directions by Macrogen INC Sequencing ( Seoul, Korea) using an ABI PRISM 3130 x l Genetic Analyzer (Applied Biosystems, Foster City, USA). Sequences were aligned using BIOEDIT version 7.2.5 ( Hall 1999). Pairwise distances were calculated with MEGA version X ( Kumar et al. 2018) using the “Distances” option and “Nucleotide: p-distance” model option for distances. The COI tree was constructed using the MEGA X ( Kumar et al. 2018). We selected T92+I with partial deletion as the most appropriate model for reconstruction (based on lowest AICc and BIC scores) and conducted a Maximum Likelihood analysis based on the Tamura 3-parameter model ( Tamura 1992). Branch supporting values were assessed using 1,000 bootstrap replications. DNA sequences of other ingroup taxa were downloaded from GenBank (https://www.ncbi.nlm.nih.gov/genbank), obtaining sequences for Sparsorythus buntawensis Batucan, Nuñeza & Lin, 2016 ; Sparsorythus gracilis Sroka & Soldán, 2008 ; and Sparsorythus sescarorum Garces, Bauernfeind & Freitag, 2018 . The species Tricorythodes explicatus Eaton, 1892 ( Ephemeroptera , Leptohyphidae ), was used to root the trees as an outgroup species (sequence available in GenBank (https://www.ncbi.nlm.nih.gov/ genbank)). The GenBank accession numbers are given in Table 1 View TABLE 1 . Vouchered specimens are deposited in the Department of Biology, Faculty of Science, Khon Kaen University (KKU), Thailand.
Results
A total of 122 nymphs and 3,160 winged specimens of Sparsorythus were found at Wang Tao waterfall. Based on morphological characteristics, all winged specimens were S. multilabeculatus Sroka & Soldán, 2008 . They were composed of female subimagines and male imagines. Female imagines were absent. Eggs of female subimagines and female nymphs showed the same characteristics. Rearing nymphs to adult stage was not successful. Genetic distances of the sequences of the mitochondrial COI gene among male and female nymphs, female subimagines, and male imagines ranged from 0 to 0.004.
In the Maximum Likelihood analysis, Sparsorythus nymphs and female subimagines from Wang Tao waterfall grouped together with males identified as S. multilabeculatus and were distinct from other species included in the analysis ( Fig. 3 View FIGURE 3 ). It showed that they were the same species, S. multilabeculatus .
