Chaetopterus bruneli, Moore & Gagnon & Petersen, 2020
|
publication ID |
https://doi.org/ 10.5852/ejt.2020.720.1111 |
|
publication LSID |
lsid:zoobank.org:pub:A1B44ABD-A7B7-40EF-A036-A0EBBC1FF360 |
|
DOI |
https://doi.org/10.5281/zenodo.4324047 |
|
persistent identifier |
https://treatment.plazi.org/id/A18C3F2E-B79B-4617-9E06-E1C665C9729A |
|
taxon LSID |
lsid:zoobank.org:act:A18C3F2E-B79B-4617-9E06-E1C665C9729A |
|
treatment provided by |
Valdenar |
|
scientific name |
Chaetopterus bruneli |
| status |
sp. nov. |
Chaetopterus bruneli View in CoL sp. nov.
urn:lsid:zoobank.org:act:A18C3F2E-B79B-4617-9E06-E1C665C9729A
Diagnosis
Small, epifaunal Chaetopterus inhabiting a pale cream to tan, laminated, crescent-shaped tube. Segmental distribution 9A+5B+4–6C. Neuropodia absent in region A. Parapodia of B1 not posteriorly displaced from segment A-last. Segment B2 short, dorsal surface not fleshy. B1 and B2 neuropodia with four completely unfused, discrete lobes. With 3–8 small, light brown cutting notochaetae on segment A4, in an inconspicuous ventral fascicle. Uncini tooth distribution as follows: A9 neuropodia absent, B1 anterior lobe with 6–8 teeth, B1 posterior lobe with 5–7, B3 piston tori with 5–6, B3 ventral lobes with 5 teeth, C1 lateral lobes with 6–7, C1 ventral lobes with 8–10 teeth.
Etymology
This species is named in honour of Dr. Pierre Brunel, Université de Montréal, who has made a significant contribution to the study of deep-water benthic invertebrate communities in the Estuary and Gulf of St. Lawrence (EGSL) during his career. The specific epithet is a noun in the genitive case.
Material examined
Holotype
CANADA • St. Lawrence Estuary , epifaunal on surface of muddy sediment; 48.7033º N, 68.5663º W; depth 350 m; 13 May 1992, Jean-Marc Gagnon leg.; CMNA 2015-0016 . GoogleMaps
Paratypes
CANADA • 10 specs; same collection data as for holotype; CMNA 2015-0001 to CMNA 2015-0004 , CMNA 2015-0011 to CMNA 2015-0015 , CMNA 2015-0017 GoogleMaps • 3 specs; same collection data as for holotype; 1 Nov. 1990; CMNA 2015-0005 to CMNA 2015-0007 GoogleMaps • 3 specs; same collection data as for holotype; 16 May 1991; CMNA 2015-0008 to CMNA 2015-0010 GoogleMaps • 1 spec.; same collection data as for holotype; 15 Oct. 1993; CMNA 2015-0018 . All type specimens and associated slide preparations are deposited in the Canadian Museum of Nature GoogleMaps .
Description (holotype)
Mean and range values are given in parentheses for the holotype and six paratypes. Uncini data are based on paratype specimen CMNA 2015-0018, and their tooth counts and measurements are given in Table 1. View Table 1
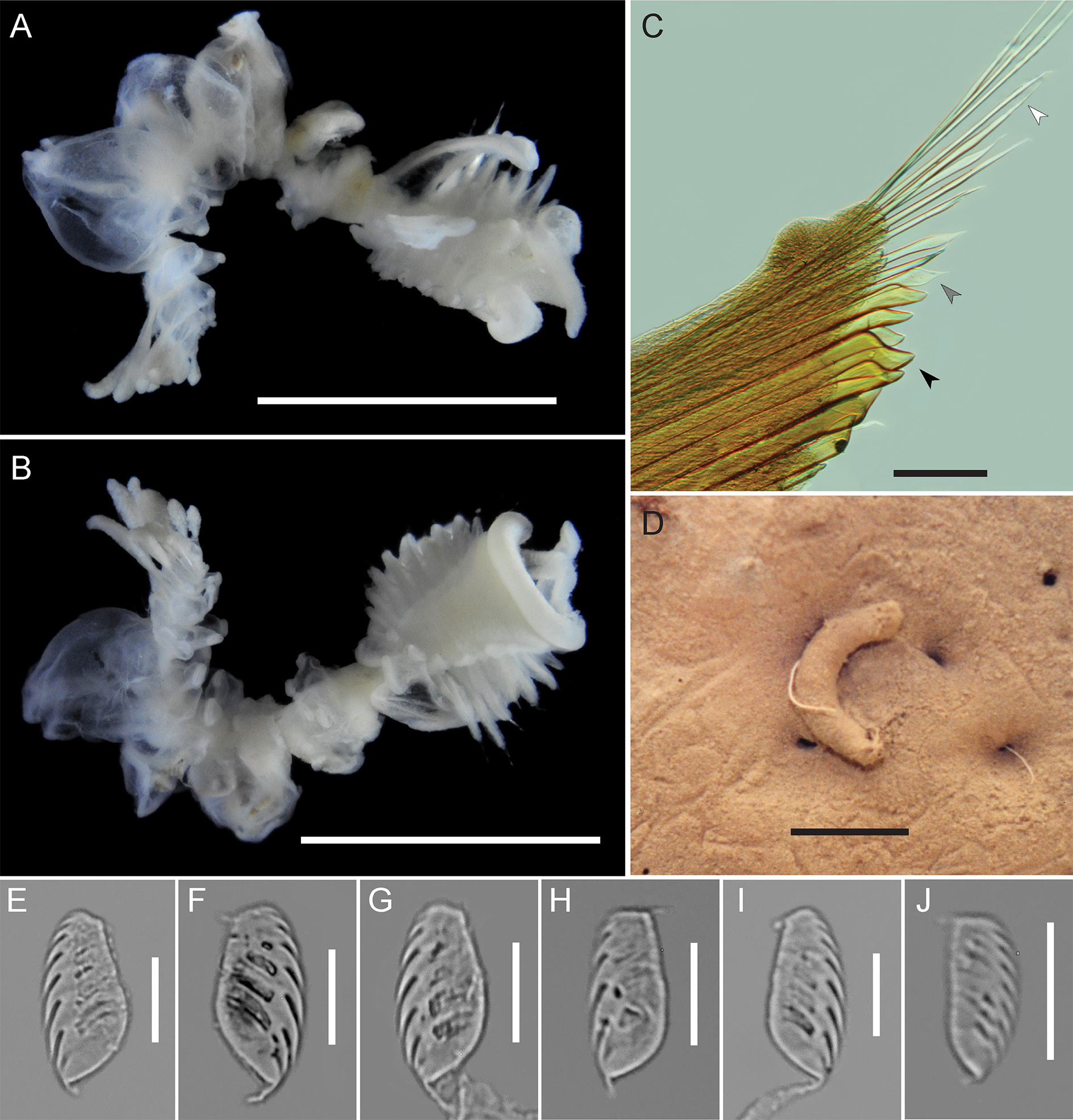
GROSS MORPHOLOGY AND PRESEGMENTAL STRUCTURES. Small, epibenthic Chaetopterus , 10.5 mm total body length for 19 chaetigers (mean 11.6 mm, range 8.0–15.0 mm, n = 7); segmental formula 9–10A+5B+5– 6C. Peristomium ventrally narrow, horseshoe-shaped in dorsal view, with narrow dorsolateral lobes that sometimes obscure A1 notopodia, without pigment in preserved specimens. Grooved palps short and stout, inserted at dorsal inner margins of peristomium, 2.1 mm in length (mean 2.1 mm, range 2.0– 2.5 mm). Eyes absent.
REGION A. Anterior region with 9 segments ( Fig. 2A View Fig ; 10 chaetigers on one side in paratype CMNA 2015-0005); 3.0 mm in length (mean 3.2 mm, range 2.0–4.0 mm, n = 7) and 4.0 mm in width (mean 4.5 mm, range 3.5–5.5 mm, n = 7), wider than long. Ventral surface of A with broad, triangular glandular shield, widest anteriorly and narrowing posteriorly to A9 ( Fig. 2B View Fig ). Region A notopodia shortest at A1 or A9, longest at A5 or A6 and decreasing in length again to segment A9; A4 notopodia shorter than neighboring notopodia. Small swellings not visible at dorsal base of A notopodia. Segment A4 notopodia bearing 3–8 relatively small, apically blunt translucent yellow cutting chaetae, with a distinct ventral tooth, on the ventral side of the notopodia ( Fig. 2C View Fig ). Other notopodia and the distal side of A4 notopodia bear lanceolate and simple chaetae ( Fig. 2C View Fig ). Region A chaetigers uniramous, A9 without neuropodia or uncini ( Figs 2B View Fig , 3B View Fig ).
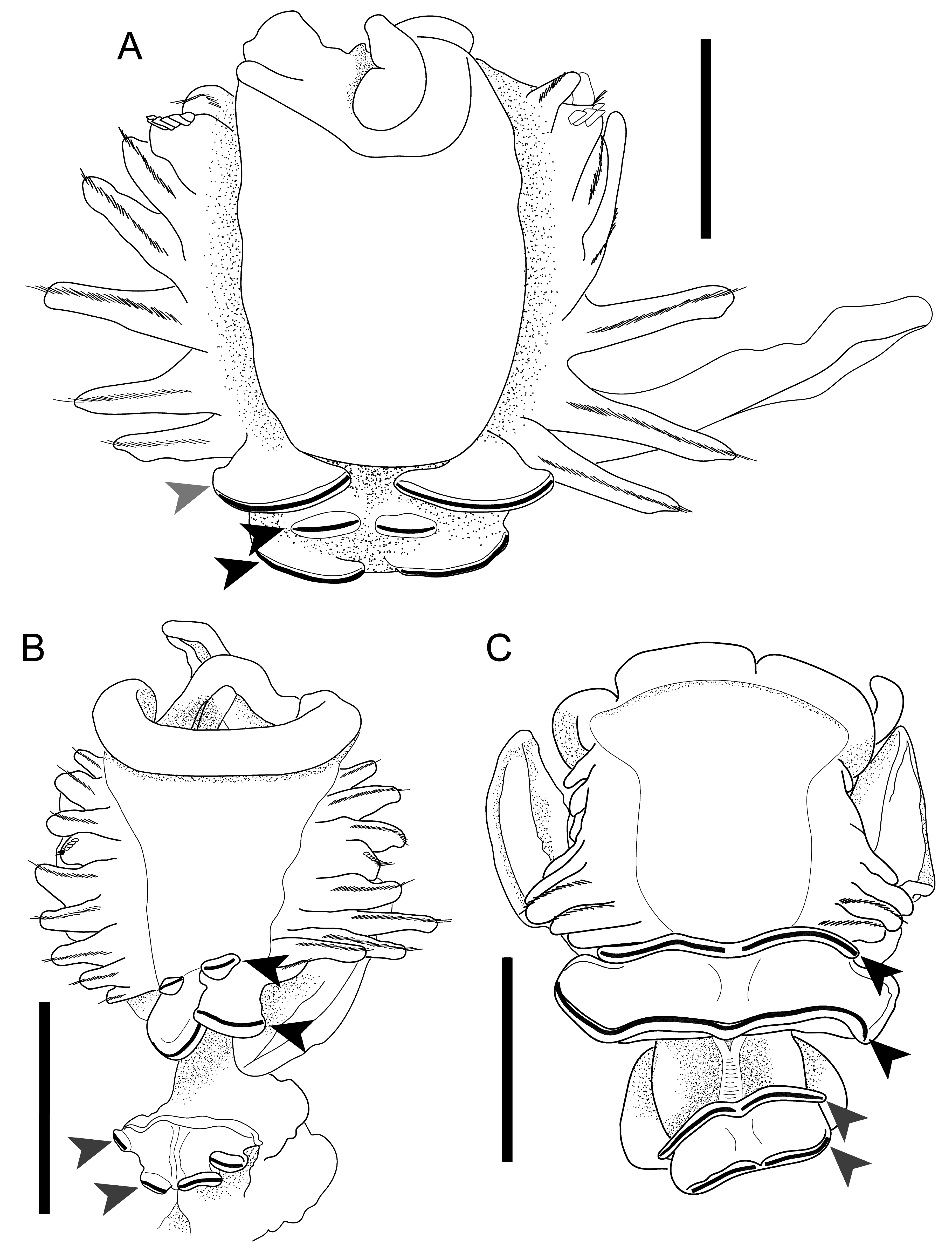
REGION B. Middle body region 6 mm in length (mean 5.9 mm, range 4.5–7.0 mm, n = 7). B1 with long aliform notopodia, approximately the length of region A (2.0–4.0 mm), reaching anteriorly to the peristomium ( Fig. 2A View Fig ). B1 and B2 neuropodia bilobed, with four completely unfused, discrete lobes, with uncini on distal lobe margins ( Figs 2B View Fig , 3B View Fig ). Anterior pair of neuropodial lobes in B1 narrow and situated medially ( Figs 2B View Fig , 3B View Fig ); uncini pyriform to D-shaped, apically rounded and pointed or rounded at base, widest slightly below middle ( Fig. 2E View Fig ). Posterior neuropodial lobes of B1 broader than anterior lobes and situated laterally ( Figs 2B View Fig , 3B View Fig ), uncini ellipsoid to D-shaped, apically blunt and rounded and pointed at base, widest at middle or slightly below ( Fig. 2F View Fig ). Segment B2 short relative to total body length; neuropodia bilobed with four completely unfused, discrete lobes; anterior pair of lobes situated more laterally than posterior pair of lobes ( Fig. 2B View Fig ). Neuropodia of B3–B5 each with a single pair of well-developed, unfused lobes, bearing uncini on posterior margins. Uncini of B3 piston tori ellipsoid to pyriform, apically rounded and rounded or pointed at base, widest at middle, proximal margin sometimes with a medial notch ( Fig. 2G View Fig ). B3 ventral lobe uncini ellipsoid to pyriform, apically blunt and pointed at base, widest at middle or slightly below, proximal margin with a medial notch ( Fig. 2H View Fig ).
REGION C. Posterior body region 1.5 mm in length (range 1.5–4.0 mm, n = 7), with 4–6 chaetigers. Region C notopodia long and club-shaped, constricted at the base and widening distally, with internal simple notochaetae ( Fig. 2 View Fig A–B); C1 notopodia 2.0 mm in length (mean 3.0 mm, range 2.0–4.0 mm, n = 7). Neuropodia bilobed, ventral lobes medially fused after C1 by a thin membrane, C1 ventral neuropodial lobes broader than those in succeeding segments; lateral lobes of C neuropodia lacking dorsal and ventral cirri. Both neuropodial lobes bear a row of uncini on distal margin. C1 lateral lobe uncini asymmetrically pyriform to ellipsoid, apically rounded and pointed at base, widest at middle or slightly below, with curved proximal margin ( Fig. 2I View Fig ). C1 ventral lobe uncini long ellipsoid to D-shaped, apically blunt and pointed at base, widest at middle or slightly above ( Fig. 2J View Fig ).
TUBE. Tube short and curved, pale cream to tan, thin and composed of laminated proteinaceous material, externally covered in mud ( Fig. 2D View Fig ).
Ecology and distribution
The new species is known only from the type locality in the lower St. Lawrence Estuary, epibenthic on fine sediments at a depth of 350 m. Tubes resembling those of this species were also observed on bottom photographs from stations 18 and 20, at 390 and 331 m depth, respectively ( Belley et al. 2010).
In the late spring to early autumn of 1990 and 1991, several physico-chemical and biological measures were studied near the type locality of C. bruneli sp. nov. in the lower St. Lawrence Estuary (Station 26 in Savenkoff et al. 1994). These measurements were collected around the same time as some of the type specimens presented here. At that time, the type locality was in an upwelling area of the Laurentian trough, characterized by moderate productivity, low vertical stratification, higher salinity, and lower temperatures compared to the more productive plume region downstream ( Savenkoff et al. 1994). The bottom water in the lower St. Lawrence Estuary is now being reported as increasingly and persistently hypoxic in recent years ( Belley et al. 2010; Gilbert et al. 2005), which may explain why attempts to recollect this species from the type locality in September 2015 and August 2020 proved unsuccessful. Future benthic surveys of the St. Lawrence Estuary and Gulf of St. Lawrence may provide revised information about the distribution of this species.
Remarks
Chaetopterus bruneli sp. nov. is likely closely related to C. norvegicus . A summary of morphological features for both species is given in Tables 1 View Table 1 and 2. These species are unusual among described species of Chaetopterus for having unfused neuropodial lobes in segments B1 and B2 (see Discussion). Chaetopterus bruneli sp. nov. differs from C. norvegicus by its smaller body size, its lack of neuropodia in tagma A, and by its club-shaped notopodia and fewer chaetigers in tagma C. Chaetopterus bruneli sp. nov. builds mud-covered tubes on the surfaces of fine sediments at upper continental slope depths, whereas C. norvegicus is found in rocky areas on hard substrates at continental shelf depths, with little sediment on the external surface of the tubes.
Some characters distinguishing these species may vary ontogenetically in Chaetopterus , including the body size and the number of segments in tagma C. There is, however, evidence from studies on the development of Chaetopterus that the neuropodia in the anterior tagma are present one day postmetamorphosis in a species that bears these structures as adults ( Irvine et al. 1999: fig. 8b). Thus, the absence of neuropodia in tagma A should be reliable for distinguishing post-settlement individuals. While no mature gonads were observed in the preserved material of C. bruneli sp. nov., long-term preservation in alcohol can sometimes make distinguishing fat deposits from gametes difficult in specimens of Chaetopterus . The specimens of C. bruneli sp. nov. described here were collected over several seasons over two years and do not vary substantially in their morphology, nor in size. Genetic evidence is not available, as the specimens were preserved in formalin and no new material is available for tissue collection. The morphological and ecological differences between these species outlined above are substantial enough to provide sufficient grounds for the establishment of a new species.
Only one other described species of Chaetopterus , C. longipes Crossland, 1904 , lacks neuropodia in tagma A ( Fig. 3C View Fig ). Chaetopterus longipes was originally described from the Maldive Archipelago and was redescribed from Japanese material ( Nishi 1996). Phylogenetic analyses suggest that C. longipes may represent a species complex distributed in both the tropical Indo-Pacific and Caribbean ( Moore et al. 2017). Like C. norvegicus , C. longipes builds epibenthic tubes; these, however, occur gregariously and are embedded within elevated crevices on rocky coral reef substrates or found attached to the undersides of rocks rather than on the surface of soft sediments ( Nishi 1996, 2001). Neither C. bruneli sp. nov. nor C. norvegicus are known to occur gregariously. C. bruneli sp. nov. lacks the fused, suckerlike neuropodia of segments B1 and B2 that are present in C. longipes and in most other species of Chaetopterus ( Fig. 3 View Fig ). There are no records of any Chaetopterus species lacking neuropodia in tagma A north of 33º N latitude. The morphological and ecological differences among these species warrant the establishment of a distinct taxon, C. bruneli sp. nov.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.