Sylvicanthon aequinoctialis (Harold, 1868) Cupello & Vaz-De, 2018
|
publication ID |
https://doi.org/ 10.5852/ejt.2018.467 |
|
publication LSID |
lsid:zoobank.org:pub:8D27AAB8-B7F2-424C-B1A6-66FEFA66EDFF |
|
DOI |
https://doi.org/10.5281/zenodo.3846309 |
|
persistent identifier |
https://treatment.plazi.org/id/A72C87FB-FFC0-FFE2-0EC8-0B4B08969114 |
|
treatment provided by |
Valdenar |
|
scientific name |
Sylvicanthon aequinoctialis (Harold, 1868) |
| status |
comb. nov. |
Sylvicanthon aequinoctialis (Harold, 1868) View in CoL comb. nov.
Figs 7A View Fig , 11B View Fig , 15 View Fig E–F, 17E, 20, 28A–B, 29A–B, 30, 31A
Canthon aequinoctialis Harold, 1868a: 2 View in CoL , 5, 14, 79, 141.
“ Coprobius aequinoctialis ” – Dejean 1833: 136.
“ Coprobius aequinoctialis ” – Dejean 1836: 151. — Harold 1868a: 79 (as synonym of C. aequinoctialis ). Nomen nudum.
Canthon aequinoctialis View in CoL – Harold 1869b: 989; 1880: 16. — Bates 1887: 33. — Gillet 1911: 27. — Schmidt 1922: 64, 72. — Balthasar 1939: 187; 1941: 341 (error: refers to S. proseni View in CoL ); 1951: 326 (error: refers to S. proseni View in CoL ). — Martínez 1949a: 290. — Vulcano & Pereira 1967: 561. — Gill 1991: 225–226, 228. — Escobar 2000a: 206 (error: refers to S. proseni View in CoL ); 2000b: 114, 121 (mixed information with S. proseni View in CoL ). — Medina et al. 2001: 135 (mixed information with S. proseni View in CoL ); 2003: 27, 51, 57–58, figs 9, 164; 2012: 93, 115, 171, 192. — Ratcliffe 2002: 12. — Solís & Kohlmann 2002: 1, 4, 6–8, 53–54, 64, figs 1f, 31; 2012: 3, 21. — Halffter 2003: 43. — Celi et al. 2004: 42 (error: refers to S. proseni View in CoL ). — Noriega-Alvarado 2004: 40 (idem). — Kohlmann et al. 2007: 9, 28; 2010: 553. — Noriega et al. 2008: 78–79 (error: refers to S. proseni View in CoL ). — Carpio et al. 2009: 462, 464, 469 (idem). — Medina & Pulido 2009: 58 (error: refers to S. proseni View in CoL ). — Arango & Montes 2010: 262. — Figueroa & Alvarado 2011: 210–211, fig. 1c (error: refers to S. proseni View in CoL ). — Solís et al. 2011: 36, 38. — Krajcik 2012: 63. — Noriega 2012: 4 (error: refers to S. proseni View in CoL ). — Ratcliffe et al. 2015: 195 (error: refers to S. proseni View in CoL ).
Canthon aequinoctiale – Blackwelder 1944: 198; 1973: 2. — Gacharná 1951: 221. — Howden & Young 1981: 20, 27–28, 153, 167, figs 36–37. — Vaz-de-Mello & Louzada 1997: 56. — Vaz-de-Mello 1999: 449–450 (error: refers to S. proseni View in CoL ). — Young 2009: 322.
Canthon cf. aequinoctialis View in CoL – Noriega et al. 2007b: 81. — Martínez et al. 2010: 24.
Canthon (Canthon) aequinoctialis View in CoL – Halffter & Martínez 1977: 62, 90–91. — Noriega et al. 2007a: 54–55 (error: refers to S. proseni View in CoL ).
Canthon (Canthon) aequinoctiale – Vaz-de-Mello 2000 (error: refers to S. proseni View in CoL ).
Canthon (Canthon) aequinotialis [sic] – Noriega-Alvarado 2009. — Culot et al. 2011: Supporting information, table S1 (error: refers to S. proseni View in CoL ).
Canthon (Glaphyrocanthon) aequinoctialis View in CoL – Howden 1966: 730.
Glaphyrocanthon (Glaphyrocanthon) aequinoctialis – Pereira & Martínez 1956: 126, 128. — Martínez et al. 1964: 3, 5, 8–10, 13. — Vulcano & Pereira 1967: 561.
Glaphyrocanthon (Glaphyrocanthon) aequinotialis [sic] – Vulcano & Pereira 1964: 661.
Etymology
From the Latin ‘ aequinoctialis ’, meaning ‘equinox’ or ‘equinoctial’. Probable reference to the type locality, region crossed by the Equator.
Material examined
Lectotype (here designated)
COLOMBIA: ♂, type locality cited by Harold (1868a) as “ Columbien, Neu-Granada ”, (“ aequinoctialis / t. Har ”, “ Nov. Grenade ”, “Ex-Musaeo / E. Harold”, “ HOLOTYPE ”, “ TIPO / Canthon / ( Canthon ) / aequinoctialis / Harold / G. H. y A. M. det . 76 ”) ( MNHN) ( Fig. 29A View Fig ).
Paralectotype
COLOMBIA: ♀, (“26381”, “ Nov Granad. Gom. ”, “Type”, “ aequinoctialis / Harold * / Nov. Granada ”, “ PARALECTOTYPE / Canthon aequinoctialis / Harold, 1868 / des. Cupello & Vaz-de-Mello, 2015”) ( ZMHB) ( Fig. 29B View Fig ).
Additional material (282 ♂♂, 210 ♀♀, 81 unsexed specimens)
COLOMBIA: 1 ♀, “ Lebas ” (collector or locality?) ( ISNB, Gillet collection); 1 ♂, illegible locality, 1921 ( MZSP); 1 ♂, “New Granada”, R. Bunch leg. ( BMNH, E.Y. Western coll.). – Antioquia: 2 ♂♂, San Luis, Sonsón, Reserva Natural Cañon del Río Claro, 1440 m, 2 Mar. 1994, Harold M. Parra leg. ( CEMT); 2 ♂♂, San Luis, Sonsón, Reserva Natural Cañon del Río Claro, 1440 m, 3 Mar. 1994, H.M. Parra leg. ( CEMT); 1 ♀, San Luis, Sonsón, Reserva Natural Cañon del Río Claro, 1440 m, 3 Mar. 1994, H.M. Parra leg. ( MCNZ). – Cesar: 1 ♂, 1 ♀, Chimichagua, Finca Señor Reyes, 09º21′9.8″ N, 73º48′22.2″ W, 42 m, 7 Mar. 2010, pitfall with human faeces, P. Delgado leg. ( CEMT). – Chocó: 2 ♂♂ (1 dissected), 1 ♀, Acandí, Capurganá, “caminho ‘Al Cielo’”, 10 m, 6 Jan. 1999, A. Vitolo leg. ( CEMT); 2 ♂♂ (1 dissected), Acandí, Capurganá, “Jardin”, 08º37′42″ S, 77º21′12″ W, 30 m, 16–18 Jan. 2008, pitfall with human faeces, Arias et al. leg. ( CEMT); 1 ♂, 4 ♀♀, Lloró, 05º31′ N, 76º33′ W, 90 m, 20 Feb. 2003, Olaya and Mosquero leg. ( TAMU); 1 ♀, Parque Nacional Natural Ensenada de Utría, 19 Jun. 1997, pitfall with human faeces, Llanos-Jurado leg. ( CEMT); 2 ♂♂, 1 ♀, Quibdó, Tutunendó (“ 20 km NE Quibdó”), 60 m, 26 Nov. 2001, J.C. Neita leg. ( TAMU); 3 ♂♂, Quibdó, Estación Ambiental Pandó, 05º42′43″ S, 76º37′59″ W, 43 m, 9–11 Jun. 2010, pitfall with human faeces, J. Arias leg. ( CEMT); 4 ♂♂, 6 ♀♀, Quibdó, Pacurita, 05º41′ N, 76º40′ W, 53 m, 25 Nov. 2001, dung, J.C. Neita leg. ( TAMU); 2 ♀♀, Quibdó, Pacurita, “Arriba de la quebrada Aguas Claras”, 05º42′ N, 76º40′ W, 43 m, 9–11 Jun. 2010, pitfall with human faeces, J. Arias leg. ( CEMT); 2 ♂♂, Unión Panamericana, 05º32′45″ N, 76º44′33″ W, 115 m, J.C. Neita leg. ( TAMU); 3 ♂♂ (1 dissected), 1 ♀, Unión Panamericana, Salero, 05º32′ N, 76º44′ W, 120 m, 3–5 Jun. 2010, pitfall with human faeces, J. Arias leg. ( CEMT). – Distrito Capital (?): 1 specimen, Bogotá ( BMNH). – Santander: 3 ♂♂, 1 ♀, Serranía de las Quinchas, Reserva El Paujil, Jul. 2006, pitfall with Alouatta seniculus dung, Santos Zarate leg. ( CEMT); 1 ♂, 1 ♀, Serranía de las Quinchas, Reserva El Paujil, Sep. 2006, pitfall with Alouatta seniculus dung, Santos Zarate leg. ( CEMT). – Tolima: 1 ♂, 300 m, Nov. 1995, human faeces, F. Escobar leg. ( CEMT). – Valle Del Cauca: 1 ♂, Estación Agro-Forestal Bajo Calima, 50 m, 2 Oct. 1995, A. Lopera leg. ( CEMT); 1 ♂ (dissected), Dagua, Río Escalerete, 31 Mar.–4 Apr. 1991, L.C. Pardo Locarno leg. ( CEMT).
COSTA RICA: Guanacaste: Volcán Rincón de la Vieja, Hacienda Guachipelín, 13 Aug. 1999, trap with human faeces, J. L. Moreno & T. Mestre leg. 1 ♂ ( CEMT). – Limón: Río Reventazón, Humburgfarm, 15 Apr. 1923, without collector – 1 ♀ ( MZSP); Río Reventazón, Humburgfarm, 27 Aug. 1925, F. Nevermann leg. 1 ♂ ( MZSP). – Puntarenas: Osa, 10–15 Aug. 1966 [trap with rotten flesh], S. Peck leg. 1 ♂ ( CNCI); 11 specimens, Península de Osa, Corcovado National Park, Sirena Biological Station, 08º30.259′ N 83º35.958′ W, 18 m, 17–20 Jul. 2009, Mehrabi et al. leg. ( OUMNH); 20 specimens, Península de Osa, Corcovado National Park, Sirena Biological Station, 08º30.502′ N, 83º36.285′ W, 29 m, 29–31 Jul. 2009, Mehrabi et al. leg. ( OUMNH); 52 ♂♂ (1 dissected), 46 ♀♀, Península de Osa, Sirena Biological Station, 08º30′ N, 83º36′ W, 30 m, Jul. 2009, swine dung, Mann leg. ( CEMT); 7 specimens, Península de Osa, Corcovado National Park, Sirena Biological Station, 08º29.891′ N 35º998′ W, 31 m, 11–14 Jul. 2009, Mehrabi et al. leg. ( OUMNH); 9 specimens, Península de Osa, Corcovado National Park, Sirena Biological Station, 08º29.46′ N 83º35.57′ W, 41 m, Jul. 2009, Mehrabi, Coals, Cowburn and Yu leg. ( OUMNH); 2 specimens, Península de Osa, Corcovado National Park, Sirena Biological Station, 08º30.897′ N 83º35.847′ W, 42 m, 31 Jul.–2 Aug. 2009, Mehrabi et al. leg. ( OUMNH); 2 specimens, Península de Osa, Corcovado National Park, Sirena Biological Station, 08º28.230′ N 83º35.275′ W, 47 m, 26–29 Jul. 2009, Mehrabi et al. leg. ( OUMNH); 8 specimens, Península de Osa, Corcovado National Park, Sirena Biological Station, 08º29.287′ N 83º35.327′ W, 49 m, 19–22 Jul. 2009, Mehrabi et al. leg. ( OUMNH); 1 specimen, Península de Osa, Osa Biodiversity Centre, 08º24′14″ N 83º20′09″ W, 115 m, Apr. 2010, Mann and Mehrabi leg. ( OUMNH); 3 ♂♂, 1 ♀, Reserva Biológica Bosque Nuboso Monteverde (“Parque Nacional Monteverde”), 13 Aug. 1999, J.L. Moreno and T. Mestre leg. ( CEMT).
HONDURAS: Atlántida: 1 ♂ (dissected), 2 ♀♀, 15 km W of La Ceiba, 15–19 Jun. 1996, flight interception trap, R. Lehman leg. ( TAMU).
NICARAGUA: Chontales: 1 specimen, T. Belt leg. ( BMNH, BCA). – Jinotega: 2 ♀♀, Reserva Natural Cerro Kilambé, 1000 m, Aug. 2001, M.A. Guatemala leg. ( SEAN); 1 ♀, Santa Maura, 1215 m, Mar. 2002, L. Pivotti leg. ( SEAN). – Matagalpa: 41 ♂♂, 17 ♀♀, Matiguas, 5–6 Dec. 2003, B. Hernández leg. ( SEAN); 3 ♂♂, 4 ♀♀, Matiguas, 8–9 Feb. 2004, B. Hernández leg. ( SEAN); 5 ♂♂, 2 ♀♀, 13– 14 Feb. 2004, B. Hernández leg. 5 ( SEAN); 18 ♂♂, 21 ♀♀, Matiguas, 10–11 Mar. 2004, B. Hernández leg. ( SEAN); 3 ♂♂, 1 ♀, Matiguas, 14–15 Mar. 2004, B. Hernández leg. ( SEAN); 1 ♀, Matiguas, 21– 22 Apr. 2004, B. Hernández leg. ( SEAN); 11 ♂♂, 17 ♀♀, Matiguas, 23–24 Apr. 2004, B. Hernández leg. ( SEAN); 27 ♂♂, 23 ♀♀, Matiguas, 26–27Apr. 2004, B. Hernández leg. ( SEAN); 9 ♂♂, 2 ♀♀, Matiguas, 3–4 Jun. 2004, B. Hernández leg. ( SEAN); 9 ♂♂, 5 ♀♀, Matiguas, 19–20 Jun. 2004, B. Hernández leg. ( SEAN); 7 ♂♂, Matiguas, 23–24 Jun. 2004, B. Hernández leg. ( SEAN); 2 ♂♂, 4 ♀♀, Matiguas, 29–30 Jun. 2004, B. Hernández leg. ( SEAN); 1 ♂, Matiguas, 2–3 Jul. 2004, B. Hernández leg. ( SEAN); 2 ♂♂, Reserva Natural Biósfera de Bosawas, Macizo de Peñas Blancas, 29–30 Apr. 2017, B. Hernández leg ( CEMT). – Río San Juan: 1 ♂, Reserva Biológica Indio Maíz, 10º58′24″ N, 84º04′52″ W, 14–15 Jun. 2002, P. Schmit and B. Hernández leg. ( SEAN).
PANAMA: Chiriquí: 1 specimen, David, San José de David, Champion leg. ( BMNH – BCA); 1 ♂, Gualaca, Hornito, Finca La Suiza, 9–15 May 1999, pitfall with human faeces, Wappes and Morris leg. ( TAMU). – Colón: 1 ♀, Chagres, Fort San Lorenzo (“San Lorenzo Forest” sic), 09º17′ N, 79º58′ W, May 2004, flight interception trap, A. Tishechkin leg. ( CEMT); 1 ♂, Colón, 2 km S of Sabanitas, 09º19′19″ N, 79º47′54″ W, 25–26 Jun. 1999, UV light, A. Gillogly leg. ( TAMU). – Panamá: 3 ♀♀, Canal Zone, Fort Kobbe, 14 Jun. 1976, E.G. Riley leg. ( TAMU); 9 ♂♂, 4 ♀♀, Canal Zone, Fort Kobbe, 4–21 Jun. 1985, pitfall with human faeces, E.G. Riley leg. ( TAMU); 1 specimen, Canal Zone, Lago Gatún, Barro Colorado Island, K.W. Cooper leg. ( MNHN); 1 ♂, Canal Zone, Lago Gatún, Barro Colorado Island, 22 Feb. 1955, R. Freund leg. ( CNCI); 1 ♀, Canal Zone, Lago Gatún, Barro Colorado Island, 13 Feb. 1960, S. Breeland leg. ( CNCI); 4 ♂♂, 3 ♀♀, Canal Zone, Lago Gatún, Barro Colorado Island, 18 Jul. 1963, L. J. Bottimer leg. ( CNCI); 7 specimens, Canal Zone, Gatún Lake, Barro Colorado Island, Mar. 1975, O.P. Young leg. ( BMNH); 1 ♂, Canal Zone, Gatún Lake, Barro Colorado Island, Jun. 1978 ( CEMT); 2 ♂♂, 1 ♀, Canal Zone, Gatún Lake, Barro Colorado Island, 9 Jul. 1978, dung trap, A. Forsyth leg. ( TAMU); 1 ♂, Canal Zone, Gatún Lake, Barro Colorado Island, 19 Jul. 1978, dung trap, A. Forsyth leg. ( TAMU); 1 ♂, Canal Zone, Gatún Lake, Barro Colorado Island, 16 Aug. 1978, dung trap, A. Forsyth leg. ( TAMU); 4 specimens, Canal Zone, Gatún Lake, Barro Colorado Island, 12–14 May 1981, B. Gill leg. ( BMNH); 1 specimen, Canal Zone, Gatún Lake, Barro Colorado Island, 20–28 May 1981, B. Gill leg. ( BMNH); 1 ♂, 1 ♀, Canal Zone, Río Changena, 21 Sep. 1961, C.E. Yunker leg. ( CNCI); 1 ♂, Canal Zone, Skunk Hollow, 13 Jul. 1975, B.C. Ratcliffe leg. ( ZMHB); 5 specimens, Canal Zone, Skunk Hollow, 6 mi. NW of Gatún Lake, 17–31 May 1980, B.C. Ratcliffe leg. ( BMNH); 4 ♂♂, 1 ♀, same collecting data as for preceding ( ZMHB); 2 ♂♂, Cerro Azul, 12–13 May 1996, Wappes, Huether and Morris leg. ( TAMU); 2 ♀♀, Cerro Azul, 21–24 May 1996, Wappes, Huether and Morris leg. ( TAMU); 1 ♀, El Llano-Carti Road, Km 7.5, 350 m, 4–6 Jun. 1995, flight interception trap, A.R. Gillogly leg. ( TAMU); 1 ♂, 1 ♀, El Llano-Carti Road, Km 8–13, 21–24 May 1996, Wappes, Huether and Morris leg. ( TAMU); 1 ♀, Nusagandi, Ina Igar (Trail), 18–21 May 1993, pitfall with human faeces, E.G. Riley leg. ( CEMT); 19 ♂♂ (1 dissected), 9 ♀♀, same collecting data as for preceding ( TAMU); 1 ♀, Parque nacional Soberanía, 23–27 May 1996, Wappes, Huether and Morris leg. ( TAMU); 1 ♀, Pipeline Road, Km 01–12, 26–30 Jun. 1997, Wappes and Morris leg. ( CEMT); 3 ♂♂, 3 ♀♀, same collecting data as for preceding ( TAMU); 1 ♂, 1 ♀, Pipline Road, Km 6.1, “near Gambba”, 40 m, 7–21 Jun. 1995, J. Ashe and R. Brooks leg. ( MCNZ).
No data: 1 ♂, 1 ♀ ( ISNB, Gillet collection), 3 ♀♀ ( ISNB, J. Thomson collection, pseudotypes).
Redescription
COLOURATION. Entire body surface bright and glossy, including the surface of pygidium. Head, pronotum, elytra, and pygidium usually purple, blue, or, occasionally, green. Metaventrite dark green or dark blue. Meso- and metafemora dark brown and, in general, with greenish reflections.
HEAD. Tegument entirely covered by alveolar microsculpture and without any trace of micropunctation or, occasionally, with very weak, almost imperceptible micropunctures among microsculpture. Clypeus with two apical teeth obtuse and contiguous at base; with a single transverse row of very short setae covering the base of both teeth. Genae with evident tooth immediately behind clypeal-genal juncture. Posterior edge of head with complete margin between eyes.
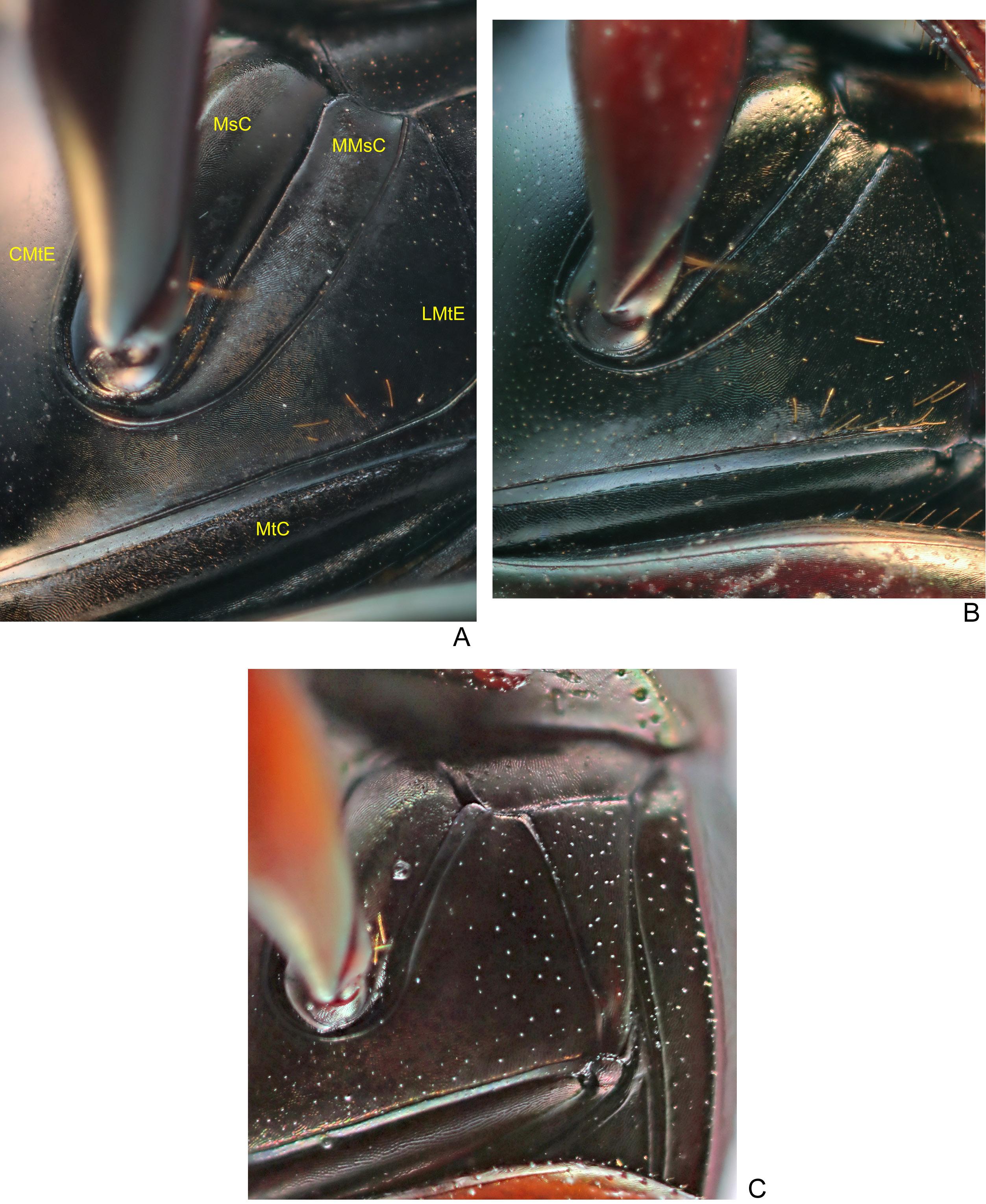
THORAX. Pronotum very shiny and with tegument at centre ranging from completely smooth, with no trace of microsculpture or micropunctation, to with very weak and effaced alveolar microsculpture and micropunctation; on the sides, always with fine alveolar microsculpture. Posterior edge, in general, without transverse line at centre; in some specimens, with traces of fine transverse line at centre. Hypomeral cavity with long setae on posterior region and as long or shorter setae at centre and on external edge; external edge with a turbercle which can be clearly present or almost imperceptible. Metaventrite entirely glabrous (or, occasionally, with few setae at lateral region close to metacoxae, Fig. 7A View Fig ); with uniform alveolar microsculpture throughout ventral region of metaventrite (i.e., between metacoxae) and with very tenuous micropunctation, which, in general, is only visible under high magnification (higher than 35 ×).
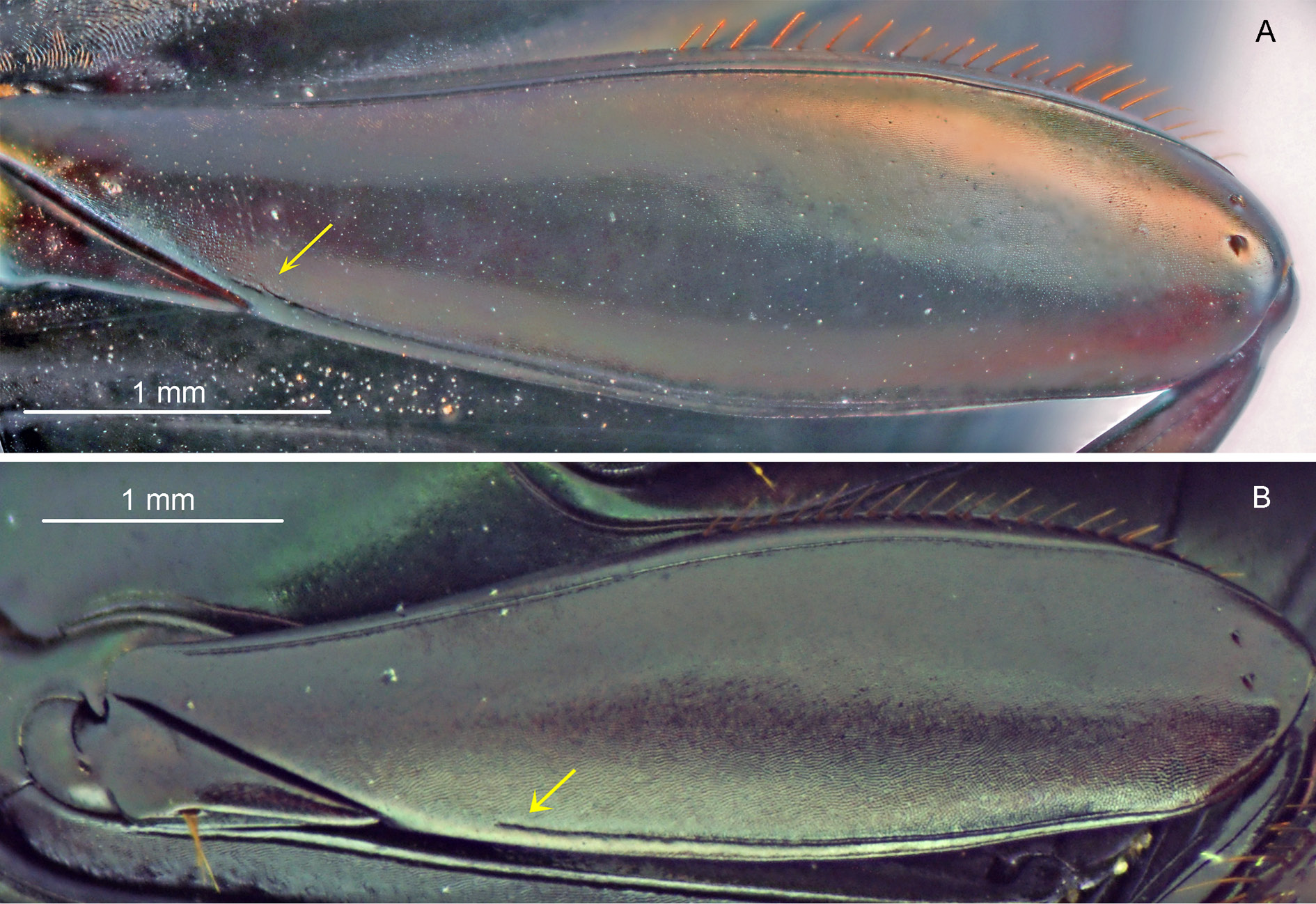
LEGS. Ventral surface of all femora bright and with silky appearance. Profemora with tegument completely covered by alveolar microsculpture. Protibiae broad and without internal expansion; at their apical half, external edge with three medium-sized, broad and acute or obstuse teeth – the two apical much larger than basal ones ( Fig. 11B View Fig ). Mesofemora margined anteriorly only at basal half; unmargined portion of anterior edge with row of very short setae; tegument with rivose microsculpture at external (anterior) half and with alveolar microsculpture at internal (posterior) half. Metafemora with both anterior and posterior margins ( Fig. 31A View Fig ); posterior margin extending from the apex of femur until, at most, little beyond the trochanter; tegument as on mesofemora, with no trace of micropunctation or coarse punctation at base. Metatarsomeres II and V subequal in length and longer than the others; metatarsomere IV shorter than the others.
ELYTRA. With only eight very subtly-marked, fine and superficial striae, all of which without carinulae; first seven striae equally marked (only seventh stria, occasionally, more effaced and discontinuous); eighth stria present only on humerus and subtly carinate (humeral carina). Tegument bright with alveolar microsculpture and micropunctation very subtle and effaced, only visible under magnification of at least 30 ×; microsculpture more evident only on the outer sides.
ABDOMEN. Ventrite V and, especially, ventrite VI smooth at centre and with weaker rivose microsculpture on the sides. Lateral foveae absent in both sexes. Pygidium with shiny tegument and very diffuse effaced rivose microsculpture; of equivalent length in both sexes.
AEDEAGUS. Parameres long, slightly shorter than phallobase, and clearly asymmetrical: left paramere laterally with apical depression more profound and wide than depression on left paramere (difference more easily seen in dorsal view). In lateral view, parameres with central angularity and without ventral keel or notch.
SEXUAL DIMORPHISM. Males: Protibial spur wide and foliaceous, external edge projected into an acute long spine, while internal edge has no prolongation or has a very short spine; area between both spines straight or slightly excavated ( Fig. 15E View Fig ). Ventrite VI strongly narrowed at centre; ventrite V usually without medial flange or only slightly indicated over anterior edge of ventrite VI. Females: Protibial spur spiniform ( Fig. 15F View Fig ). Ventrite VI broad at centre and with anterior edge slightly covered by weak medial flange of ventrite V.
Measurements
Males (N = 20). TL: AV: 10.5 ± 0.93; MX: 12.3; MN: 9.0. EW: AV: 7.1 ± 0.66; MX: 7.8; MN: 5.7. PL: AV: 3.4 ± 0.3; MX: 3.9; MN: 2.7. PW: AV: 6.3 ± 0.83; MX: 7.4; MN: 5.3. PgL: AV: 1.9 ± 0.16; MX: 2.1; MN: 1.6. PgW: AV: 3.4 ± 0.29; MX: 3.7; MN: 2.8.
Females (N = 21). CT: AV: 10.4 ± 0.88; MX: 11.8; MN: 8.7. EW: AV: 7.0 ± 0.51; MX: 7.6; MN: 6. PL: AV: 3.4 ± 0.29; MX: 3.9; MN: 2.9. PW: AV: 6.3 ± 0.47; MX: 5.3; MN: 6.8. PgL: AV: 1.7 ± 0.14; MX: 2.0; MN: 1.5. PgW: AV: 3.3 ± 0.27; MX: 3.7; MN: 2.8.
Geographical distribution
Widespread throughout the tropical forests from Honduras to northern Colombia.
Ecoregions
Central American Atlantic Moist Forests, Central American Montane Forests, Isthmian-Atlantic Moist Forests, Talamancan Montane Forests, Costa Rican Seasonal Moist Forests, Isthmian-Pacific Moist Forests, Chocó-Darién Moist Forests, Guajira-Barranquilla Xeric Scrub, Magdalena-Urabá Moist Forests, Magdalena Valley Montane Forests, Magdalena Valley Dry Forests.
Collecting sites ( Fig. 30 View Fig )
HONDURAS. Atlántida: La Ceiba.
NICARAGUA. Jinotega: Reserva Natural Cerro Kilambé, Santa Maura. Matagalpa: Matiguas, Reserva Natural Biósfera de Bosawas (Macizo de Peñas Blancas). Chontales. Río San Juan: Reserva Biológica Indio Maíz.
COSTA RICA. Guanacaste: Cerro El Hacha, Volcán Rincón de la Vieja. Alajuela: Playuelas, San Ramón de dos Ríos, Volcán Tenorio (Colonia Río Celeste). Heredia: Sarapiquí (Estación Biológica La Selva). Puntarenas: Golfitos (Las Torres), Osa (Albergue Ecoturístico Cerro de Oro), Parque Nacional Corcovado (Estación Biológica Sirena) , Reserva Biológica Bosque Nuboso Monteverde. San José: Santa Ana (Ciudad Colon), Turrúcares. Limón: Guácimo (Parque Nacional Tortuguero), Limón (Río Blanco), Pococí (Cedrales de la Rita, Cerro Cocorí, Refugio Nacional de Fauna Silvestre Barra del Colorado), Refugio de Vida Silvestre Gandoca-Manzanillo (Manzanillo), Talamanca (Bratsi: Amubri).
PANAMA. Chiriquí: Barú (Progreso), David (San José de David), Gualaca (Hornito), Colón: Chagres (Fort San Lorenzo), Colón. Panamá: Panama Canal (Fort Kobbe; Lago Gatún: Isla Barro Colorado; Skunk Hollow), Cerro Azul, Nusagandi, Parque Nacional Soberanía.
COLOMBIA: Atlántico: Juan de Acosta (Reserva de Tierra Arena). Cesar: Chimichagua. Santander: Serranía de las Quinchas (Reserva El Paujil). Antioquia: San Luis (Sonsón). Chocó:Acandí (Capurganá), Lloró, Parque Nacional Natural Ensenada de Utría, Quibdó (Tutunendó, Estación Ambiental Pandó, Pacurita), Unión Panamericana (Salero). Caldas: Norcasia (Reserva Natural Río Manso). Valle del Cauca : Estación Agro-Forestal Bajo Calima, Dagua. Cundinamarca. Tolima.
Intraspecific variation and taxonomic discussion
Despite its extensive geographical distribution and the large number of specimens studied for this work, limited morphological variation was observed in S. aequinoctialis apart from colouration and size 19. The most noticeable variation is related to the sculpture at the centre of the pronotum, which can be almost completely smooth, without any trace of alveolar microsculpture or micropunctation, in some specimens, or have microsculpture and micropunctation present, but very fine and effaced, in others. It is interesting to note that in its sister species, S. proseni , the degree to which the pronotal micropunctation is marked varies intrapopulationally at a much stronger intensity. A detailed comparison between S. aequinoctialis and S. proseni is present in the taxonomic discussion of the latter species.
Comments
The earliest references found in the literature on the species we now know as S. aequinoctialis are the records by Dejean (1833, 1836) of the presence of “ Coprobius aequinoctialis ”, from “Carthagena”, in his beetle collection. As for other nominal species firstly established in the catalogues of Dejean, this name is unavailable as it was not published along with a description or indication and, therefore, it is out of the availability criteria adopted by the Code (Articles 11 and 12). Only thirty years later, Harold (1868a), in his revision of Canthon , at last described the species, now with the name Canthon aequinoctialis , from “Columbien, Neu-Granada”.
There is no doubt that the two specimens here recognized as part of the type series of S. aequinoctialis were indeed described by Harold (1868a). The now-lectotype, besides having originated from Harold’s personal collection (nowadays deposited at the MNHN), bears a label with the information “ aequinoctialis / t. Har ” in Harold’s own handwriting ( Fig. 29A View Fig ). The paralectotype, in turn, housed at the ZMHB, has an old drawer label written “ aequinoctialis / Harold* / Nov. Granada ” ( Fig. 29B View Fig ). According to Joachim Willers (personal communication to MC, 2015), curator at ZMHB, the asterisk following the author’s name indicates that the collection has at least one type specimen of that nominal species which, in this case, should be the female here recognized as paralectotype (and it is worth noting that Harold (1868a: 10) indeed made it clear he had examined specimens from the ZMHB for his revision of Canthon ). The choice which of the two known syntypes should be designated as lectotype was not a simple one: while the female is in perfect condition, the male is significantly damaged around the pin, although no known diagnostic characters of the species have been lost. On the other hand, the male belongs to the Edgar von Harold collection, has a label handwritten by the nominal species’ author and, the most important fact, it is of the same sex as all the other name-bearing types in Sylvicanthon (i.e., holotypes and lectotypes). Therefore, the male syntype was chosen instead of the female to be here designated as lectotype.
As S. aequinoctialis was for a long time confused with S. proseni , much of what is published under the former name actually refers to the latter species (see the discussion of S. proseni for more details). In particular, the geographical distribution cited in the literature for S. aequinoctialis suffered from this problem. In general, the species is cited as occurring in large parts of Central America and throughout the Amazon region, including Brazil, Peru and Bolivia. In reality, however, in South America , S. aequinoctialis is present only in the portion west of the Cordillera Oriental 20, in the Colombian Andes, specifically on the Caribbean coast (department of Atlántico), in the Chocó biogeographical region (maybe also present in Ecuador, although not recorded from there yet) and in the valleys between the Central and Oriental Cordilleras, in places such as Tolima and Caldas. In Central America , the species occurs at least from Panama northwards to Honduras ( Fig. 30 View Fig ). Howden & Young (1981), Kohlmann & Solís (2002) and Halffter (2003), without citing their primary sources, said that S. aequinoctialis was also present in Belize, but this information is probably incorrect, since we could not find any specimens with this provenance in none of the studied collections (including the former private collection of Henry Howden, today housed at the CMNC; François Génier, personal communication to MC, 2015), nor researchers having recently collected in that country found the species living there (Latha Thomas, University of Belize, personal communication to MC, 2015). Halffter (2003) added that this species occurred in Guatemala and most probably also in southern Mexico, but these records were equally unsupported by more detailed information.
Natural history
In contrast to the other species of Sylvicanthon , of which only fragmentary biological information has been published, it is possible to find far more detailed data about S. aequinoctialis in the literature. Howden & Young (1981), for example, in their important monograph on the Panamanian dung beetles, described several aspects of the life of this species based on populations from the island of Barro Colorado, in the Gatun Lake of the Panama Canal. According to the authors, S. aequinoctialis breeds throughout the year and is one of the most abundant dung beetle species on the island, not showing any seasonal variation in its population density. During the rainy season (May to December), they feed on both dung and carrion, with the consumption of carcasses of animals such as the agouti [ Dasyprocta punctata (Gray, 1842) ], collared peccary [ Pecari tajacu (Linnaeus, 1758]), and rat, as well as human faeces and coati [ Nasua nasua (Linnaeus, 1758) ], jaguarundi [ Puma yagouaroundi (Geoffroy Saint-Hilaire, 1803) ] and tapir [ Tapirus bairdii (Gill, 1865) ] dung, being reported. During the dry season (December to May), however, when carcasses are scarcer and consumed mainly by vertebrates or specialist necrophagous dung beetles ( Young 1978, 1980), S. aequinoctialis is found only on dung. Regarding their circadian cycle, S. aequinoctialis , as the other species in the genus, is crepuscular and nocturnal, with an activity period between 5 and 9 pm (Howden & Young 1980). In Barro Colorado, S. aequinoctialis is the most abundant roller dung beetle, having been responsible for the consumption of at least one-fourth of the total volume of tapir dung utilized in experiments by Young (2009) on the island.
Farther north, we have information about some populations of S. aequinoctialis from Costa Rica given by Kohlmann & Solís (2002). Like in Panama, Costa Rican adults of S. aequinoctialis from both sides of the Continental Divide (i.e., both on the Caribbean and the Pacific coasts of Costa Rica) are active throughout the year and are among the most abundant nocturnal dung beetles of humid lowland forests. There, they live up to 800 m altitude on the Atlantic side of the Continental Divide, and up to 900 m on the Pacific side. Specimens were collected on human and equine dung, as well as using flight interception traps and through active collections on the ground or beneath fallen tree trunks.
Lastly, we have data for populations in Colombia. Medina et al. (2012) also highlighted S. aequinoctialis as one of the most common species in the Magdalena River Valley region at an altitude between 180 and 300 m, and stated that the species was collected with traps baited with human faeces and cow dung, mushrooms and, occasionally, with rotten fish. Additionally, Solís et al. (2011) stated that this species was present in the Atlántico department in altitudes between 160 and 500 m.
Several of the specimens examined also have bionomic information on their labels. There are records of specimens collected using traps baited with swine, human and howler monkey [ Alouatta seniculus (Linnaeus, 1758) ] dung, as well as rotten flesh, and also in flight interception traps and with ultraviolet light. Specimens were collected in all the months of the year, but there is an apparent higher concentration during the rainiest months (March: 49; April: 87; May: 52; June: 76; July: 63), when 327 out of the 573 studied specimens were caught. In relation to the altitudinal amplitude, there are representatives of S. aequinoctialis collected from at approximately the sea level up to areas as elevated as 1000 m in the Reserva Natural Cerro Kilambé (Jinotega, Nicaragua) and 1440 m in San Luis (Antioquia, Colombia). Therefore, after combining all the available data, what we see is that S. aequinoctialis is, throughout its distribution, an opportunistic species that explores several food sources and habitats and that is resistant to the seasonal changes, characteristics that make it one of the most abundant and dominant species among the roller Scarabaeinae of the New World tropical forests.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Scarabaeinae |
|
Tribe |
Deltochilini |
|
Genus |
Sylvicanthon aequinoctialis (Harold, 1868)
| Cupello, Mario & Vaz-De, Fernando Z. 2018 |
Canthon cf. aequinoctialis
| Martinez N. J. & Canas L. M. & Rangel J. L. & Barraza J. M. & Montes J. M. & Blanco O. R. 2010: 24 |
| Noriega J. A. & Solis C. & Escobar F. & Realpe E. 2007: 81 |
Canthon (Canthon) aequinoctialis
| Noriega J. A. & Realpe E. & Fagua G. 2007: 54 |
| Halffter G. & Martinez A. 1977: 62 |
Canthon (Glaphyrocanthon) aequinoctialis
| Howden H. F. 1966: 730 |
Glaphyrocanthon (Glaphyrocanthon) aequinotialis
| Vulcano M. A. & Pereira F. S. 1964: 661 |
Glaphyrocanthon (Glaphyrocanthon) aequinoctialis
| Vulcano M. A. & Pereira F. S. 1967: 561 |
| Martinez A. & Halffter G. & Halffter V. 1964: 3 |
| Martinez A. & Pereira F. S. 1956: 126 |
Canthon aequinoctiale
| Young O. P. 2009: 322 |
| Vaz-de-Mello F. Z. 1999: 449 |
| Vaz-de-Mello F. Z. & Louzada J. N. C. 1997: 56 |
| Howden H. F. & Young O. P. 1981: 20 |
| Blackwelder R. E. 1973: 2 |
| Gacharna G. C. 1951: 221 |
| Blackwelder R. E. 1944: 198 |
Canthon aequinoctialis
| Ratcliffe B. C. & Jameson M. L. & Figueroa L. & Cave R. D. & Paulsen M. J. & Cano E. B. & Beza-Beza C. & Jimenez-Ferbans L. & Reyes-Castillo P. 2015: 195 |
| Krajcik M. 2012: 63 |
| Noriega J. A. 2012: 4 |
| Figueroa L. & Alvarado M. 2011: 210 |
| Solis C. & Noriega J. A. & Herrera G. 2011: 36 |
| Arango L. & Montes J. M. 2010: 262 |
| Carpio C. & Donoso D. A. & Ramon G. & Dangles O. 2009: 462 |
| Medina C. A. & Pulido L. A. 2009: 58 |
| Noriega J. A. & Cubillos A. M. & Castaneda C. & Sanchez A. M. 2008: 78 |
| Kohlmann B. & Solis A. & Elle O. & Soto X. & Russo R. 2007: 9 |
| Celi J. & Terneus E. & Torres J. & Ortega M. 2004: 42 |
| Noriega-Alvarado J. A. 2004: 40 |
| Halffter G. 2003: 43 |
| Ratcliffe B. C. 2002: 12 |
| Solis A. & Kohlmann B. 2002: 1 |
| Medina C. A. & Lopera-Toro A. & Vitolo A. & Gill B. 2001: 135 |
| Escobar F. 2000: 206 |
| Gill B. D. 1991: 225 |
| Vulcano M. A. & Pereira F. S. 1967: 561 |
| Martinez A. 1949: 290 |
| Balthasar V. 1941: 341 |
| Balthasar V. 1939: 187 |
| Schmidt A. 1922: 64 |
| Gillet J. J. E. 1911: 27 |
| Bates H. W. 1887: 33 |
| Harold E. 1880: 16 |
| Harold E. 1869: 989 |
Canthon aequinoctialis
| Harold E. 1868: 2 |
Coprobius aequinoctialis
| Harold E. 1868: 79 |
Coprobius aequinoctialis
| Dejean P. F. M. A. 1833: 136 |
Canthon (Canthon) aequinoctiale
| Vaz-de-Mello 2000 |
Canthon (Canthon) aequinotialis
| Culot et al. 2011 |