Mature nymphs: Male average body length ( Fig. 4 View FIGURE 4 ) 3.05 mm (n = 40), female average body length ( Fig. 5 View FIGURE 5 ) 3.48 mm (n = 76). Body colouration pale yellowish, dorsal side with black marking.
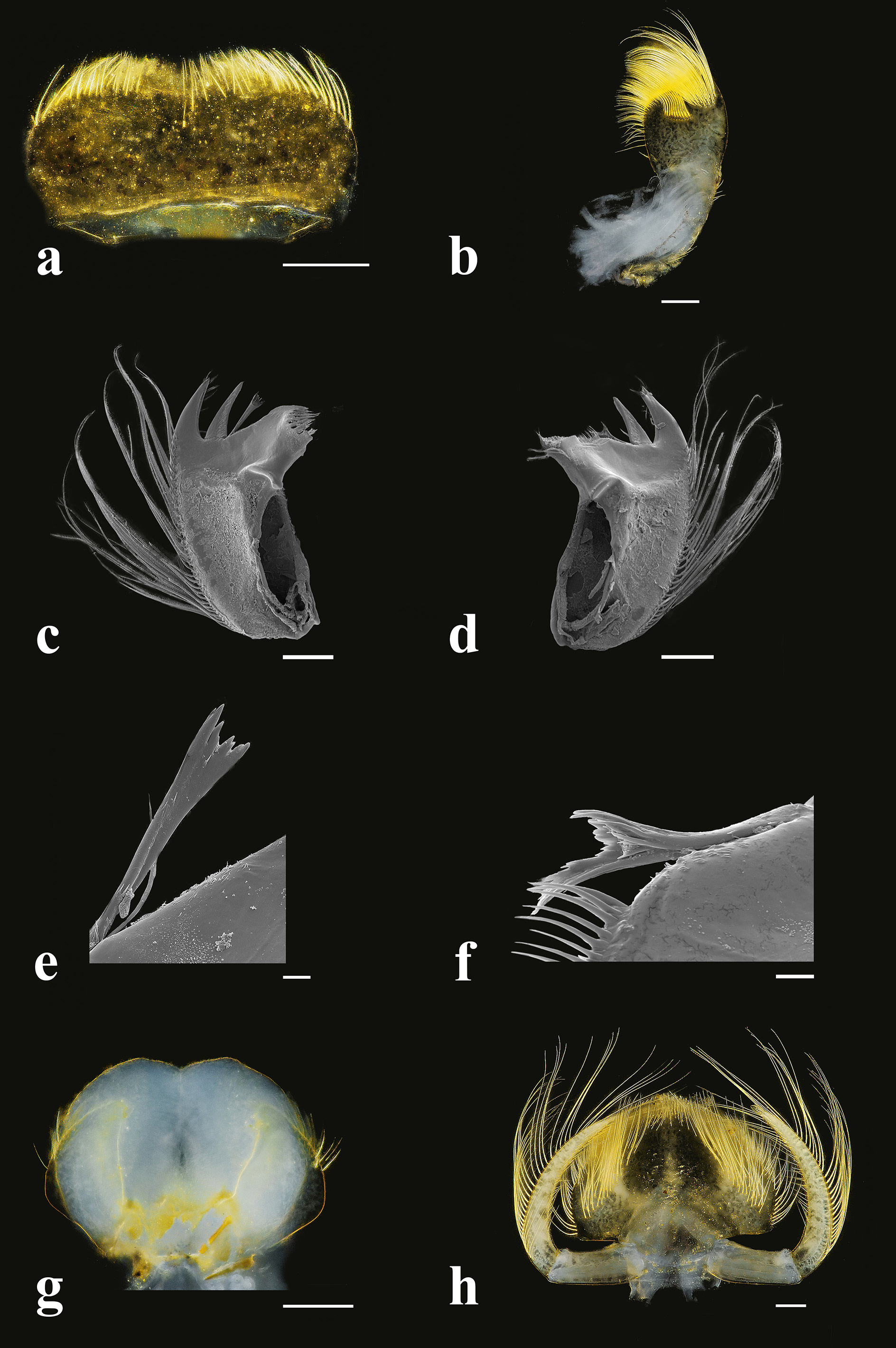
Head: Head wider than long and dark blackish-brown. Head widths 0.62–0.94 mm (male) and 0.63–1.21 mm (female). Antennae longer than head length 2.4:1 (male) and 1.9:1 (female). Scapes about one half length of pedicels. Compound eyes black, ocelli greyish. Eyes of both sexes about same size. Ratio of distance between compound eyes to eye width of male = 3.42:1, female = 4.32:1. Labrum ( Fig. 6a View FIGURE 6 ) oval, approximately twice as wide as long, with scattered bristles over entire dorsal surface and dense tiny bristles on ventral side. Anterior margin covered by numerous bristles diminishing in length medially. Mandibles ( Figs. 6c, 6d View FIGURE 6 ) robust, each with outer margin bearing regular row of long filtering setae, lateral end of this row curved toward ventral side of mandible; outer incisor of each mandible triangular, with numerous bristles on ventral side; apex with pair of projections; ventral side of inner incisor with bristles and dorsal side with tiny branched setae; left prostheca ( Fig. 6e View FIGURE 6 ) asymmetrically wider apically, with several pointed teeth and with one bristle-like process at base; length of left prostheca approximately as long as inner incisor; right prostheca ( Fig. 6f View FIGURE 6 ) notched, triangular, with several pointed teeth, no setae on inner side; length of right prostheca approximately 2/3 as long as inner incisor. Maxillae ( Fig. 6b View FIGURE 6 ) each with rough oblong shape, approximately 1/3 longer than wide, apical part truncate. Maxillary palps absent. Hypopharynx ( Fig. 6g View FIGURE 6 ) with lingua rounded and wider than long and with shallow emarginate in middle. Labial plate ( Fig. 6h View FIGURE 6 ) without small nick at middle of anterior margin. Glossae and paraglossae fused into rounded triangular plate and with two groups of setae on lateral submargin.
