Lynceus insularis, Olesen & Pöllabauer & Sigvardt & Rogers, 2016
|
publication ID |
https://doi.org/10.5852/ejt.2016.224 |
|
DOI |
https://doi.org/10.5281/zenodo.3853009 |
|
persistent identifier |
https://treatment.plazi.org/id/AA38C433-FFEC-FF97-121A-FBF20E83FD24 |
|
treatment provided by |
Valdenar |
|
scientific name |
Lynceus insularis |
| status |
sp. nov. |
Lynceus insularis View in CoL sp. nov.
urn:lsid:zoobank.org:act:AC898BFD-F299-45AD-B6BA-D820B24C693E
Figs 2–8 View Fig View Fig View Fig View Fig View Fig View Fig View Fig
Material examined
Holotype
NEW CALEDONIA: Ƌ, South Province, Mont-Dore , doline (sinkhole), 22°19'32.38" S, 166°54'07.26" E, 30 Apr. 2009, C. Pöllabauer coll. (DOL-03, stored as ZMUC-CRU-4783). GoogleMaps
Allotype
NEW CALEDONIA: ♀, same collection data as holotype (ZMUC-CRU-4784). GoogleMaps
Paratypes
NEW CALEDONIA: 2 spec., same collection data as holotype (ZMUC-CRU-4785); 8 ƋƋ, 6 ♀♀, same location as holotype, 14 May 2010 (ZMUC-CRU-4786); 2 ƋƋ, 1 ♀, same location as holotype, 14 May 2010, prepared for SEM (ZMUC-CRU-4787).
Etymology
The species epithet ‘ insularis ’ is the genitive form of the Latin word for ‘island’ (insula), literally ‘of an island’ in reference to the insular distribution of this species.
Description
Male (holotype; Figs 2–3 View Fig View Fig , 5A View Fig , 6 View Fig )
LENGTH RANGE. 4.8–5.9 mm.
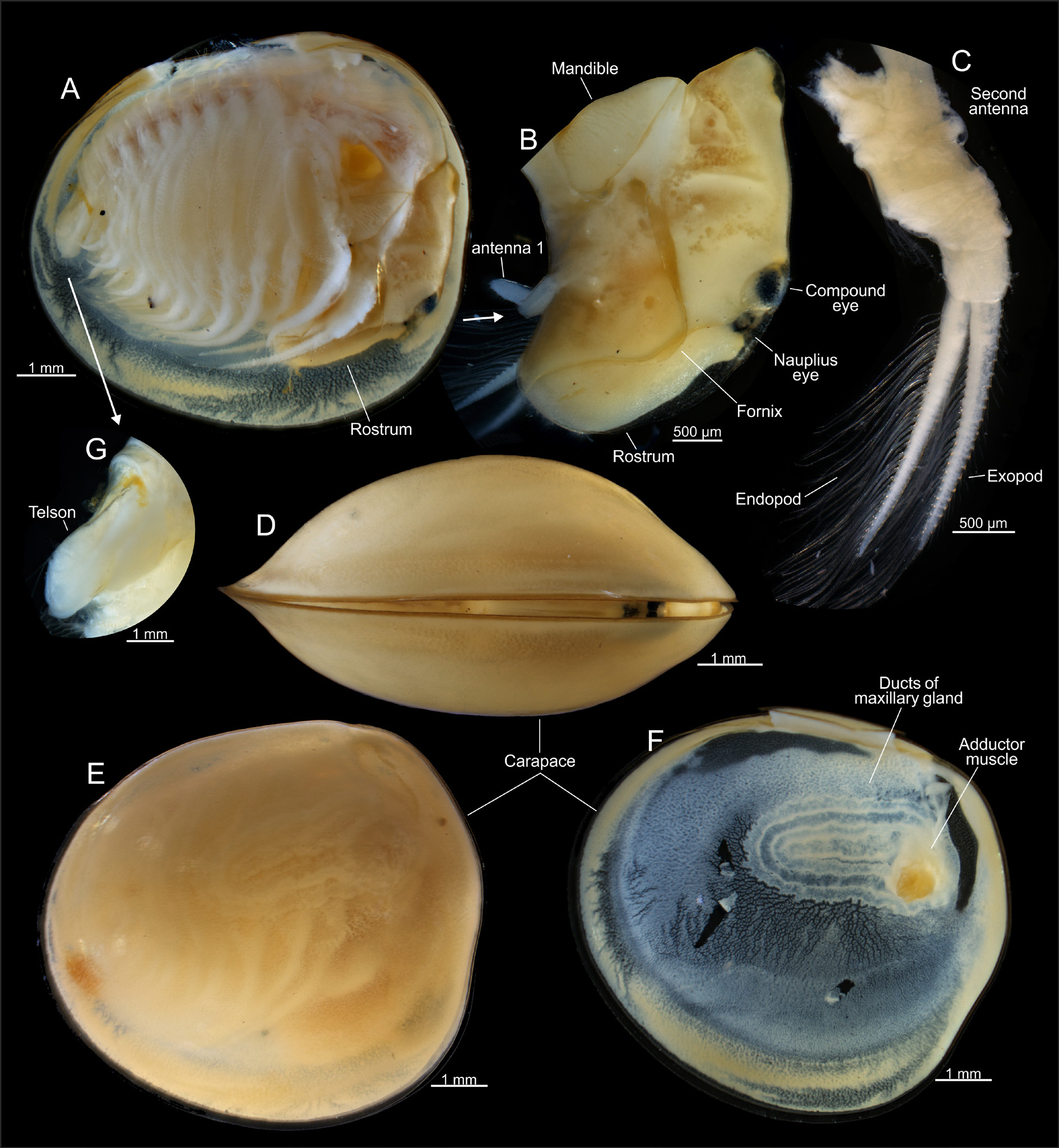
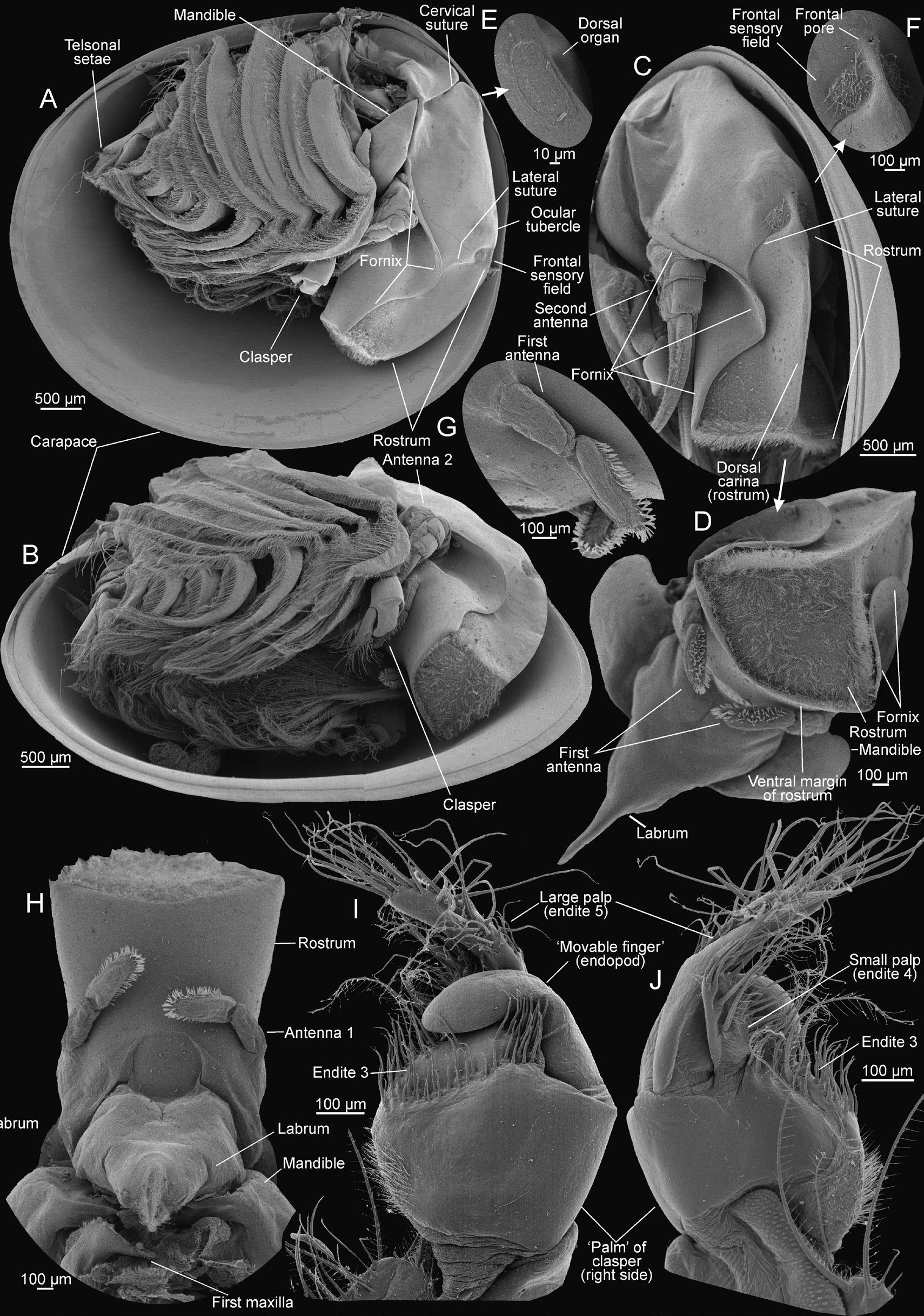
HEAD. 0.75 to 0.80 of body length. Occipital condyle rounded, longitudinal. Fornices broad posteriorly, rounded above second antennae, folded anteriorly over sides of rostrum base. Fornices project anteriorly as sharp ridges on each side of rostral constriction and extend to ends of rostral carina bifurcation. Ocular tubercle somewhat prominent, with shallow concavity posterolaterally between tubercle and occipital condyle. Frontal setal fields subcircular, separated by rostral carina, about ¾ size of compound eye. Dorsal organ narrowly oval, elongate. Rostrum constricted basally, bearing pronounced medial carina. Greatest rostral width 0.8 times rostral length. Rostral carina simple, projecting and narrow along margin. Rostral carina bifurcated distally, with each branch continuing to fornices. Rostrum distad of carina bifurcation bent nearly 90° posteriorly. Carina bifurcations and rostral anterior surface to apex densely setose. Rostrum apex subequal in width to distance between compound eyes and rostral constriction. Apex margin projecting slightly, lacking setae and arcing between fornix margins.
FIRST ANTENNA.With two antennomeres. Proximal antennomere cylindrical, twice as long as broad. Distal antennomere cylindrical, 5 times as long as broad, with apex rounded and bearing numerous short sensory setae (olfactory papillae sensu Martin et al. 1986) in a fringe around anterior surface.
SECOND ANTENNA. Peduncle with proximal coxa with transverse row of 10–12 plumose setae directed anteriorly. Peduncle basis with three or four short, acute setae between endopod and exopod (flagellae). Exopod (anterior flagellum) with 26 flagellomeres, each bearing a dorsolateral, pectinate, natatory seta bearing numerous setulae. Endopod (posterior flagellum) with 38 flagellomeres, with lateral, pectinate natatory seta bearing numerous setulae.
LABRUM. Large, smooth, apically tapering to elongated spine. Labrum apex with ventral surface bearing fine setae. Mandible broadly spatulate, molar surface with 18 transverse ridges becoming larger in size posteriorly. Posteriormost three ridges more broadly spaced than other ridges, with penultimate ridge separated from previous ridge by its basal width and projecting as two spines, posteriormost ridge separated by twice its basal width and prolonged into a single spine; semicircularly arranged row of 7 bent spines present anterior to anteriormost small ridge. Possible paragnaths posterior to mandibles: lobiform, with dense setae.
MAXILLA I. Typical for the genus ( Martin et al. 1986; Martin & Belk 1989; Fryer & Boxshall 2009), elongate, broad-margined, distally with 10–15 stout setae, each with double pectinate row in proximal half and densely plumose in distal half. With three posterior, intermediate-length, robust setae lacking plumose portion, but with two rows of spiniform denticles, and one short robust seta terminating in 4–5 spines. Posterior margin fringed with fine setae.
MAXILLA II. Absent.
CARAPACE. 8–10 mm in length, globose, subspherical, smooth and without ornamentation, subtriangular in lateral outline, with anterior margin being less arcuate than other margins. Maxillary gland ducts arranged transversely, posterior to adductor muscle scar.
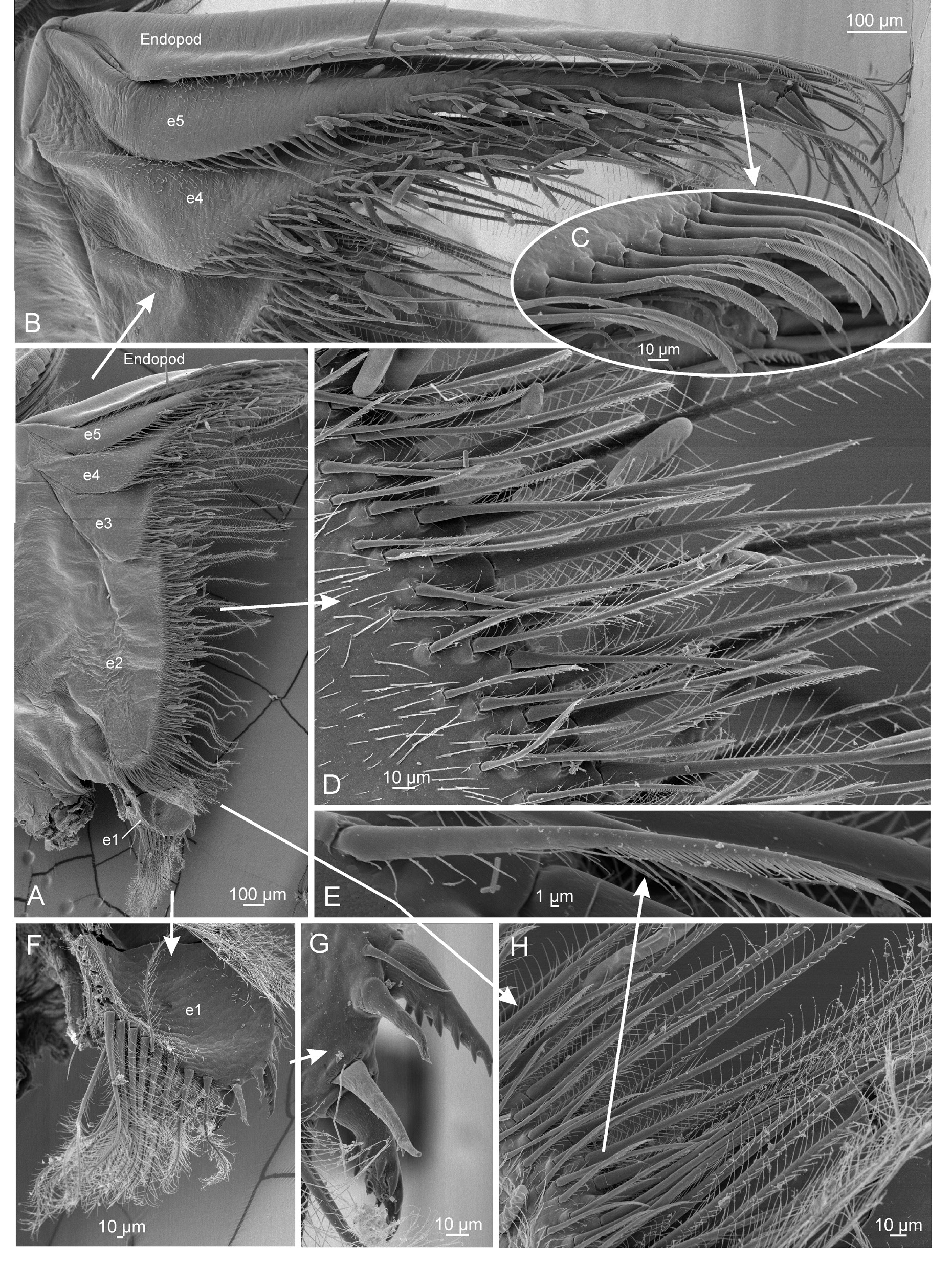
THORACOPODS. 10 pairs, first pair modified as claspers. Right and left claspers equal in size and shape, of typical Lynceus form (clasper terminology follows Sigvardt & Olesen 2014 and Kaji et al. 2014). Endite 1 of clasper limb typical for genus, lobiform, margined with short setae. Endite 2 broadly transverse, margined with several stout, long (~100–700 μm) setae, each margined with sparse setules separated by at least half their length. Endite 3 broad, oval, laterally compressed, with proximal anterior margin bearing patch of fine plumose setae. Median margin or “palm” region (“gripping area”) of clasper with slender, relatively short setae (~100 μm) bearing sparse lateral setulae (type 3 or 4 setae of Sigvardt & Olesen 2014), positioned in semicircular arrangement leaving glabrous area under endopod apex. Anterior margin of glabrous area partially obscured by endopod, with short row of 4 cylindrical peglike spines (type 2 setae of Sigvardt & Olesen 2014). Endite 4 (small palp) lobiform proximally, with dorsal branch bearing five long (~100–400 μm) setae, each margined with sparse long setules along anterior margin to apex, and additional ~20 setae of varying length along posterior margin. Endite 5 (large palp) 50% longer than endite 4, subcylindrical, straight in proximal half, with medial bend, straight in distal half. Posterior margin of distal half with row of long (~100–400 μm) setae, each margined with sparse setules separated by at least half their length. Setae continue to dorsal surface of rounded apex. Endopod (movable finger) stout, straight in proximal half, with distal half abruptly decurved, tapering to subacute apex. Endopod apex extending ¼ to ½ of endite 3 “palmar” region (“gripping area”). Remaining thoracopods and body typical for the genus ( Martin et al. 1986; Martin & Belk 1989). Remaining nine thoracopod pairs in general serially similar, becoming gradually smaller posteriorly (significant differences between limbs in the series mentioned below). General aspects of setation of anterior thoracopods are as follows. Margin of endites 1–5 with two rows of setae, margined on distal and proximal sides with smaller setulae. Marginal setae encompass a posterior row of elongate plumose setae, a row of shorter pectinate scraping setae and fine, short, scattered setae. Posterior row of plumose setae three or four times longer than anterior pectinate row. Endite 1 oval, with two to four large pectinate spines at apex. Endite 2 broadly transverse. Endite 3 transverse and lobiform on distolateral margin. Endite 4 projecting, elongate, digitiform. Endite 5 elongate, digitiform, straight. Endopod elongate, digitiform, straight, with stout setae along dorsal margin and apically, similar in form to setae of endite 5. Endites 4–5 and distal margin of endopod with scattered fine, filiform setae, with pectinate scraping setae present apically. Exopod distally elongate, digitiform and apically acute. Exopod proximolaterally broadly oval. Epipod projecting dorsally, truncated. Significant variation in limb components between thoracopods from anterior to posterior as follows. One epipod per appendage present in thoracopods 1 through 7, becoming gradually longer posteriorly, reaching maximum length in thoracopod 4, after which they become smaller until thoracopod 7; epipod absent in thoracopods 8–10. Proximolateral part of exopod broadly oval in thoracopods 2 through 4, from thoracopod 5 gradually more narrow and curved distally; thoracopod 10 lacks a proximolateral part. Endites 4–5 and endopod project significantly, digitiform in thoracopods 2 through 6, from thoracopod 7 gradually shorter and more lobate posteriad.
TELSON. Broad, smooth, lacking denticles. Telson terminating in a pair of triangular setule-covered protrusions. Dorsoposterior angles with a posterior, transverse fringe of setae. Posterior surface of telson pilose, with pelage short and posteriorly directed. Telsonal setae each born on low conical mound. Setal base set in shallow, circular recesses. Telsonal setae filiform, elongate, longer than telson, slightly tumid at base.
Female ( Figs 4 View Fig , 5 View Fig B–F, 7–8)
LENGTH RANGE. 4.8–5.9 mm. Generally similar to male in appearance.
HEAD. Occipital condyle rounded, longitudinal. Fornices broad posteriorly, rounded above second antennae, folded anteriorly over sides of rostrum base. Fornices project anteriorly as sharp ridges on each side of rostral constriction, extend just short of distolateral rostral corners. Distal apices of fornix rounded. Ocular tubercle less pronounced than in male. Setal fields subcircular, separated by rostral carina, about ¾ size of compound eye. Dorsal organ as in male. Rostrum constricted basally, bearing pronounced medial carina. Greatest width of rostrum 0.8 times rostral length. Rostral carina simple, projecting, narrow along margin. Rostral carina not bifurcated distally. Apex margin rounded, projecting as a ridge, lacking setae.
ANTENNAE AND MOUTHPARTS. As in males.
CARAPACE. 8–10 mm in length, globose, subspherical, smooth, without ornamentation. Anterior margin nearly straight. Maxillary gland ducts as in male.
THORACOPODS. Twelve pairs, claspers absent. General shape of many thoracopods and setal patterns of endites, endopod and exopod as in male. Epipod becoming gradually longer from thoracopod 1 through 4, in thoracopod 4 of maximum size, from thoracopod 5 through 7 decreasing in size, absent on thoracopods 8 through 12. Exopod increases in size posteriad from thoracopod 1 until thoracopod 3 or 4, after which it becomes smaller, entirely absent in thoracopods 11–12. Proximolateral part of exopod broadly oval in thoracopods 1–2, from thoracopod 3 gradually more narrow and curved distally; proximolateral part of exopod in throacopods 9–10 distinctly modified into curved lobe with distal setation to which egg clusters are attached.
LAMINA ABDOMINALIS. Present, broad, directed laterally, bearing three marginal extensions and two dorsal extensions. Anterior marginal extension sinuate, longer than other marginal extensions by one fourth. Medial marginal extension tapering, nearly straight. Posterior marginal extension broadly triangular, with posterior margin arcing underneath lamina abdominalis. Dorsal extensions of lamina abdominalis directed anteriorly. Anterior dorsal extension sinuate, posterior dorsal extension digitiform, roughly ¾ length of anterior extension. Eggs held between lamina abdominalis and exopods, attached to distal setae of modified exopods of thoracopods 9–10.
TELSON. As in male.
Type locality
Temporary body of water (doline or sinkhole DOL-03; see Table 1 View Table 1 , Fig. 1 View Fig ) with a perimeter at about 290 m, a surface area of about 3800 m ² and a maximum depth at about 1.2 to 2.6 m. Melaleuca quinquenervia (Cav.) S.T. Blake trees grow both along the margin and in the deeper parts of the sink hole, and there are some scattered spots of Eleocharis spiralis (Rottb.) Roem. and Schult. and Lepironia articulata (Retz.) (both Cyperaceae ); the muddy bottom is covered with smaller, submersed macrophytes like the New Caledonian endemic Eriocaulon neocaledonicum Schltr. Lynceus insularis sp. nov. occurs among vegetation or is free swimming.
Habitat
All sites where this species occurs are dolines (sinkholes) in Pliocene/Quarternary laterite deposits ( Lillie & Brothers 1970) ( Fig. 1 View Fig ). All localities have Cyperaceae growing on the bottom, sometimes being more than 2 m in length. The soil of the region is nutrient poor but rich in heavy metals (Mg, Fe, Cr, Co and Ni) and is ultrabasic.
Distributional range
This species is only known from New Caledonia. To date it is known from five sites, all in the Mont- Dore and Yaté communities of Southern Province. The species was first recognized in April 2000 (DOL- 03). However, since it was not found in 2004, a larger survey of 17 additional, similar localities was undertaken in 2009, of which 5 had either empty carapaces or living Lynceus specimens. All localities are within a distance of 2 km from each other (in mining area), except one (DOL-16) which is situated approximately 9 km northeast of DOL-03 (the type locality).
Activity Period
The entire life cycle takes 3–4 months (from February to May/June). Larvae usually hatch in February, which is in the middle of the rainy season (from December to April). February is the warmest month, with average water temperatures ranging from 24–26°C. The highest population density and largest
mating intensity was observed at the end of April. From about June, Lynceus is absent from the ponds even if water still remains. The ponds are dry from about September to November.
IUCN Red List status
Lynceus insularis sp. nov. currently meets the red list definition ( IUCN 2001) as a critically endangered species, with its distribution area being only about 1.7 ha. Furthermore, the distribution area of L. insularis sp. nov. is severely fragmented into small subpopulations, each of which shows extreme fluctuations in population size ( IUCN Red List Criteria B2a, c). The probability of extinction is estimated to be at least 50% within 50 years due to a high risk of degradation of the biotope of the species which might result as a cumulative effect of a number of factors: hydrological changes, reduction of water supply, acidification of fresh water, invasive species ( Cervus timorensis rusa Müller & Schlegel, 1845 ) and mining activities in the vicinity (less than 1 km).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |