Sylvicanthon bridarollii (Martínez, 1949)
|
publication ID |
https://doi.org/ 10.5852/ejt.2018.467 |
|
publication LSID |
lsid:zoobank.org:pub:8D27AAB8-B7F2-424C-B1A6-66FEFA66EDFF |
|
DOI |
https://doi.org/10.5281/zenodo.3846317 |
|
persistent identifier |
https://treatment.plazi.org/id/A72C87FB-FFFE-FFC7-0D38-0F6B0FBC91CD |
|
treatment provided by |
Valdenar |
|
scientific name |
Sylvicanthon bridarollii (Martínez, 1949) |
| status |
|
Sylvicanthon bridarollii (Martínez, 1949) View in CoL
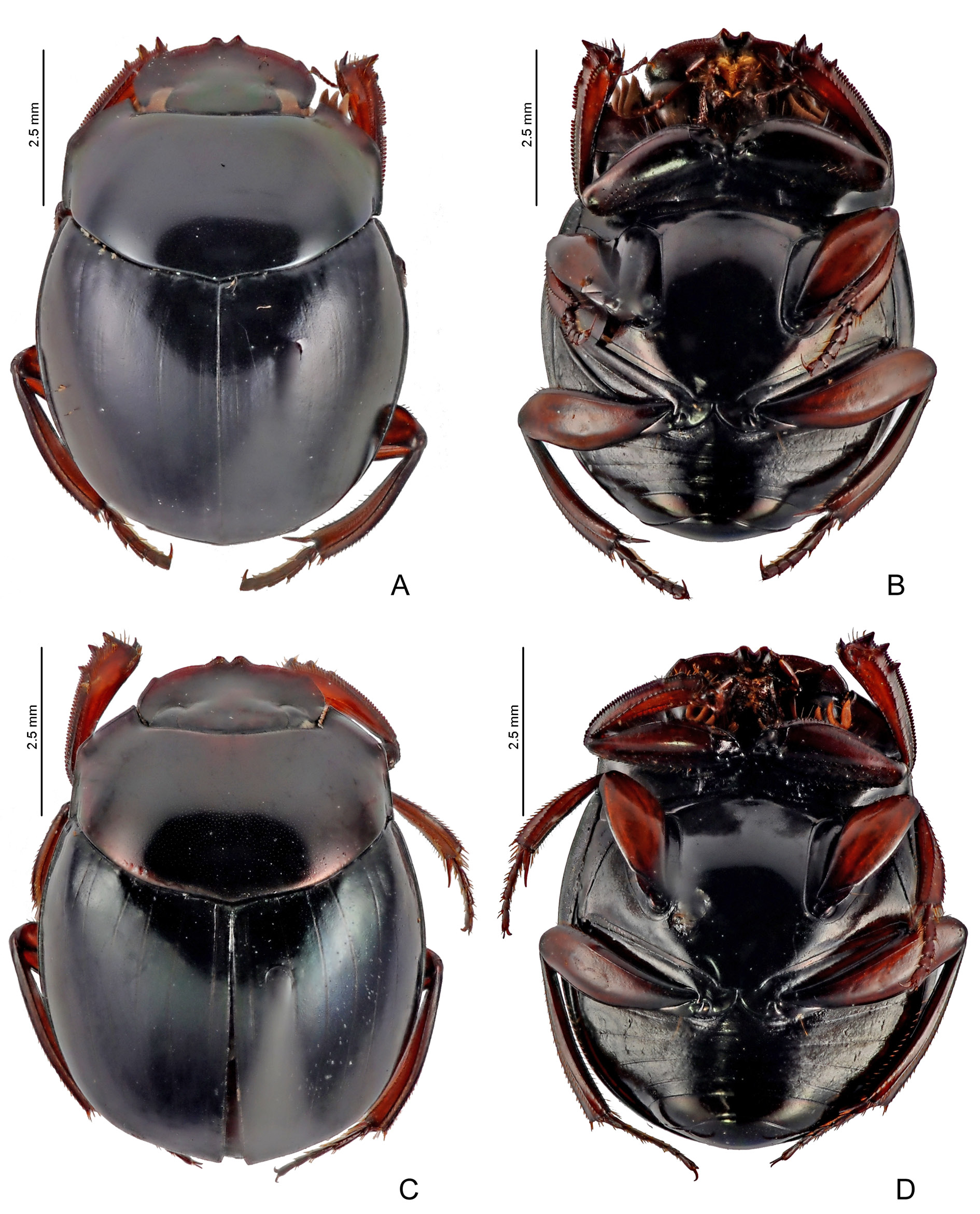
Figs 8A View Fig , 9A View Fig , 11 View Fig F–G, 13G–H, 15I, 18A, 20, 32–34, 35C–D, 36
Glaphyrocanthon bridarollii Martínez, 1949a: 282–287 View in CoL , 290.
Glaphyrocanthon bridarollii View in CoL – Halffter & Martínez 1977: 63.
Glaphyrocanthon (Glaphyrocanthon) bridarollii View in CoL – Martínez 1950: 170–171. — Pereira & Martínez 1956: 126, 128. — Martínez et al. 1964: 5, 8, 10, 14. — Vulcano & Pereira 1964: 661; 1967: 561. — Martínez & Pereira 1967: 53.
Sylvicanthon bridarollii View in CoL — Halffter & Martínez 1977: 63. — Amézquita et al. 1999: 119–120. — Medina & Lopera-Toro 2000: 312, fig. 9d, h. — Vaz-de-Mello 2000: 195. — Escobar 2000a: 210; 2000b: 121. — Medina et al. 2001: 137; 2003: 44, fig. 100; 2013: 464–466, 469, 471, figs 89, 117, 131, 240. — Carpio et al. 2004: 464, 469. — Celi et al. 2004: 46. — Larsen 2004: 261. — Horgan 2006: 364. — Medina & Pulido 2009: 59. — Carvajal et al. 2011: 117, 316. — Price & Feer 2012: 327 (error: referring to S. seag View in CoL sp. nov.). — Ratcliffe et al. 2015: 196. — Tarasov & Génier 2015: 21–24, 54, figs 4–7, 29g.
Silvicanthon bridarollii [sic] – Horgan 2005a: 609–610; 2005b: 131; 2009: 3532, 3537. — Chamorro et al. 2018: 98.
Sylvicanthon bridarolli [sic] – Figueroa & Alvarado 2011: 210–211, fig. 1b. — Chamorro et al. 2018: 86, fig. 9D. — Espinoza & Noriega 2018: 146, 149.
Sylvicanthon View in CoL sp. – Kirk 1992: 54 (tentative).
Canthon bridarollii – Krajcik 2012: 63.
Etymology
Eponym refers to the Argentinian naturalist and Jesuit priest Albino J. Bridarolli (1903–1949) (Martínez 1949).
Material examined
Holotype
BOLIVIA: ♂, Cochabamba, Chapare, Coni River, 400 m (“ BOLIVIA / Dep. Cochabamba / Chapare - 400mts. / R. Zischka - leg. / Coll. Martínez ”, “ BOLIVIA / Chapare / 400 M / Zischka”, “ HOLOTIPO Ƌ ”, “ Glaphyrocanthon / bridarollii / ♂ / sp. n. / A. Martínez det. 19 49 ”, “ FICHADO ”, “MACN-En / 937”), genital capsule removed and glued to a triangular card point ( MACN) ( Fig. 33A View Fig ).
Paratypes (3♂♂ and 4 ♀♀ examined)
Two paratypes, male and female, could not be located (they were possibly deposited at the Zischka collection, now housed at the Zoologische Staatssammlung München, Munich, Germany; see comments below).
BOLIVIA: 1 ♀ (allotype) (“CHAPARE / BOLIVIA / ZISCHKA col. / Coll. Martínez / 24-V-48 ”, “ ALOTIPO ♀ ”, “ Glaphyrocanthon / bridarollii / ♀ / sp. n. / A. Martínez det. 19 49 ”, “ FICHADO ”, “MACN-En / 938”) ( MACN) ( Fig. 33B View Fig ); 1 ♂ (dissected) (“ BOLIVIA / Dep. Cochabamba / Chapare 400mts. / R. Zischka-leg. / Coll. Martínez ”, “ Bolivia / Region subandina / Prov. Chapare – 400m / ex coll Zischka”, “ Glaphyrocanthon / bridarollii ♂ / sp. n. / A. Martínez-det 19 49 ”, “ PARATYPE ”, “ PARATIPO ♂”, “H. & A. Howden / Collection / ex. A. Martínez coll.”, “Canadian Museum of / Musée canadien de la / NATURE / CMNEN 00012714”) ( CMNC); 1 ♂ (“ BOLIVIA / Dep. Cochabamba / Chapare 400mts. / R. Zischka-leg. / Coll. Martínez ”, “ Glaphyrocanthon / bridarollii ♂ / sp. n. / A. Martínez-det 19 49 ”, “ PARATIPO ♂”, “ PARATYPE ”, “H. & A. Howden / Collection / ex. A. Martínez coll.”, “Canadian Museum of / Musée canadien de la / NATURE / CMNEN 00019064”) ( CMNC), 1 ♀ (“ BOLIVIA / Dep. Cochabamba / Chapare 400mts. / R. Zischka-leg. / Coll. Martínez ”, “ Glaphyrocanthon / bridarollii ♀ / sp. n. / A. Martínez-det 19 49 ”, “ PARATYPE ”, “ PARATIPO ♀ ”, “H. & A. Howden / collection / ex. A. Martínez coll.”, “Canadian Museum of / Musée canadien de la / NATURE / CMNEN 00019065”) ( CMNC); 1 ♂ (dissected) (“♂”, “ BOLÍVIA / tropica / Region CHAPARE / (400 Mtr.) / DIRINGS”, “ BOLIVIA / Dep. Cochabamba / Chapare 400mts. / R. Zischka-leg. / Coll. Martínez ”, “ Glaphyrocanthon / bridarollii ♂ / sp. n. / A. Martínez-det 19 49 ”, “ PARATIPO ♂”) ( MZSP); 1 ♀ (“ ♀ ”, “ BOLIVIA / tropica / Region CHAPARÉ / (400 Mtr.) / DIRINGS”, “ BOLIVIA / Dep. Cochabamba / Chapare 400 mts. / R. Zischka-legit. / Coll. Martínez ”, “ PARATIPO ♀ ”, “ Glaphyrocanthon / bridarollii ♀ / sp. n. / A. Martínezdet. 19 49 ”) ( MZSP); 1 ♀ (“ ♀ ”, “ BOLIVIA / tropica / Region CHAPARÉ / (400 Mtr.) / DIRINGS”, “ BOLIVIA / Dep. Cochabamba / Chapare 400 mts. / R. Zischka-legit. / Coll. Martínez ”, “ PARATIPO ♀ ”, “ Glaphyrocanthon / bridarollii ♀ / sp. n. / A. Martínez-det. 19 49 ”) ( MZSP).
Additional material (382 ♂♂, 256 ♀♀)
BOLIVIA: 1 ♀, eastern Bolivia (“Ost Bolivien”), Steinbach S.V. leg. ( ZMHB, labelled as syntype of Canthon obscurus by the ZMHB staff, but, very likely, a pseudotype). – Beni: 3 ♂♂, 4 ♀♀, Mamoré, San Ramón, Estancia San Lorenzo, 13º25′36″ S, 64º26′06″ W, 140 m, 11 Oct. 2003, pitfall with human faeces, A.C. Hamel and K. Walker leg. ( OUMNH); 2 ♂♂, 1 ♀, Mamoré, San Ramón, Estancia San Lorenzo, 13º25′36″ S, 64º26′06″ W, 140 m, 12 Oct. 2003, pitfall with human faeces, A.C. Hamel and K. Walker leg. ( OUMNH); 2 ♂♂, 3 ♀♀, Mamoré, San Ramón, Estancia San Lorenzo, 13º25′36″ S, 64º26′06″ W, 140 m, 13 Oct. 2003, pitfall with human faeces, A.C. Hamel and K. Walker leg. ( OUMNH). – Cochabamba: 2 ♂♂, 1 ♀, Cercado, 124 km E of Cochabamba, Río Espírito Santo, 17º03′45″ S, 65º38′38″ W, 700 m, 6–8 Feb. 1999, dung trap, F. Génier leg. ( CMNC); 2 ♂♂, 1 ♀, Chapare, Villa Tunari, Oct. 1992, Arnagada(?) leg. ( CMNC); 21 ♂♂ (1 dissected), 10 ♀♀, Estación Biológica Villa Carmen, Universidad San Simon, 67.5 km E of Villa Tunari, 17º06′19″ S, 64º46′57″ W, 300 m, 7–9 Feb. 1999, F. Génier leg. ( CMNC); 14 ♂♂ (1 dissected), 7 ♀♀, Estación Biológica Villa Carmen, Universidad San Simon, 67.5 km E of Villa Tunari, 17º06′19″ S, 64º46′57″ W, 300 m, 9–13 Feb. 1999, F. Génier leg. ( CMNC); 3 ♂♂, 4 ♀♀, José Carrasco, Chimoré, 250 m, Jan. 1972, A. Martínez leg. ( CMNC); 3 ♀♀, “PD Altamachi”, 16º02′ S, 66º40′ W, 1150 m, 25 Sep. 2004, trap with human faeces, A.C. Hamel leg. ( OUMNH); 1 ♀, “PD Altamachi”, 16º02′ S, 66º40′ W, 1150 m, 27 Sep. 2004, trap with human faeces, A.C. Hamel leg. ( OUMNH). – La Paz: 1 ♂, 1 ♀, Larecaja, Guanay, San José, 17 Oct. 2001, G. Castillo leg. ( CEMT); 1 ♀, Larecaja, Guanay, Uyapi, 15 Oct. 1995, G. Arriágada leg. ( CEMT); 1 ♂, 2 ♀♀, Larecaja, Guanay, 10 Nov. 2004, A. U-Peña leg. ( CEMT); 1 ♀, Nor Yungas, Coroico ( MZSP); 1 ♀, Parque Nacional Madidi, 13º38′ S, 68º44′ W, 260 m, 26 Jul. 2004, trap with human faeces, C. Hamel leg. ( OUMNH); 2 ♂♂ (1 dissected), 7 ♀♀, Parque Nacional Madidi, 13º38′ S, 68º44′ W, 260 m, 27 Jul. 2004, trap with human faeces, C. Hamel leg. ( OUMNH). – Santa Cruz: 1 ♀, Andrés Ibáñez, El Espejo(?), Feb. 1961, Martínez leg. ( CMNC); 2 ♀♀, Andrés Ibáñez, Santa Cruz de la Sierra, Jardín Botánico, 29 Sep. 2006, T. Vidaurre, M. Amaya and G. Mollos leg. ( CEMT); 4 ♂♂, Andrés Ibáñez, Santa Cruz de la Sierra, Jardín Botánico, 17º47′02″ S, 63º03′47″ W, 400 m, W.D. Edmonds leg. ( TAMU); 2 ♂♂, 2 ♀♀, Andrés Ibáñez, Santa Cruz de la Sierra, Jardín Botánico, 17º46′00″ S, 63º04′13″ W, 420 m, 5–6 Nov. 2006, pitfall with human faeces, Mann and Hamel leg. ( OUMNH); 3 ♂♂ (1 dissected), 2 ♀♀, Andrés Ibáñez, Santa Cruz de la Sierra, Jardín Botánico, 17º46′00″ S, 63º04′13″ W, 420 m, 7–8 Nov. 2006, pitfall with human faeces, Mann and Hamel leg. ( OUMNH); 3 ♂♂, Andrés Ibáñez, Santa Cruz de la Sierra, Jardín Botánico, 17º46′00″ S, 63º04′13″ W, 420 m, 8–9 Nov. 2006, pitfall with human faeces, Mann and Hamel leg. ( OUMNH); 4 ♀♀, Andrés Ibañez, Santa Cruz de la Sierra, Jardín Botánico, “8.5 Km Carretera a Cotoca”, 17º45′51.3″ S, 63º39′30.8″ W, 10–12 Nov. 2006, Scarabnet leg. ( CEMT); 1 ♀, Ichilo, Buena Vista (“4–6k SSE Buena Vista”), Hotel Fauna and Flora, 420–450 m, 2–12 Feb. 2000, pitfall with dung/ carrion, J.E. Wappes leg. ( TAMU); 1 ♀, Ichilo, Buena Vista (“ 3.7 km SSE Buena Vista”), Hotel Fauna and Flora, 17º29′ S, 63º33′ W, 29Apr.–6 May 2004, flight interception trap, A.R. Cline leg. ( CMNC); 3 ♀♀, Obispo Santistevan, General Saavedra, “CIMCA”(?), 9 Sep. 1988, C.J. Pruetti leg. ( CMNC); 1 ♀, San Pedro(?), 12 Sep. 1997, C. J. Pruetti leg. ( CMNC).
BRAZIL: Acre: 8 ♂♂, 4 ♀♀, Manoel Urbano, Parque Estadual Chandless, 09º22′26″ S, 63º55′20″ W, 24 Jun. 2013, T.F. Brito leg. ( CEMT); 1 ♂, 1 ♀, Manoel Urbano, Parque Estadual Chandless, 09º22′26″ S, 63º55′20″ W, 1 Jul. 2013, T.F. Brito leg. ( CEMT); 1 ♀, Rio Branco, Jul. 1996, M. Castro leg. ( CEMT); 1 ♂, 1 ♀, Senador Guiomard, 67º37′ W, 10º04′ S, 14 Apr. 2017, pitfall W ith human faeces, Bruna S. Bittencourt leg. ( CEMT); 1 ♂, 2 ♀♀, Tarauacá, Nov. 1956, W erner leg. ( MZSP); 2 ♂♂, 2 ♀♀, Tarauacá, Dec. 1956, Dirings leg. ( MZSP);5 ♂♂, 3 ♀♀ (1 dissected), Xapuri, Reserva Chico Mendes, 500 m, 19 Oct. 2008, Rafael Andrade leg. ( CEMT); 1 ♂, Xapuri, Reserva Chico Mendes, 10º17.607′ S, 68º41.638′ W, 500 m, 20 Oct. 2008, pitfall W ith human faeces, J. Silveira leg. ( CEMT). – Rondônia: 1 ♂, 1 ♀, Cacoal, Loteamento Pichek, 11º26′26″ S, 61º25′22″ W, 228 m, 14 Jan. 2017, pitfall W ith human faeces, R. Silva leg. ( CEMT); 13 ♂♂, 6 ♀♀, Colorado do Oeste, “Laticínio”, 13º07′05.49″ S, 60º33′28.04″ W, 16–18 Dec. 2016, pitfall W ith human faeces, C.B.S. Souza leg. ( CEMT); 6 ♂♂, 3 ♀♀, Colorado do Oeste, “Laticínio”, 13º07′05.49″ S, 60º33′28.04″ W, 20–22 Feb. 2017, pitfall W ith human faeces, C.B.S. Souza leg. ( CEMT); 2 ♂♂, Porto Velho, Abunã, 09º36′38″ S, 65º21′33″ W, 200 m, 19 Sep. 2010, pitfall W ith human faeces, J.C.F. Falcão leg. ( CEMT); 1 ♂, 1 ♀, Porto Velho, ESEC Cuniã, 08º04′11.82″ S, 63º28′34.64″ W, 83 m, 10–12 Nov. 2013, pitfall W ith human faeces, M.A.P.A. Silveira leg. ( CEMT); 2 ♀♀, Porto Velho, Nova Mutum-Paraná [“Mutum”], 09º35′46″ S, 65º02′27″ W, Jan. 2012, R.V. Nunes leg. ( CEMT); 1 ♂, Porto Velho, Nova Mutum-Paraná [“Mutum”], 09º35′46″ S, 65º02′27″ W, 200 m, Sep. 2012, pitfall with human faeces, R.V. Nunes leg. ( CEMT); 1 ♀, Rolim de Moura, 11º44′04.87″ S, 61º55′08.64″ W, 214 m, 27–29 Jul. 2015, pitfall with human faeces, D.C. Castro leg. ( CEMT); 1 ♂, 2 ♀♀, Rolim de Moura, 11º44′04.93″ S, 61º55′09.19″ W, 215 m, 8–10 Dec. 2015, pitfall with human faeces, D.C. Castro leg. ( CEMT); 4 ♂♂, Rolim de Moura, 11º44′4.96″ S, 61º55′10.4″ W, 217 m, 8–10 Dec. 2015, pitfall with human faeces, D.C. Castro leg. ( CEMT); 2 ♂♂, Rolim de Moura, 11º44′03.77″ S, 61º55′09.74″ W, 218 m, 27–29 Jul. 2015, pitfall with human faeces, D.C. Castro leg. ( CEMT); 1 ♂, 1 ♀, Rolim de Moura, 11º44′03.77″ S, 61º55′09.74″ W, 218 m, 8–10 Dec. 2015, pitfall with human faeces, D.C. Castro leg. ( CEMT); 1 ♂, Rolim de Moura, 11º44′05″ S, 61º55′11.62″ W, 219 m, 27–29 Jul. 2015, pitfall with human faeces, D.C. Castro leg. ( CEMT); 2 ♂♂, 3 ♀♀, Rolim de Moura, 11º44′04.42″ S, 61º55′11.59″ W, 220 m, 8–10 Dec. 2015, pitfall with human faeces, D.C. Castro leg. ( CEMT); 1 ♂, 1 ♀, Rolim de Moura, 11º44′05.02″ S, 61º55′12.19″ W, 220 m, 27–29 Jul. 2015, pitfall with human faeces, D.C. Castro leg. ( CEMT); 4 ♂♂, 1 ♀, Rolim de Moura, 11º44′05.04″ S, 61º55′12.78″ W, 221 m, 8–10 Dec. 2015, pitfall with human faeces, D.C. Castro leg. ( CEMT); 5 ♂♂, 1 ♀, Rolim de Moura, 11º44′04.48″ S, 61º55′12.75″ W, 222 m, 8–10 Dec. 2015, pitfall with human faeces, D.C. Castro leg. ( CEMT); 6 ♂♂, 3 ♀♀, Rolim de Moura, 11º43′43.42″ S, 61º53′32.13″ W, 255 m, 7–9 Dec. 2015, pitfall with human faeces, D.C. Castro leg. ( CEMT); 3 ♂♂, 3 ♀♀, Rolim de Moura, 11º43′43.42″ S, 61º53′32.13″ W, 255 m, 27–29 Jul. 2015, pitfall with human faeces, D.C. Castro leg. ( CEMT); 2 ♀♀, Rolim de Moura, 11º44′25.01″ S, 61º55′24.14″ W, 272 m, 27–29 Jul. 2015, pitfall with human faeces, D.C. Castro leg. ( CEMT); 3 ♂♂, 3 ♀♀, São Francisco do Guaporé, Bacabalzinho, REBIO Guaporé, Campo do Murundu, 12º31′ S, 63º26′ W, 2 Oct. 2013, S.E. Silva leg. ( CEMT).
COLOMBIA: Caquetá: 1 ♂ (dissected), Parque Nacional Natural Sierra de Chiribiquete, 300 m, pitfall with human faeces, Feb. 2000, J. Noriega leg. ( CPJN).
ECUADOR: Morona Santiago: 3 ♂♂ (1 dissected), Untsuants, Sítio 1, 700 m, 8 Dec. 2001, J. Celi and J. Torres leg. ( CMNC); 1 ♂, Untsuante, Sítio 3, 700 m, 19 Jan. 2002, pitfall with human faeces, J. Celi and M. Ortega leg. ( CMNC); 4 ♂♂, Untsuante, Sítio 5, 600 m, 22 Jan. 2002, J. Celi and M. Ortega leg. ( CMNC); 3 ♂♂ (1 dissected), 4 ♀♀, Untsuante, Sítio 6, 600 m, 22 Jan. 2002, pitfall with human faeces, J. Celi and M. Ortega leg. ( CMNC). – Napo: 1 ♂, Jatun Sacha Biological Station, 21 km E of Puerto Napo, 400 m, 8 Jul. 1994, dung trap, F. Génier leg. ( CMNC); 2 ♂♂, 1 ♀, Puerto Misahualli, Jungle Hotel, 8–20 Sep. 1997, D.G. Marqua leg. ( TAMU); 3 ♂♂, 7 ♀♀, Tena, 400 m, 15–21 Feb. 1986, human faeces trap, François Génier leg. ( CMNC); 11 ♂♂ (1 dissected), 8 ♀♀, Tena (“ 5 km W Tena”), 500 m, 6–9 Jul. 1976, dung trap, S. Peck leg. ( CMNC); 3 ♂♂, 1 ♀, Tena (“ 20 km S Tena”), 600 m, 9–11 Jul. 1976, S. Peck leg. ( CMNC). – Orellana: 2 ♂♂ (1 dissected), 1 ♀, Tiputini Biodiversity Station, 0º38′ S, 76º09′ W, 220 m, Sep. 2000, carrion trap, D. Inward leg. ( BMNH). – Sucumbíos: 1 ♂ (dissected), Shushufindi, Reserva Biológica Limoncocha (“Limoncocha”), 0º28′ S, 76º36′ W, 300 m, 31 Mar. 1974, H.P. Stockwell leg. ( CMNC); 3 ♂♂, 2 ♀♀, Shushufindi, “Limoncocha”, 10–15 Mar. 1975, J.M. Campbell leg. ( CMNC); 3 ♂♂, 5 ♀♀, Shushufindi, “Limoncocha”, 250 m, 18–24 Jun. 1976, dung trap, S. Peck leg. ( CMNC).
PERU: 1 ♂, “Peru mont. / O. Thieme V.” ( ZMHB, labelled as Syntype of Canthon obscurus by the ZMHB Staff, but, very likely, a pseudotype). – Amazonas: 1 ♀, Rodríguez de Mendoza, Quebrada Huancabamba, 06º35′30″ S, 77º33.2′ W, 2360 m (“ 2100m ”), 14 Jun.–23 Jul. 2010, D. Chunga leg. ( MUSM). – Cuzco: 1 ♀,La Convención, Echarate, Centro Poblado Tunquio, 12º15′44.30″ S, 72º52′37.08″ W, 960 m, 26 Sep.–1 Oct. 2010, C. Carranza and S. Cavero leg. ( MUSM); 1 ♂, 1 ♀, La Convención, Echarate, Comunidad Tupac Amaru, 12º06′49.78″ S, 72º49′35.47″ W, 371 m, 22 Oct. 2009, M. Alvarado and E. Rázuri leg. ( MUSM); 1 ♂, La Convención, Echarate, Comunidad Tupac Amaru, 11º56′52.58″ S, 72º54′50.06″ W, 536 m, 17–19 Jan. 2010, C. Espinoza and E. Razuri leg. ( MUSM); 1 ♂, La Convención, Echarate, Campamento Segakiato, 11º45′38.6″ S, 73º14′57.7″ W, 908 m, 2 Mar. 2011, M. Alvarado and E. Rázuri leg. ( MUSM); 1 ♂, 1 ♀,La Convención, Echarate, Campamento Segakiato, 11º45′38.6″ S, 73º14′57.7″ W, 908 m, 1–4 May 2011, S. Cavero and C. Espinoza leg. ( MUSM); 1 ♂, 1 ♀, La Convención, Echarate, Quebrada Pomoreni, 65º06′09.8″ S, 77º41′51.97″ W, 488 m, 21–24 Apr. 2010, L. Figueroa and D. Chunga leg. ( MUSM). – Huánuco: 1 ♀, Estación Biológica Panguana (Forschungsstation Panguana), Rio Pachitea, Rio Yuyapichis, 09º37′ S, 74º56′ W, 260 m, 28 Aug.– 14 Sep. 1986, Listabarth leg. ( NHMW); 4 ♂♂ (1 dissected), 5 ♀♀, Leoncio Prado, Rupa-Rupa, Tingo María, Universidad Nacional Agraria de la Selva (“Tingo María Universidad”), Jul. 1974 ( CMNC); 1 ♂, 2 ♀♀, Leoncio Prado, Rupa-Rupa, Tingo María, Universidad Nacional Agraria de la Selva Dec. 1974 ( CMNC); 1 ♂, 2 ♀♀, Puerto Inca, Clayton, 09º11′53.37″ S, 74º55′12.1″ W, 243 m, 10–12 Apr. 2009, C. Carranza leg. ( MUSM); 2 ♀♀, Puerto Inca, Clayton, 09º11′53.37″ S, 74º55′12.1″ W, 243 m, 16–18 Jun. 2009, C. Carranza leg. ( MUSM); 1 ♂, 1 ♀, Puerto Inca, Tournavista, 08º56′11.75″ S, 74º43′23.3″ W, 178 m, 18–19 Jun. 2009, C. Carranza leg. ( MUSM). – Junín: 2 ♂♂, Chanchamayo, San Ramón, pitfall, F.G. Horgan leg. ( MUSM); 1 ♂,Chanchamayo, San Ramón, May 2002, human faeces pitfall, F.G. Horgan leg. ( MUSM); 1 ♂, 1 ♀, Chanchamayo, San Ramón, 8 May 2002, human faeces pitfall, F.G. Horgan leg. ( MUSM); 1 ♂, 5 ♀♀, Chanchamayo, San Ramón, 17 May 2002, human faeces pitfall, F.G. Horgan leg. ( MUSM); 1 ♂ (dissected), 2 ♀♀, Chanchamayo, San Ramón, Oct. 2002, human faeces pitfall, F.G. Horgan leg. ( MUSM); 1 ♂, Chanchamayo, San Ramón, Oct. 2002, human faeces pitfall, F.G. Horgan leg. ( MUSM); 1 ♀, Chanchamayo, San Ramón, Catarata El Tirol (“El Tirol”), 820–1000 m, Jul. 2000, dung trap, C. Torpoco leg. ( CMNC); 2 ♂♂, 2 ♀♀ (1 dissected), Satipo ( CEMT); 1 ♂, 1 ♀, Satipo, 600 m, 23 May–3 Jun. 2004, A. Santibañez leg. ( TAMU); 1 ♂, 1 ♀, Satipo, 11º14′22″ S, 74º39′37″ W, 1008 m, 28 Aug.–2 Sep. 2011, I. Medina and L. Figueroa leg. ( MUSM). – Loreto: 1 ♂, Alto Amazonas, Teniente César López Rojas, 02º35′39.6″ S, 76º06′55″ W, 230–305 m, 18–26 Jul. 1993, flight interception trap, R. Leschen leg. ( CMNC); 3 ♂♂,Reserva Nacional Pacaya Samiria, Rio Samiria, Cocha Shinguito, 26 Aug. 1991, T.L. Erwin Exp. Res. Pacaya-Samiria, G.E. Ball and D. Shpeley leg. ( CMNC); 2 ♂♂, Reserva Nacional Pacaya Samiria, Rio Samiria, Cocha Shinguito, 26–29 Aug. 1991, T.L. Erwin Exp. Res. Pacaya-Samiria, G.E. Ball and D. Shpeley leg. ( CMNC); 1 ♀, Zona Reservada Sierra del Divisor, 07º04′01″ S, 74º01′21″ W, 213 m, 16–19 Feb. 2009, C. Espinoza leg. ( MUSM); 1 ♂, 1 ♀, Zona Reservada Sierra del Divisor, 2 km from Rio Hubuya, 196 m, 13–14 Oct. 2008, C. Carranza leg. ( MUSM); 1 ♂, Reservada Sierra del Divisor, Quebrada Ubuya, 06º57′19″ S, 74º01′24″ W, 202 m, 2–3 Mar. 2009, C. Espinoza leg. ( MUSM). – Madre De Díos: 2 ♂♂, Manu, Manu, Salvación (“near Salvación”), 13º50′37″ S, 71º19′57″ W, 650 m, Nov. 1999, human faeces pitfall, T. Larsen leg. ( CMNC); 3 ♂♂ (1 dissected), 4 ♀♀, Manu, Parque Nacional del Manu (“Manu National Park”), 15– 30 Aug. 1986, A. Forsyth leg. ( CMNC); 10 ♂♂, 2 ♀♀, Manu, Parque Nacional del Manu, Estación Biológica Cocha Cashu, 11º55′ S, 77º18′ W, 380 m, 18 Aug.–5 Sep. 1986, D.C. Darling & A.B. Forsyth leg. ( MUSM); 7 ♂♂ (1 dissected), 1 ♀, Manu, Parque Nacional del Manu, Estación Biológica Cocha Cashu, 11º53′45″ S, 71º24′24″ W, 350 m, 17–19 Oct. 2000, flight interception trap, R. Brooks leg. ( CMNC); 1 ♂, Manu, Reserva Comunal Amarakaeri, 12º56′32.48″ S, 70º48′23.30″ W, 333 m, 24– 26 Oct. 2010, J. Costa and M. Vilchez leg. ( MUSM); 1 ♀, Río Patuyacu, Oculto Camp, 12º39′00″ S, 68º55′33″ W, 400 m, 25–26 Mar. 1999, human faeces pitfall, T. Larsen leg. ( MUSM); 1 ♀, Tambopata, 290 m, 21 Mar. 1987, pitfall, P. Lozada leg. ( MUSM); 1 ♂, 2 ♀♀, Tambopata, Las Piedras, fundo vivero El Bosque, 12º27′49.27″ S, 69º07′30.69″ W, 17–19 Apr. 2011, O. Huaches leg. ( MUSM); 1 ♀, Tambopata, Puerto Maldonado, Madama, 12º31′20″ S, 69º03′44″ W, 29 Mar. 2009, L. Figueroa leg. ( MUSM); 3 ♂♂, 2 ♀♀, Tambopata, Puerto Maldonado, Madama, 12º31′20″ S, 69º03′44″ W, 19–20 Jul. 2009, 182 m, M. Alvarado leg. ( MUSM); 5 ♂♂, 3 ♀♀, Tambopata, Puerto Maldonado (“ 15 km N.E. Puerto Maldonado”), Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 13 Jun. 1989, flight interception trap, Ashe and Leschen leg. ( CMNC); 1 ♂, Tambopata, Puerto Maldonado (“ 15 km N.E. Puerto Maldonado”), 13 Jul. 1989, J. Ashe and R. Leschen leg. ( MUSM); 2 ♂♂, Tambopata, Puerto Maldonado (“ 15 km N.E. Puerto Maldonado”), 200 m, 15 Jul. 1989, J. Ashe and R. Leschen leg. ( MUSM); 1 ♂,Tambopata, Puerto Maldonado (“ 15 km N.E. Puerto Maldonado”), 200 m, Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 13 Jun. 1989 ( CMNC); 10 ♂♂, 4 ♀♀, Tambopata, Puerto Maldonado, Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 15 Jun. 1989, flight interception trap, Ashe and Leschen leg. ( CMNC); 1 ♂, Tambopata, Puerto Maldonado, Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 16 Jun. 1989, flight interception trap, Ashe and Leschen leg. ( CMNC); 12 ♂♂, 5 ♀♀, Tambopata, Puerto Maldonado, Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 17 Jun. 1989, flight interception trap, Ashe and Leschen leg. ( CMNC); 18 ♂♂, 8 ♀♀, Tambopata, Puerto Maldonado, Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 20 Jun. 1989, flight interception trap, Ashe and Leschen leg. ( CMNC); 1 ♂, Tambopata, Puerto Maldonado, Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 22 Jun. 1989, flight interception trap, Ashe and Leschen leg. ( CMNC); 26 ♂♂ (1 dissected), 13 ♀♀, Tambopata, Puerto Maldonado, Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 24 Jun. 1989, flight interception trap, Ashe and Leschen leg. ( CMNC); 7 ♂♂, 2 ♀♀,Tambopata, Puerto Maldonado, Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 26 Jun. 1989, flight interception trap, Ashe and Leschen leg. ( CMNC); 7 ♂♂, 2 ♀♀, Tambopata, Puerto Maldonado, Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 28 Jun. 1989, flight interception trap, Ashe and Leschen leg. ( CMNC); 14 ♂♂ (1 dissected), 4 ♀♀,Tambopata, Puerto Maldonado, Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 30 Jun. 1989, flight interception trap, Ashe and Leschen leg. ( CMNC); 2 ♂♂, Tambopata, Puerto Maldonado, Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 4 Jul. 1989, flight interception trap, Ashe and Leschen leg. ( CMNC); 3 ♂♂, 1 ♀, Tambopata, Puerto Maldonado, Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 7 Jul. 1989, flight interception trap, Ashe and Leschen leg. ( CMNC); 1 ♀, Tambopata, Puerto Maldonado, Reserva Cuzco Amazonica, 12º33′ S, 69º03′ W, 200 m, 16 Jul. 1989, flight interception trap, Ashe and Leschen leg. ( CMNC); 1 ♂, Tambopata, Puerto Maldonado, Reserva Nacional Tambopata, 18–22 Apr. 1983, Enrique Perez leg. ( MUSM); 1 ♂, 1 ♀, Tambopata, Puerto Maldonado, Reserva Nacional Tambopata (“Rio Tambopata Res. / 30 km (air) Sw. Pto. Malonato”), 12º50′ S, 69º20′ W, 3 Oct.–15 Nov. 1983, 290 m, N.E. Stork leg. ( BMNH); 1 ♂, Tambopata, Puerto Maldonado, Sector Triunfo, 69º11′47″ W, 12º33′42″ W, 198 m, 25 Mar. 2009, L. Figueroa leg. ( MUSM); 6 ♂♂, 1 ♀, Tambopata, Puerto Maldonado, Sector Triunfo, 69º11′47″ W, 12º33′42″ W, 198 m, 23 Jul. 2009, M. Alvarado leg. ( MUSM); 3 ♂♂, 3 ♀♀, Tambopata, Reserva Nacional Tambopata, Explorer’s Inn, 12º50′30″ S, 69º17′31″ W, 161 m, 15–18 May 2009, L. Figueroa and M. Alvarado leg. ( MUSM). – Pasco: 1 ♂, 2 ♀♀, Oxapampa, Parque Nacional Yanachaga-Chemillén, Puesto Huampal, 10º10′57″ S, 75º34′25.6″ W, 1001 m, 7–10 Nov. 2010, C. Carranza and J. Peralta leg. ( MUSM). – San Martín: 1 ♂, Picota, Pilluana, Fundo Mishquiyacu, 06º04′20.1″ S, 76º58′33.8″ W, 990 m, 11–12 Dec. 2008, C. Albujar leg. ( MUSM). – Ucayali: 3 ♂♂, 2 ♀♀, Coronel Portillo, Masisea, C.N. Betel(?) leg., 08º25′39.01″ S, 74º15′53.55″ W, 118 m, 8 Aug. 2008, C. Espinoza leg. ( MUSM); 1 ♂, Coronel Portillo, Puerto Alegre, 08º44′06.76″ S, 74º09′4.54″ W, 196 m, 21–22 May 2008, L. Figueroa leg. ( MUSM); 7 ♂♂, 7 ♀♀, Coronel Portillo, Puerto Purin, 08º44′59.2″ S, 74º08′19.52″ W, 122 m, 19 May 2008, L. Figueroa leg. ( MUSM); 1 ♀, Coronel Portillo, Puerto Purin, 08º44′59.2″ S, 74º08′19.52″ W, 196 m, 21–22 May 2008, L. Figueroa leg. ( MUSM); 1 ♂, Coronel Portillo, Puerto Purin, 08º44′59.2″ S, 74º08′19.52″ W, 122 m, 17 Jul. 2008, L. Figueroa leg. ( MUSM); 1 ♂, Coronel Portillo, Río Inamapuya, 08º44′33.7″ S, 74º06′15.9″ W, 135 m, 11–12 Jul. 2008, M. Alvarado leg. ( MUSM); 1 ♂, 1 ♀, Padre Abad, Irázola, Alexander von Humboldt, 08º49′04.72″ S, 75º04′14.88″ W, 233 m, 1–2 Apr. 2009, C. Carranza leg. ( MUSM); 1 ♂, 1 ♀, Padre Abad, Irázola, Alexander von Humboldt, 08º49′04.72″ S, 75º04′14.88″ W, 233 m, 8–10 Jun. 2009, C. Carranza leg. ( MUSM).
Ambiguous data: PERU?: 1 ♂, “ 7-8.XI.87 / CUPERJALI ” ( MUSM).
Erroneous data: BRAZIL: Pará: 1 ♂ (dissected), Itaituba, Rio Tapajós, Mar. 1964 , Dirings leg. ( MZSP); 1 ♀, Redenção, Pinkaiti-Aik, 07º46′ S, 51º58′ W, Nov. 1999, P.Y. Scheffler leg. ( CEMT).
Redescription
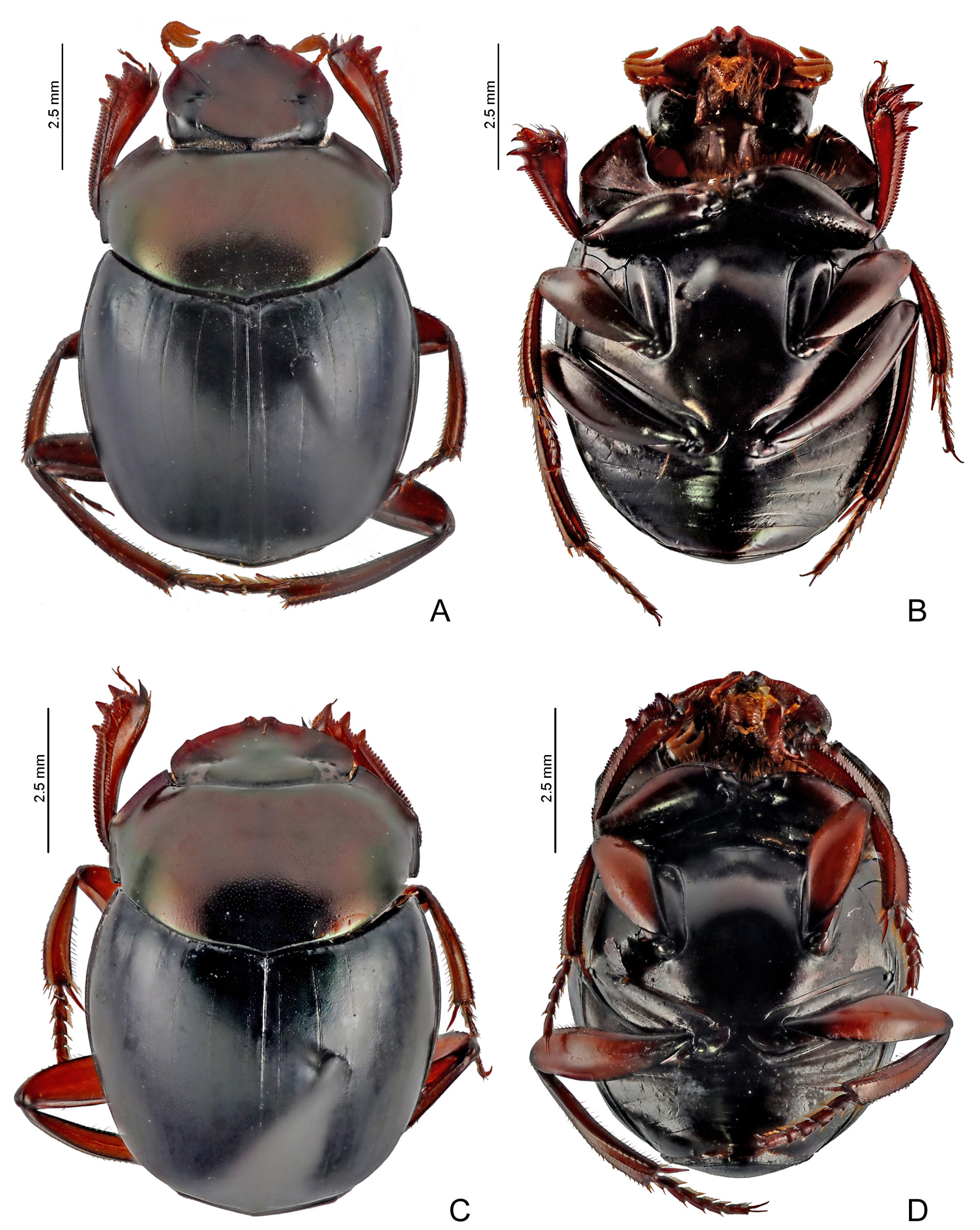
COLOURATION. Head and pronotum dark purple or coppery; occasionally, with weak greenish reflections. Elytra dark green or dark blue; when green, striae sometimes with dark blue colouration and slightly more contrasting with elytral tegument. Metaventrite black or coppery; usually with slight greenish reflections. Meso- and metafemora ranging in a north-south cline from dark brown ( Bolivia) to reddishbrown ( Peru and Brazil) and orangish or yellowish (northern Peru, Ecuador, and Colombia), but base always distinctly darker than at least apical two-thirds. Pygidium, sometimes, with shine predominantly greenish and with some coppery reflections, especially at base.
HEAD. Tegument with little shine, with strong alveolar microsculpture obliterating almost completely micropunctation which is almost imperceptible or even absent across the outer edge of head. Clypeus with two apical teeth obtuse and only slightly separated from one another; with a single transverse row of short setae covering base of both teeth. Genae with strong tooth just behind clypeal-genal juncture. Posterior edge of head without margin between eyes, or margin very fine and tenuous.
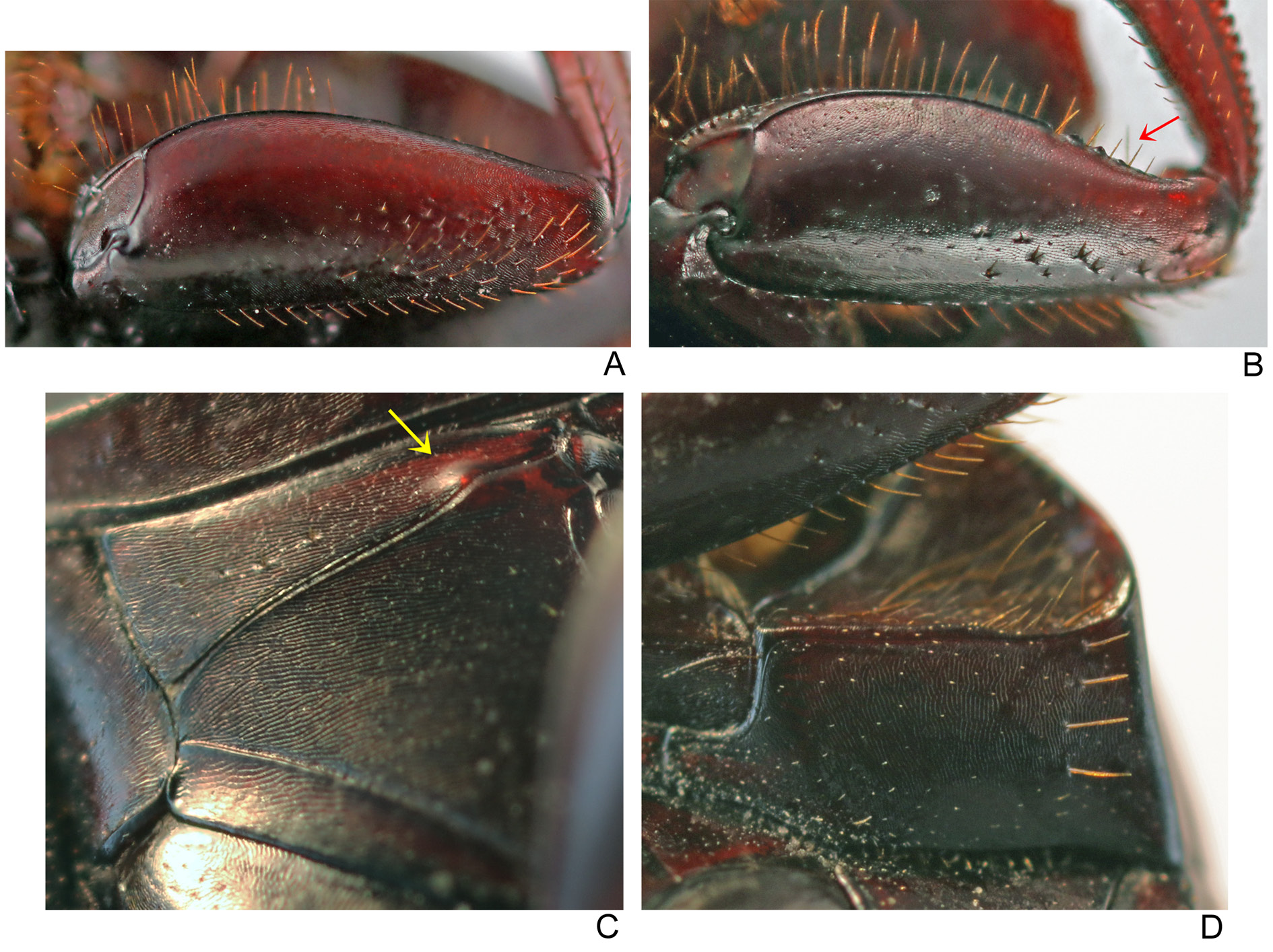
THORAX. Pronotum with tegument with diffuse shine and alveolar microsculpture very fine and strongly marked obliterating micropunctation, which is usually very weak at centre and completely absent on sides. Posterior edge may have an evident fine transverse line at centre (generally extending beyond second elytral stria), or transverse line very tenuous and short, or even absent. Hypomeral cavity with long yellowish setae at centre ( Fig. 35C View Fig ); external margin with weak tubercle. Metaventrite glabrous, including lateral region; tegument with strong rivose microsculpture on anterior region and adjacent to internal margin of mesocoxae and with strong alveolar microsculpture and fine micropunctation at centre and on posterior region.
LEGS. Ventral surface of all femora and tibiae with diffuse shine. Profemora with tegument with strong rivose microsculpture and without micropunctation ( Fig. 9A View Fig ). Protibiae narrow and with expansion on internal edge, which can be evident (southern populations in Bolivia and Peru, Fig. 11F View Fig ), or only slightly indicated (especially in Ecuador and Colombia, Fig. 11G View Fig ); at their apical third, external edge with three small acute teeth on external edge, two apical ones of subequal size and longer than the basal. Mesofemora margined anteriorly only at their basal half; unmargined portion of anterior edge with row of very short setae; posterior margin absent; tegument with strong rivose microsculpture. Metafemora margined only anteriorly, posterior margin absent; apical half of anterior edge covered by row of setae; tegument entirely covered by strong rivose microsculpture and with coarse elongate punctation at base geographically variable: individuals from southern populations in Bolivia with coarse punctation evident ( Fig. 13G View Fig ) which becomes progressively finer and imperceptible northwards until being completely absent in populations in Ecuador and Colombia ( Fig. 13H View Fig ). Metatarsomeres II and V subequal in length and longer than the others; metatarsomere IV shorter than the others.
ELYTRA. With at most nine visible striae: in general, the first two to four striae well marked, finely carinulate, and without basal widening; remaining striae progressively more effaced and interrupted; eighth and ninth striae observable only in specimens with well-marked striae and, in those cases, always very subtle; all striae lack their carinulae before reaching the apex of elytra, where they are completely indistinct; humeral carina absent. Tegument of interstriae with diffuse shine, with strong alveolar microsculpture throughout its surface; micropunctation obliterated by microsculpture and almost imperceptible.
ABDOMEN. Ventrite VI with rivose microsculpture diffuse at centre and weak on the sides; both sexes without lateral foveae. Pygidium with tegument with diffuse shine and entirely covered by alveolar microsculpture; micropunctation, if present, completely obliterated by microsculpture and difficult to see.
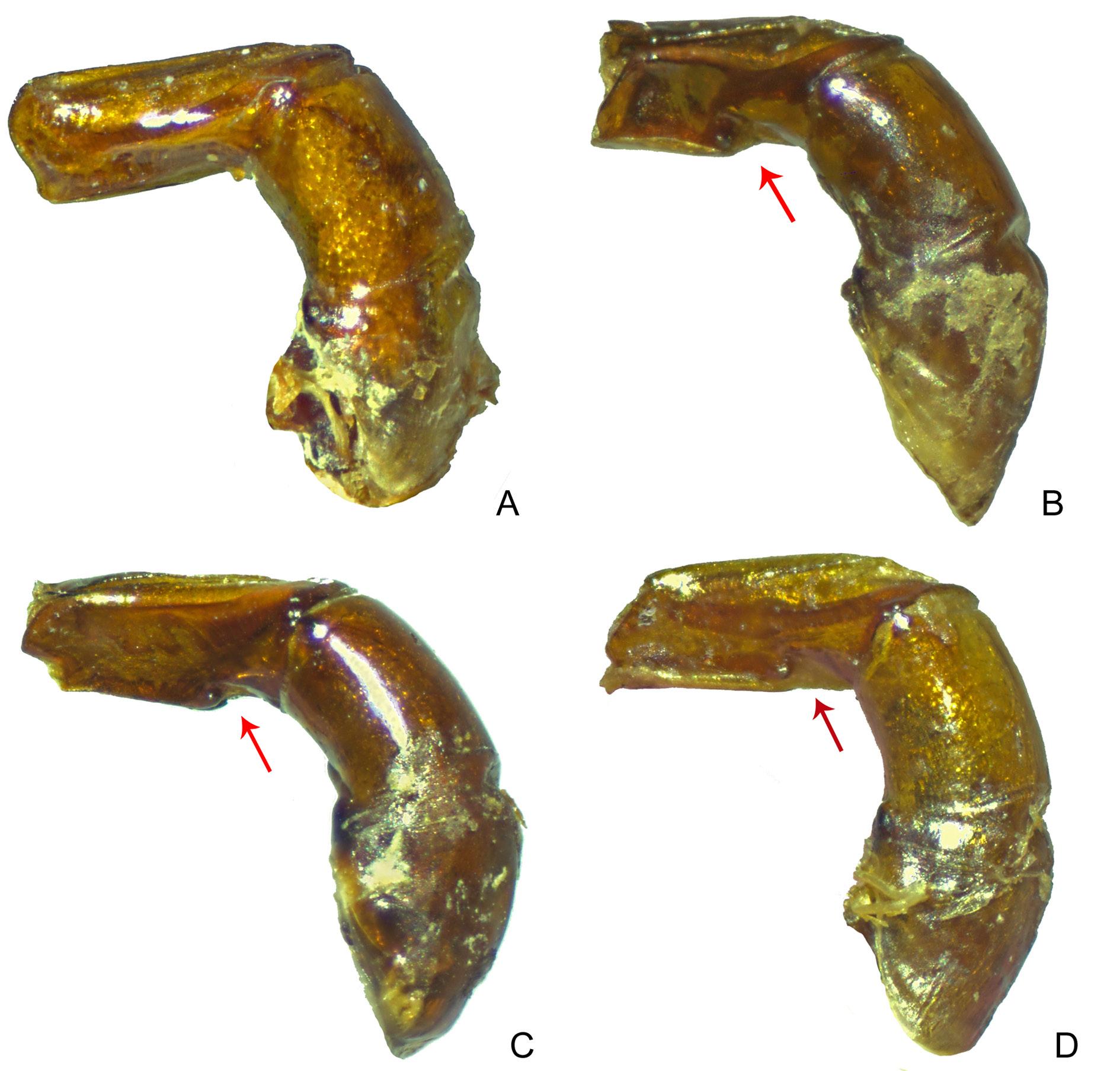
AEDEAGUS. Parameres at least half as long as phallobase and symmetrical, both faces flat. In lateral view, parameres simple, with truncate apex and without ventral keel or notch ( Fig. 18A View Fig ).
SEXUAL DIMORPHISM. Males: Protibial spur narrow and widely bifid at apex, with spiniform projections, the external projection much longer than the internal one ( Fig. 15I View Fig ). Ventrite VI with posterior edge strongly narrowed at middle; anterior edge covered very slightly by weak medial expansion of ventrite V. Females: Protibial spur simple, spiniform. Ventrite VI much wider at middle; anterior edge subtly covered by weak medial expansion of posterior edge of ventrite V.
Measurements
Males (N =14). TL: AV: 8.1 ± 0.76; MX: 9.2; MN: 6.6. EW: AV: 5.7 ± 0.43; MX: 6.3; MN: 4.8. PrL: AV: 2.4 ± 0.27; MX: 3.0; MN: 1.9. PrW: ME: 4.8 ± 0.83; MX: 5.5; MN: 2.2. PgL: ME: 1.4 ± 0.10; MX: 1.5; MN: 1.2. PgW: AV: 2.3 ± 0.24; MX: 2.6; MN: 2.0.
Females (N = 12). TL: AV: 8.1 ± 0.54; MX: 8.9; MN: 7.5. EW: AV: 5.6 ± 0.48; MX: 6.4; MN: 4.9. PrL: ME: 2.4 ± 0.26; MX: 2.9; MN: 2.1. PrW: AV: 4.9 ± 0.43; MX: 5.6; MN: 4.3. PgL: ME: 1.2 ± 0.11; MX: 1.5; MN: 1.1. PgW: AV: 2.3 ± 0.25; MX: 3.0; MN: 2.1.
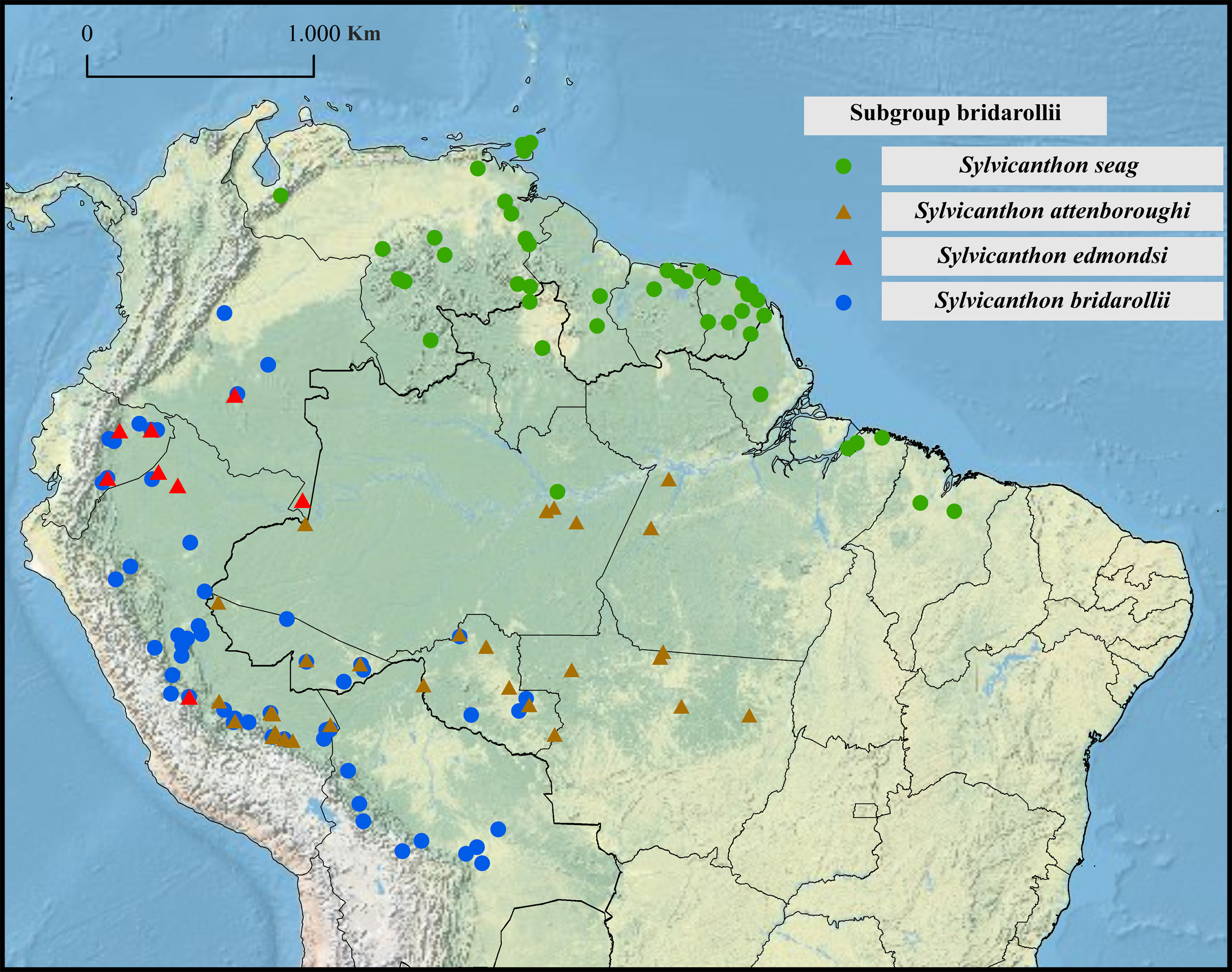
Geographical distribution
Western Amazonia in Colombia, Ecuador, Brazil, Peru, and Bolivia.
Ecoregions
Llanos, Caquetá Moist Forests, Napo Moist Forests , Cordillera Oriental Montane Forests, Iquitos Varzea, Ucayali Moist Forest, Peruvian Yungas, Southwest Amazon Moist Forests, Bolivian Yungas, Bolivian Montane Dry Forest, Chiquitano Dry Forests.
Collecting sites ( Fig. 34 View Fig )
COLOMBIA. Meta: Puerto Colombia. Caquetá: Parque Nacional Natural Sierra de Chiribiquete. Guaviare: San José del Guaviare (Parque Nacional Natural Nukak).
ECUADOR. Sucumbíos: Shushufindi (Reserva Biológica Limoncocha). Napo: Reserva Biológica Jatun Sacha , Puerto Misahualli, Tena. Orellana: Tiputini Biodiversity Station. Morona Santiago: Cordillera Cutucú, Untsuants.
PERU. Loreto: Alto Amazonas (Teniente César López Rojas), Reserva Nacional Pacaya Samiria, Zona Reservada Sierra del Divisor. Amazonas: Rodríguez de Mendoza (Quebrada Huancabamba). San Martín: Picota (Pilluana: Fundo Mishquiyacu). Ucayali: Coronel Portillo (Puerto Alegre, Puerto Purin), Padre Abad (Irázola: Alexander von Humboldt). Huánuco: Estación Biológica de Panguana, Leoncio Prado (Rupa-Rupa: Tingo María), Puerto Inca. Pasco: Oxapampa (Parque Nacional Yanachaga- Chemillén). Junín: Chanchamayo (San Ramón), Satipo. Cuzco: La Convención (Echarate, Santuario Nacional Megantoni). Madre de Díos: Manu (Manu: Salvación; Parque Nacional del Manu: Estación Biológica Cocha Cashu; Reserva Comunal Amarakaeri), Tambopata (Las Piedras; Puerto Maldonado: Madama, Reserva Cuzco Amazonica, Reserva Nacional Tambopata, Sector Triunfo).
BRAZIL. Acre: Manoel Urbano (Parque Estadual Chandless), Rio Branco, Senador Guiomard, Tarauacá, Xapuri (Reserva Extrativista Chico Mendes). Rondônia: Cacoal, Porto Velho, Rolim de Moura, São Francisco do Guaporé.
BOLIVIA. Beni: Mamoré (San Ramón). La Paz: Larecaja (Guanay), Nor Yungas (Coroico), Parque Nacional Madidi. Cochabamba: Cercado (Cochabamba), Chapare (Villa Tunari), Estación Biológica Villa Carmen, José Carrasco (Chimoré). Santa Cruz: Andrés Ibáñez (Santa Cruz de la Sierra), Ichilo (Buena Vista), Obispo Santistevan (General Saavedra).
Intraspecific variation and taxonomic discussion
Although the four species composing the bridarollii subgroup are very similar to one another, S. bridarollii is precisely the one that we can more readily differentiate from the others. The following characteristics are unique to S. bridarollii in its subgroup: pronotum and pygidium with alveolar microsculpture strongly marked and with a very weak micropunctation, which, in general, is indistinct; hypomeral cavity with long setae at centre ( Fig. 35 View Fig C–D); metaventrite with a strong alveolar microsculpture at centre; protibiae with internal margin expanded at their apical half ( Fig. 11 View Fig F–G; see below discussion about the geographical variation of this character); and parameres elongated, simple (i.e., without any ventral keel or notch) and symmetric, both with external faces equally flat ( Fig. 18A View Fig ). Furthermore, the metafemora with a dark brown colour and with well-impressed coarse punctures at base is a characteristic exclusive of the southern populations of S. bridarollii ( Fig. 13G View Fig ; see more details below).
As can be seen on the map of Fig. 34 View Fig , S. bridarollii is distributed parallel to the Andes throughout western Amazonia, in altitudes between 100 and 2360 m. The only representative of the bridarollii subgroup with which it is never found in sympatry is S. seag sp. nov., a species exclusive to the northern Amazon region. Apart from the characteristics listed above, these two species are different by the head and pronotal micropunctation, shape of the male protibial spur ( Fig. 15 View Fig I–J), shape of ventrites V and VI of females, the tegument of pygidium and, especially, the totally distinct shape of the parameres ( Fig. 18 View Fig A–B) (see Table 4). Pronotum and elytra with a bright green or dark blue colouration typical of the northern populations of S. seag sp. nov. ( Fig. 37 View Fig B–C) are not seen in S. bridarollii ; on the other hand, specimens of S. seag sp. nov. collected on the banks of the Amazon River and in Maranhão have a similar colouration to the one observed in S. bridarollii ( Fig. 37A View Fig ).
The other two species of the bridarollii subgroup, S. edmondsi sp. nov. and S. attenboroughi sp. nov., by contrast, can be found in sympatry with S. bridarollii , respectively, in Colombia, Ecuador and Peru, and in Brazil and Peru. From S. edmondsi sp. nov., S. bridarollii can be promptly differentiated by its dorsal colouration ( Figs 32A, C View Fig , 38A View Fig ) and the shape of its parameres ( Fig. 18A, C View Fig ), but the populations of both species also differ in the average of the total body length ( Table 3). It is worth noting that Celi et al. (2004), having placed pitfall traps across an altitudinal gradient in Morona Santiago ( Ecuador), collected twice as many specimens of S. edmondsi sp. nov. (116) as of S. bridarollii (57) (the former species was collected at altitudes between 600 and 1000 m and the latter between 500 and 900 m); at one point at 700 m altitude, they collected 114 S. edmondsi sp. nov. vs only 25 S. bridarollii . In order to test whether this result is indeed a real general pattern of relative abundance between these two species, it would be interesting if future ecological works evaluated other sympatric populations of S. bridarollii and S. edmondsi sp. nov. Finally, S. attenboroughi sp. nov. is the species most similar to S. bridarollii . Nevertheless, both species are distinguished by the unique characteristics of S. bridarollii listed above and also by the degree of excavation of the hypomeral cavity ( Fig. 35 View Fig B–C) and by the head micropunctation ( Table 3).
Within Sylvicanthon , S. bridarollii is the species that shows the most remarkable form of morphological variation, which is intimately associated with the species’ geographical distribution in a clear northsouth cline ( Fig. 36 View Fig ). Colouration and presence of coarse punctures on femora, shape of protibiae, and the presence of a fine transverse line on the posterior edge of the pronotum vary along this cline in the following way: individuals from southern populations in the Bolivian provinces of Santa Cruz and Cochabamba have meso- and metafemora black at the base and dark brown on the rest of their surface, metafemora with strong, coarse punctures of irregular shape at their base ( Fig. 13G View Fig ), protibiae with internal margin with an evident expansion at their apical half ( Fig. 11F View Fig ) and pronotum without any trace of a transverse line on its posterior edge. Towards the north of Bolivia, in places like Guanay and the Madidi National Park (province of La Paz) and southern Peru, in the Madre de Díos region, the colouration of meso- and metafemora becomes lighter, being dark brown or reddish-brown; the metafemur punctures are shallower and sparser (in southern Peru, they are almost imperceptible); and the posterior edge of the pronotum starts to show some indications of a medial transverse line; the protibiae, on the other hand, continue to be essentially similar to the shape seen farther south. In central Peru, in the regions of Cuzco, Junín, Pasco, Huánuco, Ucayali, Amazonas and San Martínez, and, in a lesser degree, in the states of Acre and Rondônia, Brazil, individuals have clearly bicolour meso- and metafemora, with a dark brown basal area and the rest of the surface reddish-brown; the punctures at the base of the metafemora are very tenuous and almost imperceptible; in most of the specimens, the transverse line on the posterior edge of the pronotum is clearly seen; and the internal expansion of the protibiae becomes less pronounced. Then, from Zona Reservada Sierra del Divisor, in the Peruvian region of Loreto, north to Ecuador and Colombia, specimens have meso- and metafemora with a much lighter colouration pattern, the base being dark brown and the rest of the surface orange or yellowish ( Fig. 13H View Fig ); the punctures at the base of the metafemora are extremely tenuous and almost imperceptible (Loreto) or totally absent ( Ecuador and Colombia); and all the examined specimens show a clear transverse line on the posterior edge of the pronotum. In those northern populations, the internal expansion of the protibiae is much more tenuous than those in the southern populations ( Fig. 11G View Fig ). If not directly compared with the protibiae of the species that really lack any expansion (i.e., the other members of the bridarollii subgroup and those of the candezei subgroup), one might be misled to think that those populations of S. bridarollii do not possess a protibial expansion either.
Although the previous description was presented by separating the distribution of S. bridarollii into four distinct parts, the clinal variation found is completely continuous in that north-south axis ( Fig. 36 View Fig ), indicating that this cline is most likely the case of a primary intergradation rather than a secondary intergradation due to secondary contact of formerly allopatric demes, although this possibility should not be ruled out based only on our morphological observations (see the discussion by Mayr 1963 on how complex the history of intergradation gradients can be). Therefore, a question is raised: what were the evolutionary forces that ultimately led to the emergence of this clinal pattern? To give a proper answer to this question, it will be necessary first to acquire further knowledge on the biological roles of the varying features and the environmental factors changing at an equivalent rate along the north-south axis. Other species of Sylvicanthon also show noticeable geographical variations, such as S. seag sp. nov. and S. obscurus (in relation to colouration) and S. candezei (the pattern of pronotal microsculpture), which may have some relation to those seen in S. bridarollii .
Comments
The male from MZSP allegedly collected in Itaituba, in the Brazilian state of Pará, is certainly mislabelled, since S. bridarollii does not occur so far east ( Fig. 34 View Fig ). This specimen originates from the former collection of the German-Brazilian amateur entomologist Richard von Diringshofen (cited as “Dirings” on specimen labels), which was incorporated in the MZSP collection in 1987 ( Costa 1999; Ferreira et al. 2016), and this is not the only specimen housed there with erroneous collecting data. Cupello & Vaz-de-Mello (2014), for instance, found in that same collection two individuals of Coprophanaeus saphirinus ( Sturm, 1826) , a species present only in the southern Atlantic Forest, labelled as coming from Pará; one of them, curiously enough, also from Itaituba. As a great part of the collection of Diringshofen was bought unprepared ( Costa 1999; Ferreira et al. 2016), it is possible that, over time, specimens from different envelopes have been accidentally mixed up, so bringing us to this situation where mislabelling seems not to be such a rare problem.
The type series of Glaphyrocanthon bridarollii is composed of 10 specimens (Martínez 1949): the male holotype ( Fig. 33A View Fig ), the female allotype ( Fig. 33B View Fig ) and eight other paratypes, four males and four females. As expected, both the holotype and the allotype are deposited in the MACN, and, of the paratypes, two males and one female were found in the CMNC, while a male and two females were found in the MZSP, probably deposited there via Padre Francisco Pereira. Therefore, we did not find two of the paratypes, male and female. Martínez (1949) stated he deposited “two couples” of paratypes in the collection of Rodolfo Zischka (1895–1980), from Cochabamba, Bolivia, the type series’ collector. Since 1979, Zischka’s collection is housed at the Zoologische Staatssammlung München, Munich, Germany ( ZSM 2014) and, thus, this museum is probably the place where the missing pair of paratypes (not ‘two couples’, as said by Martínez) is being conserved.
In their list of the dung beetles occurring in Colombia, Medina et al. (2001) and Medina & Pulido (2009) cited S. bridarollii for the departments of Casanare, Guainía, Guaviare, Meta and Vichada. For Meta ( Amézquita et al 1999) and Guaviare ( Escobar 2000b), it was possible to find more precise literature records and, therefore, those departments were included in the geographical distribution given above. On the other hand, for the other three departments, no accurate locality records were found; consequently, we preferred to include them neither in the geographical list above nor on the map.
Natural history
Following S. aequinoctialis and S. proseni , S. bridarollii has the greatest amount of bionomic information available in the literature and on specimen labels. After compiling all these data, it is possible to say that S. bridarollii does not differ from the genus pattern, having food habits preferentially coprophagous, although the species can occasionally be attracted to carrion, as shown by the specimens collected by D. Inward at the Tiputini Biodiversity Station (Orellana, Ecuador). On the other hand, Figueroa & Alvarado (2011), collecting at the Reserva Nacional Tambopata (Madre de Díos, Peru), although having used pitfall traps baited with both dung and carrion, caught S. bridarollii only with the first kind of bait. A large number of the specimens here studied were collected with flight interception traps.
Having collected during the dry season in Puerto Colombia (Meta, Colombia), Amézquita et al. (1999) found that S. bridarollii was the third most abundant species in that region, corresponding to more than 13% of the collected specimens, following only “ Onthophagus haematopus Harold, 1975 ” 21, with 15%, and an unidentified species of Uroxys , with 28%. On the other hand, four other inventories – Figueroa & Alvarado (2011), at the Reserva Nacional Tambopata, in the Peruvian department of Madre de Díos; Larsen (2004), at the Zona Reservada Megantoni, in Cuzcu, Peru; Celi et al. (2004), in the Ecuadorian province of Morona Santiago; and Carpio et al. (2009), in Sucumbíos, also in Ecuador – did not find S. bridarollii among the most abundant species. In fact, in the third paper, another species of the same genus, S. proseni (cited as Canthon aequinoctialis ), was the most abundant dung beetle in the region.
Regarding habitat preferences, it seems that S. bridarollii is restricted to dense rainforests, be it either primary or secondary. Larsen (2004) also collected specimens in a bamboo ( Guadua Kunth and Chusquea Kunth ) forest. Carpio et al. (2009), in a study on the effect of the opening of a new road at the centre of a pristine forest in Sucumbíos, saw that S. bridarollii was among the five dung beetle species that have their abundance progressively increased from the road towards the forest interior.
As for the altitudinal amplitude, specimens studied for this work were mostly collected between 140 and 1100 m, but one female was caught in Rodríguez de Mendoza (Amazonas, Peru) at about 2300 m a.s.l. The specimens examined also show that adults of S. bridarollii are active throughout the year, although a much higher number of individuals has been collected between May and October.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Scarabaeinae |
|
Tribe |
Deltochilini |
|
Genus |
Sylvicanthon bridarollii (Martínez, 1949)
| Cupello, Mario & Vaz-De, Fernando Z. 2018 |
Canthon bridarollii
| Krajcik M. 2012: 63 |
Sylvicanthon bridarolli
| Chamorro W. & Marin-Armijos D. & Granda V. & Vaz-de-Mello F. Z. 2018: 86 |
| Espinoza V. R. & Noriega J. A. 2018: 146 |
| Figueroa L. & Alvarado M. 2011: 210 |
Silvicanthon bridarollii
| Chamorro W. & Marin-Armijos D. & Granda V. & Vaz-de-Mello F. Z. 2018: 98 |
| Horgan F. G. 2009: 3532 |
| Horgan F. G. 2005: 609 |
| Horgan F. G. 2005: 131 |
Sylvicanthon
| Kirk A. A. 1992: 54 |
Glaphyrocanthon bridarollii
| Halffter G. & Martinez A. 1977: 63 |
Sylvicanthon bridarollii
| Ratcliffe B. C. & Jameson M. L. & Figueroa L. & Cave R. D. & Paulsen M. J. & Cano E. B. & Beza-Beza C. & Jimenez-Ferbans L. & Reyes-Castillo P. 2015: 196 |
| Tarasov S. & Genier F. 2015: 21 |
| Price D. L. & Feer F. 2012: 327 |
| Carvajal V. & Villamarin S. & Ortega A. M. 2011: 117 |
| Medina C. A. & Pulido L. A. 2009: 59 |
| Horgan F. G. 2006: 364 |
| Celi J. & Terneus E. & Torres J. & Ortega M. 2004: 46 |
| Larsen T. 2004: 261 |
| Medina C. A. & Scholtz C. H. & Gill B. D. 2003: 44 |
| Medina C. A. & Lopera-Toro A. & Vitolo A. & Gill B. 2001: 137 |
| Medina C. A. & Lopera-Toro A. 2000: 312 |
| Vaz-de-Mello F. Z. 2000: 195 |
| Escobar F. 2000: 210 |
| Escobar F. 2000: 121 |
| Amezquita S. J. & Forsyth A. & Lopera A. & Camacho A. 1999: 119 |
| Halffter G. & Martinez A. 1977: 63 |
Glaphyrocanthon (Glaphyrocanthon) bridarollii
| Vulcano M. A. & Pereira F. S. 1967: 561 |
| Martinez A. & Pereira F. S. 1967: 53 |
| Martinez A. & Halffter G. & Halffter V. 1964: 5 |
| Vulcano M. A. & Pereira F. S. 1964: 661 |
| Martinez A. & Pereira F. S. 1956: 126 |
| Martinez A. 1950: 170 |
Glaphyrocanthon bridarollii Martínez, 1949a: 282–287
| Martinez A. 1949: 287 |