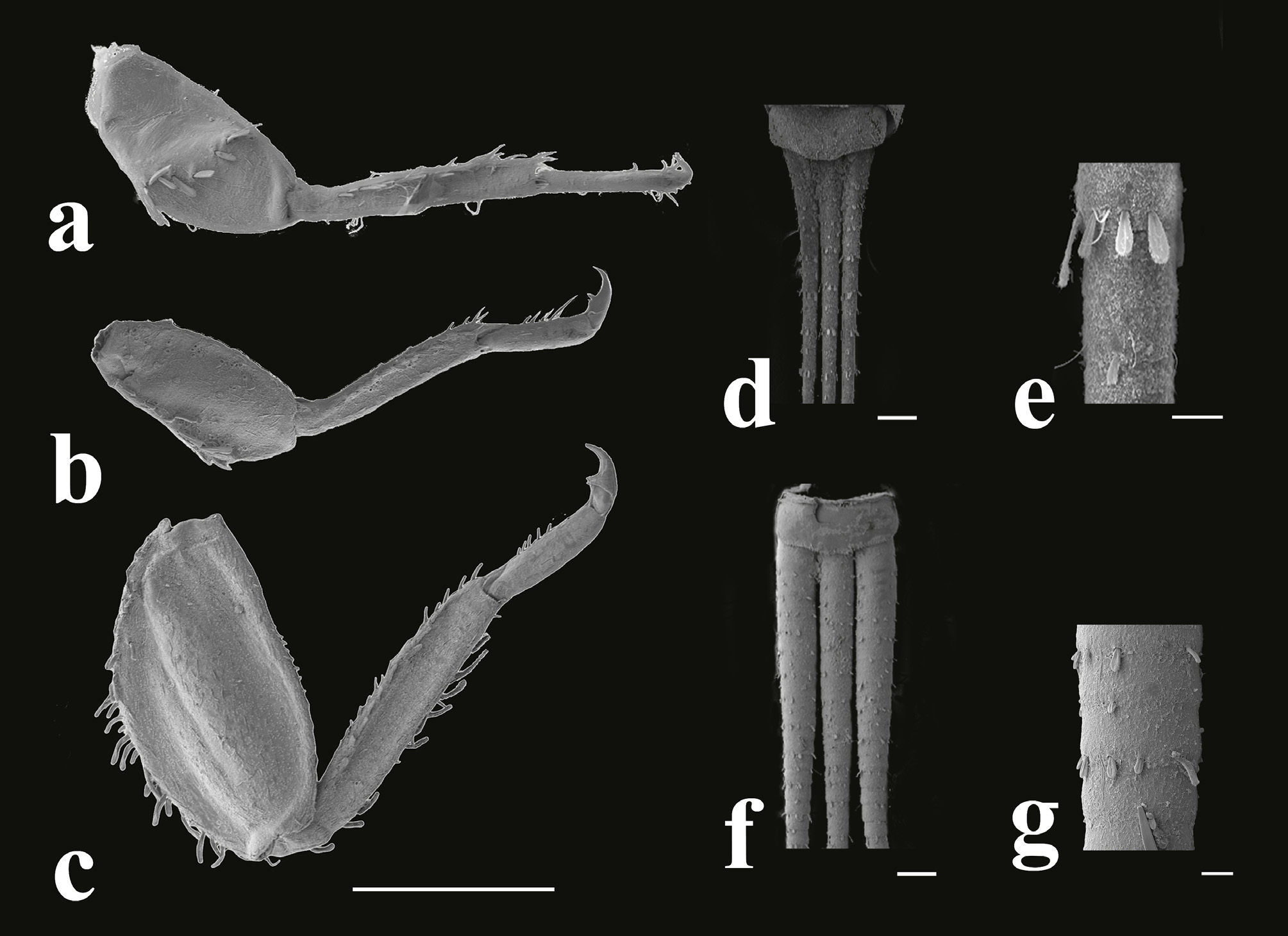
Thorax: Width of pronotum subequal to width of head in both sexes. Anterior margin of pronotum straight but anterolateral angles round and slightly projected forward. Forewing pads fused to their apices, overlapping most of abdominal segment IV of female and segment V of male. Wing pads dark in all nymph stages. Femora flat and shorter than tibiae. Hind legs larger than forelegs and midlegs. Forefemora ( Fig. 7a View FIGURE 7 ) each with conspicuous transverse row of stout setae. Setae rounded at apex and about 4–5 times longer than wide. Mid- and hind femora ( Figs. 7b, 7c View FIGURE 7 ) each with long stout setae at outer margin and short setae at inner margin, surface of mid- and hind femora covered by very small spines. Inner and outer margins of all tibiae with longitudinal row of setae. Tarsal claws hooked, bearing 2 median denticles and one lateral subapical tooth. Length ratio of femur:tibia:tarsus = 1.6:1.7:1 (forelegs); 1.5:1.4:1 (midlegs); and 2: 2:1 (hind legs).
Abdomen: Abdomen not flat, lateral sides on segments II–VII concave, segments VIII–IX cylindrical. Denticles of hind margin of abdominal tergum VII long, either pointed or blunt, or terminated by several points ( Fig. 8 View FIGURE 8 ). Gills absent on abdominal segment I; gills present on abdominal segments II–VII. Gills each with dorsal lamella and ventral portion consisting of two branches with numerous lateral filaments. Rudimentary gill on abdominal segment VII bifurcated, Y shaped. Caudal filaments: cerci as long as paracercus. Sexual dimorphism: cerci and paracercus of female simple, but wider and thicker at proximal part of male. Segments of cerci and paracercus ( Figs. 7 View FIGURE 7 d– 7g) with rounded setae. Posterior margin of caudal filaments with small setae, ratio of maximal length of setae on caudal filaments:segment length in both male and female sexes = 1:2.4.
Male imago: Body length 3–3.5 mm ( Fig. 9 View FIGURE 9 ).
Head: Head wider than long, dark blackish-brown. Eyes small, stalked. Ratio of distance between compound eyes to eye width = 2.5:1. Pedicel of each antenna much longer than scape, length ratio of flagellum:pedicel:scape = 7.7:2.5:1.
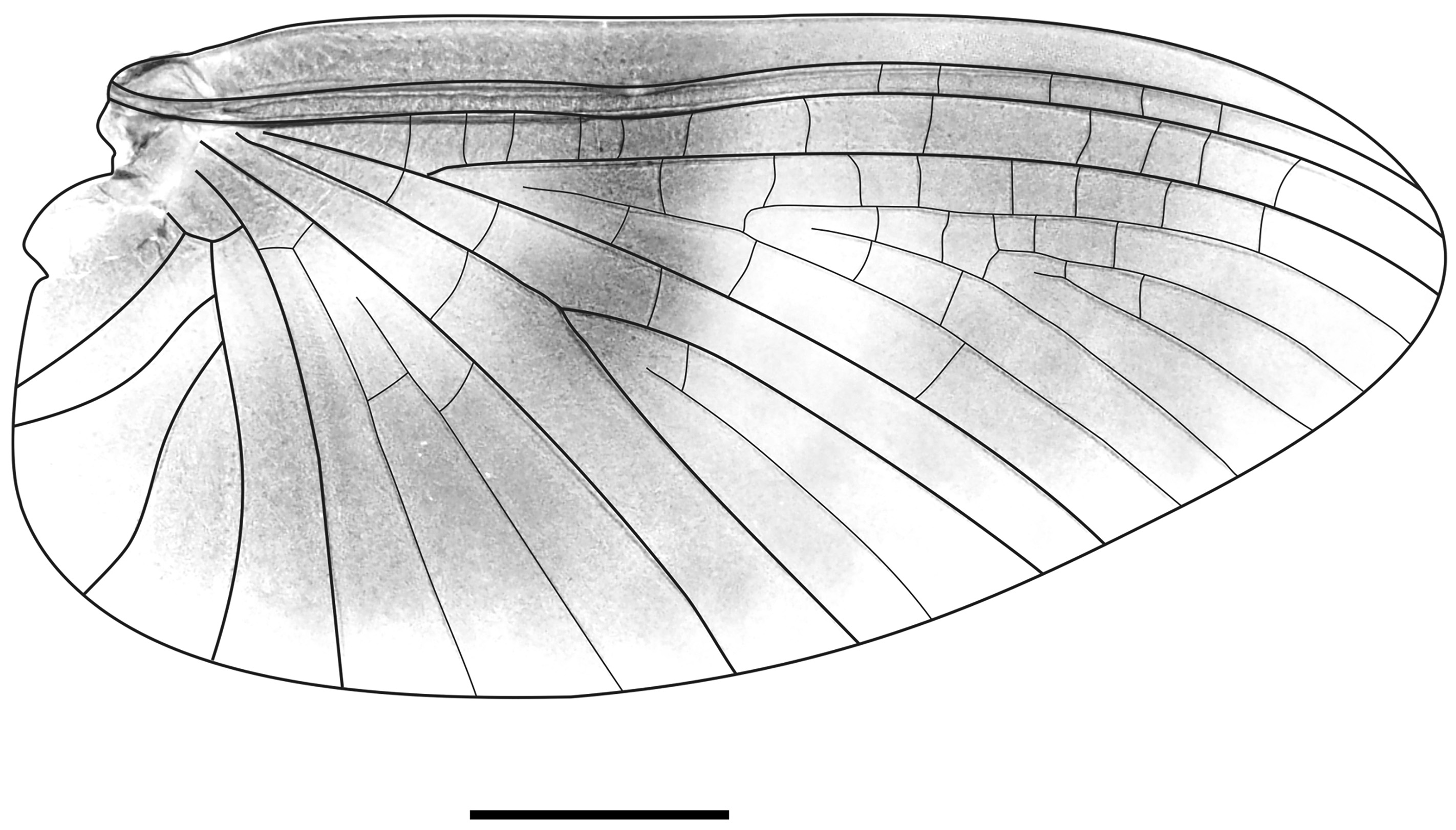
Thorax: Pronotum brownish-grey with black markings, narrower than mesonotum. Mesonotum and metanotum brown. Forewings translucent with typical Y-shaped cubital fork (Tricorythid fork). Forewings with dark grey blots in fields C, Sc, R, and near MA1–MA2 fork. Lighter blotches in fields R1 and MP1 ( Fig. 10 View FIGURE 10 ). Fine hairs cover posterior margin, diminishing distally. Forewing length 3.97 mm, width 1.83 mm. Ratio of forewing length:width = 2.2:1. Hind wings absent. Midlegs of male imagoes shorter than fore-and hind legs. Length ratios of femur:tibia: tarsus = 2:1.9:1 (forelegs); 2.8:2.7:1 (midlegs); 3.5:3.3:1 (hind legs). Ratio of femur length:width = 5:1 (forelegs); 5.7:1 (midlegs); 5.9:1 (hind legs).
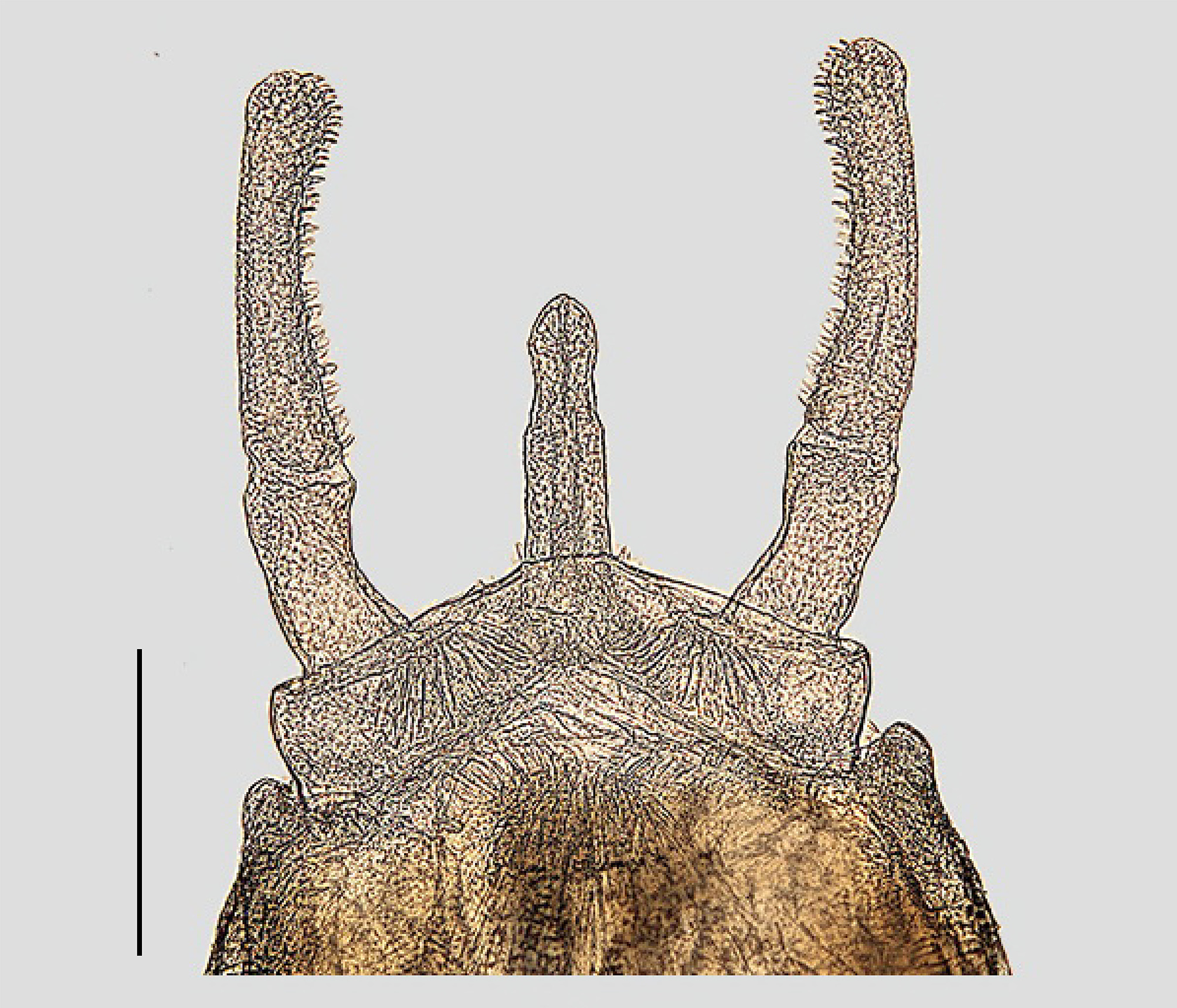
Abdomen: Abdominal shape of male more slender than that of female, with small, dark greyish, spot on tergum IX. Subgenital plate not divided. Penis ( Fig. 11 View FIGURE 11 ) completely fused, forming rod-like structure and apex with apparent medial nick. Forceps two-segmented, basal segment shorter than distal segment. Distal segment of forceps bearing rows of numerous attached structures along inner side. Penis reaching to approximately 1/3 length of second forceps segment. Subgenital plate near penis base with small thorn-like structure. Cerci glabrous, paracercus sparsely covered with fine setae, paracercus longer than cerci, cerci approximately 4x body length.
Female subimago: Body length is 3–4 mm ( Fig. 12 View FIGURE 12 ).
Head: Head dark blackish-brown, wider than long. Eyes small, stalked. Ratio of distance between compound eyes to eye width = 4.1:1.
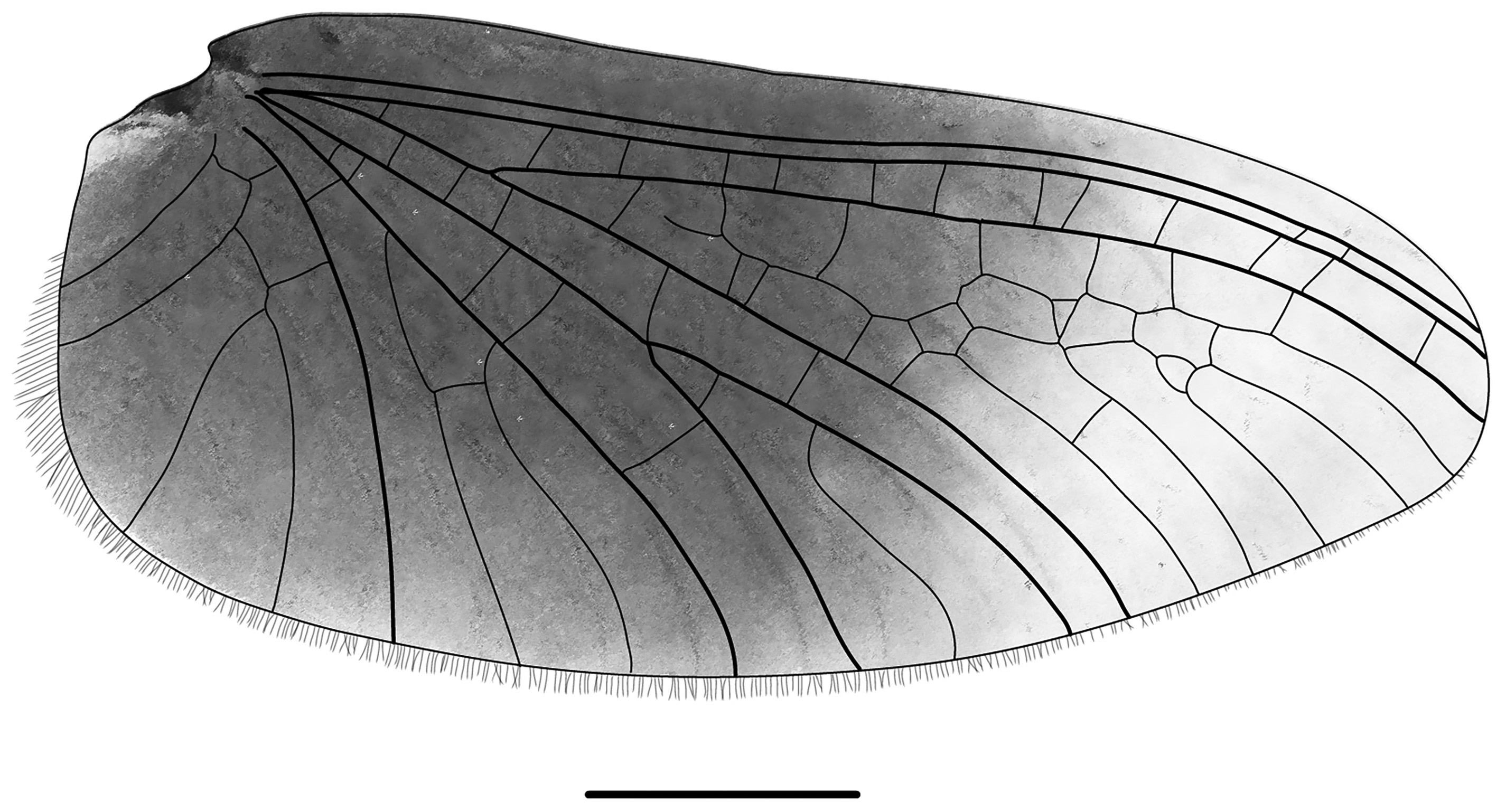
Thorax and abdomen: Prothorax and legs brownish-grey with black markings, cerci light grey. Venation of forewings dark in basal area, more than half of each wing dark, distal portion translucent ( Fig. 13 View FIGURE 13 ). Forewing length 5.67 mm, width 2.33 mm. Ratio of forewing length:width = 2.4:1. Length ratio femur:tibia:tarsus = 2.5:3.1:1 (forelegs), 2.6:2.6:1 (midlegs), 3.3:3.4:1 (hind legs). Ratio of femur length:width = 2.9:1 (forelegs), 3.7:1 (midlegs), 4.3:1 (hind legs). Abdomen dark greyish brown, containing 300– 700 eggs (n = 10). Cerci and paracercus approximately half of body length, covered with fine setae. Paracercus longer than cerci.
Egg: Oval, longer than wide, mean size 175.6 µm long and 109.9 µm wide (n = 10). Egg pale yellowish, with polar cap of same colour. Polar cap rounded at apex and covering about 1/4 of egg length ( Fig. 14 View FIGURE 14 ). Egg surface covered with hexagonal structures.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |