Penthetria Meigen
|
publication ID |
https://doi.org/10.11646/zootaxa.4926.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:3ADD4A2B-A3F9-4379-A8FE-39DD867531F7 |
|
DOI |
https://doi.org/10.5281/zenodo.4558063 |
|
persistent identifier |
https://treatment.plazi.org/id/A157FD05-FFBF-4B4C-FF2F-FBDFFC0F2236 |
|
treatment provided by |
Plazi |
|
scientific name |
Penthetria Meigen |
| status |
|
Genus Penthetria Meigen View in CoL View at ENA
Amasia Meigen, 1800: 20 . Unavailable name; work suppressed for purposes of zoological nomenclature by I.C.Z.N., 1963: 339.
Penthetria Meigen, 1803: 264 View in CoL . Type species: Penthetria funebris Meigen, 1804: 104 View in CoL (by monotypy in Meigen 1804: 104).
Threneste Wiedemann, 1830: 618 . Nomen nudum.
Eupeitenus Macquart, 1838: 88 (also 1838: 84). Type species: Penthetria atra Macquart, 1834: 175 View in CoL (by monotypy), [examined; BMNH].
Crapitula Gimmerthal, 1845: 330 . Type species: Crapitula motschulskii Gimmerthal 1845: 330 View in CoL (by monotypy).
* Bibiopsis Heer, 1849: 228 . Type species: Bibiopsis cimicoides Heer, 1849: 229 View in CoL (designated by Carpenter 1992: 414).
* Mycetophaetus Scudder, 1892: 20 . Type species: Mycetophaetus intermedius Scudder, 1892: 20 (by monotypy), [examined; MCZC]. [synonymized by Fitzgerald 1999].
Amasia Meigen in Hendel, 1908: 50. Type species: Penthetria funebris Meigen View in CoL as “ Amasia funebris (Meig.) ,” by subsequent monotypy in Bezzi, 1911: 39. [synonymized by Evenhuis & Pape 2017].
Pleciomyia Brunetti, 1911: 269 . Type species: Penthetria melanaspis Wiedemann, 1828: 72 View in CoL (by monotypy).
Parapleciomyia Brunetti, 1912: 446 . Type species: Parapleciomyia carbonaria Brunetti, 1912: 447 View in CoL (by monotypy).
Nomenclatural notes. The genus Bibiopsis Heer , based on a fossil, has historically been treated as a junior synonym of Penthetria ( Loew 1868) , as a distinct genus ( Scudder 1891), and as a junior synomym of a broadly-defined Penthetria (including Plecia Wiedeman and Protomyia Heer ; Handlirsch 1907). More recently, Evenhuis (1994, 2014) treated Bibiopsis as a junior synonym of Plecia . However, Heer’s (1849; Plate XV, Fig. 24b View FIGURES 24–29 ) illustration depicts a stout-bodied fly with R 2+3 elongate and subparallel to R 4+5, rather than short and angled, which indicates that this taxon belongs to the genus Penthetria .
Generic Diagnosis. The following combination of characters will distinguish adults of extant members of the genus Penthetria from other Bibionidae : Fore tibia simple (without apical mucron or spines), R 2+3 elongate and subparallel to R 4+5, and antennal flagellomeres short, compact.
The larva of Penthetria ( Fig. 58 View FIGURES 58–60 ) can be distinguished from the larva of other bibionids by the combination of the following characters: presence of fleshy tubercles (each abdominal segment with two transverse rows of two tubercles each), posterior spiracle on segment eight, posterior spiracle with a single ecdysial scar, and the mentum not fused to the anterior margin of the cranium or to the posterior margin of the labium (Fitzgerald 2004, Fig. 36b; pm). Pupae ( Figs. 59–60 View FIGURES 58–60 ) can be distinguished from all other bibionid genera except Plecia by the presence of fleshy tubercles.
Discussion supporting generic diagnosis. The generic diagnosis presented above is based on previous phylogenetic and descriptive work on the family Bibionidae (Fitzgerald 2004) and readers are referred there for further information concerning material examined or the broader context from which the diagnosis was developed. The diagnosis is designed for extant species, but reference to problems and exceptions when diagnosing fossil species are provided below. Additionally, characters not necessarily considered diagnostic, but that may be useful in helping to distinguish the genus, are also discussed.
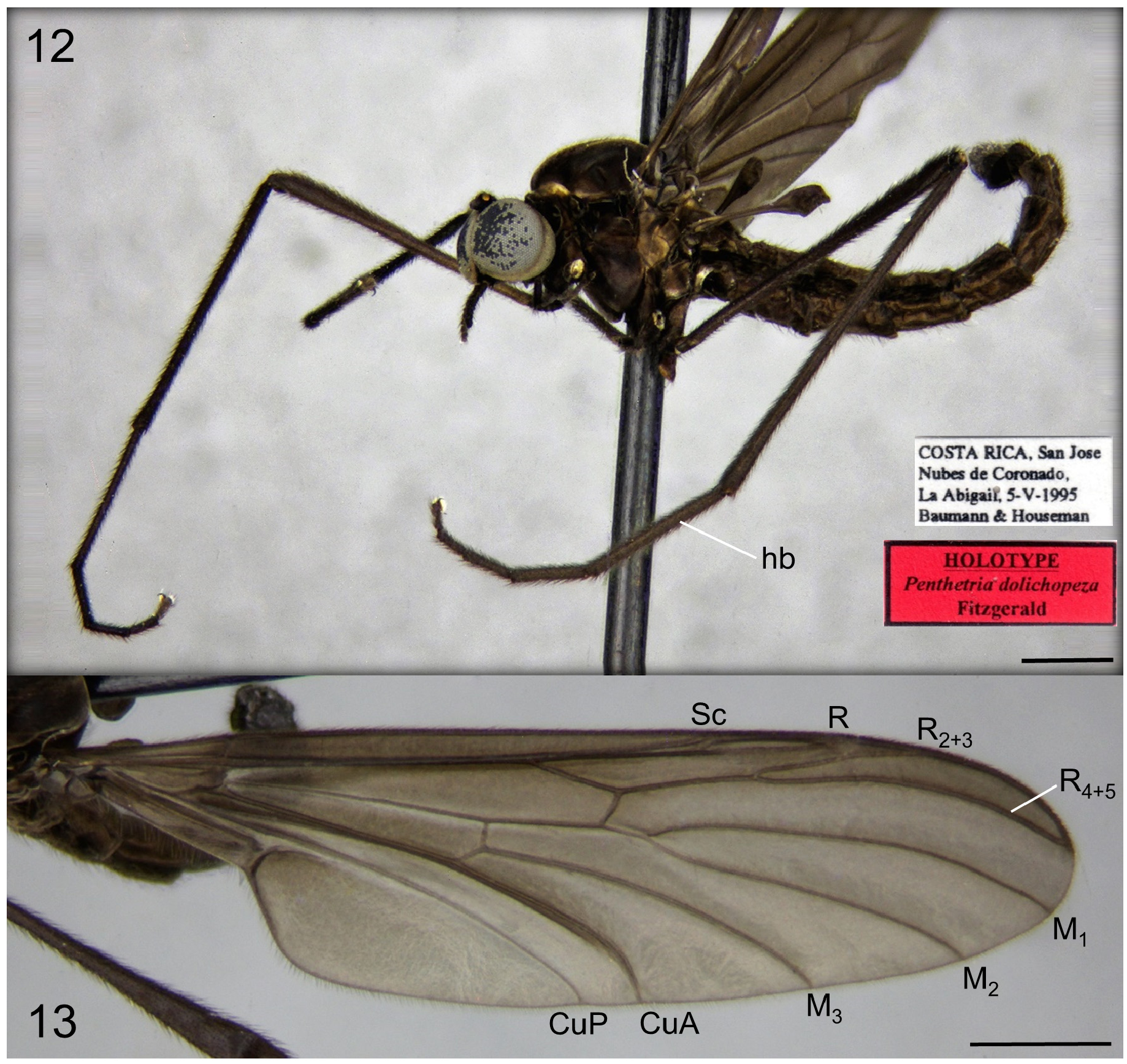
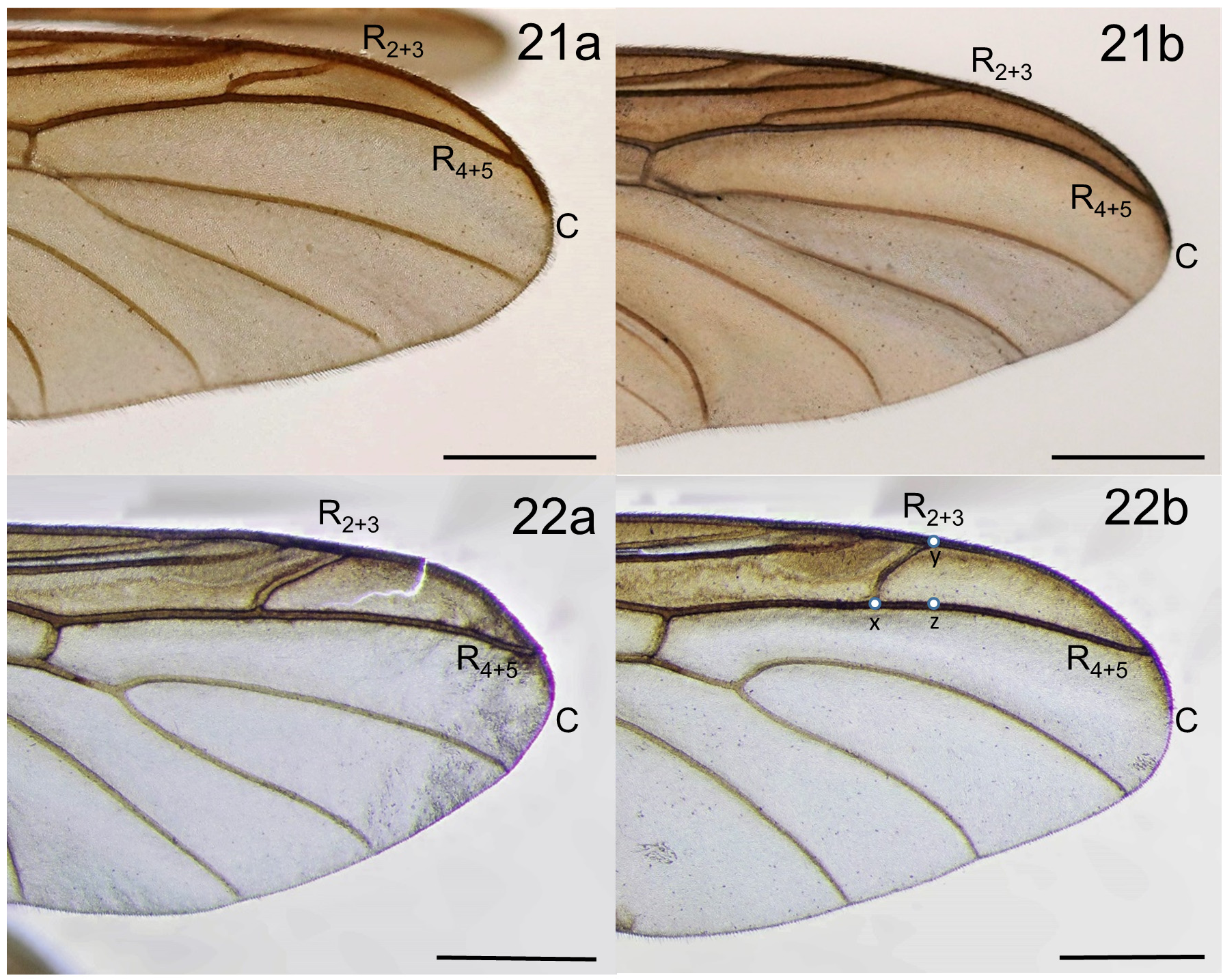
The relatively large adults of Penthetria are usually matte-black, with dark wings. They are sometimes confused with some of the large species of tropical Sciaridae , but are easily distinguished from them by the absence of a narrow eye bridge and the presence of vein R 2+3: in Penthetria males are holoptic and females broadly dichoptic ( Fig. 1 View FIGURE 1 ) (though the odd Palearctic species, P. funebris Meigen , has males with eyes that do not quite meet dorsomedially). Adults of Penthetria are distinguished from other Bibionidae by the apically simple fore tibia ( Bibioninae all have apex of fore tibia modified into a long mucron or a circlet of shorter spines), relatively short antennae with compact antennal flagellomeres ( Hesperinus with flagellomeres elongate), and vein R 2+3 elongate and subparallel to R 4+5 (e.g., Figs. 2 View FIGURES 2–3 , 13 View FIGURES 12–13 , 21b View FIGURES 21–22 , 23 View FIGURE 23 ) (R 2+3 absent in Bibioninae ). The elongate R 2+3 subparallel to R 4+5 is the character typically employed in keys for distinguishing Penthetria from the similar genus Plecia , which has R 2+3 shorter and more oblique or vertical ( Fig. 22 View FIGURES 21–22 ). Despite the fact that some species/specimens of Penthetria have R 2+3 approaching the condition found in Plecia (e.g., compare Figs. 21a & 22a View FIGURES 21–22 ) and that the form of R 2+3 is essentially evaluated by gestalt rather than any discrete measurement, the extant species can typically be distinguished in this way with confidence.
The use of R 2+ 3 in distinguishing these genera becomes more problematic with fossils, where there is a continuum in the form of R 2+3 between typical Penthetria and Plecia ( Collomb et al. 2008, and e.g. see discussion of Penthetria by Skartveit & Nel 2017). It may be useful, based on extant taxa, to develop a more objective way of describing the form of R 2+ 3 in order to help more accurately place fossil taxa. For example, in the wing variation of the extant species presented in Figs. 21–22 View FIGURES 21–22 , Penthetria can be defined by the slope of R 2+3 less than 0.43 and Plecia by the slope of R 2+3 greater than 0.74 (slope defined here as rise/run, where rise = measure of imaginary line between points y and z, and where run = measurement of imaginary line between points x and z (see Fig. 22b View FIGURES 21–22 )). However, when slope is applied to the continuum of the form of R 2+3 found in fossil species, it is not as clear. For example, if slope is measured for the 15 Plecia and 15 Penthetria wings illustrated by Skartveit & Nel (2017), with a line discriminating Plecia with slope>= 0.50, it would correctly assign alone 27 of the 30 wings (i.e., without evaluating any other aspect of morphology). More variation should be studied before determining whether such a definition could be employed, but utilizing a discrete definition of slope and understanding the breadth of variation in the form of R 2+3 (or other veins) of extant species would undoubtably help to objectify the generic placement of fossil taxa.
In addition to differences in the shape of R 2+3, Penthetria and Plecia differ in the form of the male terminalia; the male terminalia in Penthetria are very simple, plesiomorphic (e.g., see Fig. 7 View FIGURES 7–8 ), relatively homogenous, with a stout, simple, gonostylus articulating in a socket formed from a relatively elongate gonocoxite. The male terminalia in Plecia are much more complex, show great interspecific variability (see e.g., Hardy 1945, Fitzgerald 1998) and tend to have the tubular, gonostyus-socket-portion of the gonocoxite greatly shortened. However, this is less true in fossil specimens. As Skartveit and Nel (2017) point out, the male terminalia of some fossil Plecia are very plesiomorphic and Penthetria -like (e.g., see Collomb et al. 2008).
Additionally, Penthetria has subtle parapsidal sutures (longitudinal sutures on the mesonotum corresponding to the position of dorsocentral setae), while they are often present as distinct grooves in Plecia . Females of the two genera can be also be distinguished by Penthetria having two segmented-cerci and Plecia with one-segmented cer-ci—though some fossil Plecia (e.g., P. clavifemur Skartveit 2009 ) have been described with two-segmented cerci.
It is also noteworthy that Penthetria and the bibionid genus Hesperinus cannot be reliably distinguished based on wing venation alone ( Skartveit 2009), which is critical in situations where the fossil specimen is represented only by a preserved wing. In fossils where more than the wing has been preserved, Penthetria has shorter antennae, with more compact flagellomeres and tends to be more stout-bodied, with shorter legs than Hesperinus . Additionally, males of Penthetria are typically holoptic while both sexes of Hesperinus are dichoptic. See also “Intraspecific Variation” (below) with respect to the delimitation of fossil species.
Classification and phylogenetic placement. Some studies have placed Penthetria , along with the genus Plecia , in the subfamily Pleciinae of Bibionidae (e.g., Hardy 1981, Blaschke-Berthold 1994) or in the separate family Pleciidae (e.g., Krivosheina 1986) based, in part, on similarities in the larvae of these genera including the “clearly separated frons and clypeus, presence of an additional lobe of the maxillae, and primitive spiracles” ( Krivosheina 1969) or musculature of the male terminalia ( Ovtshinnikova 1994).
More recent morphological phylogenetic studies, however, have not supported these classifications, instead placing Penthetria in its own subfamily (Penthetriinae), which is considered the most basal lineage of bibionids after Hesperinus ( Hesperinus + ( Penthetria + ( Plecia + Bibioninae ))) ( Pinto & Amorim 2000, Fitzgerald 2004). This latter hypothesis was not corroborated by the molecular study by Ševčík et al. (2016), which found Penthetria (along with Hesperinus ) to be less basal within the bibionid family tree ( Bibioninae + ( Plecia + ( Penthetria + Hesperinus ))). Skartveit & Ansorge (2020) propose placing Hesperinus and Penthetria together in the subfamily Hesperininae based on the Ševčík et al. (2016) study, which finds these two genera to be sister taxa. Further work is needed to reconcile the contradictory hypotheses of these morphological and molecular studies.
Characters to support the monophyly of Penthetria are sparse. Fitzgerald (2004) suggested the larval mesothorax with two pseudosegments as a synapomorphy, but so few larvae are known that this character will have to be further vetted as the larvae of more species are discovered.
Comments on generic description. The adult and larval generic description provided below is based, in part, on previous phylogenetic and descriptive work on the family Bibionidae (Fitzgerald 2004) and readers are referred there for further discussion concerning homology of structures, material examined, and the broader context from which the description was developed. Examination of all the New World species, as well as a small number of species from the Palearctic and Oriental bioregions were examined.
A sclerotized aedeagus is considered absent in Penthetria by Fitzgerald (2004) and it has been presented this way in the generic description (below). Blaschke-Berthold (1994, Fig. 6 View FIGURES 4–6 ) suggests that the aedeagus is fused with the parameres in this genus but whether it is absent or indistinguishably fused, a distinct, separate aedeagal sclerite cannot be identified. Sperm transfer in Penthetria is apparently not achieved via an intromittent organ, but probably by spermatophore (Blaschke-Bethold 1994), as is known in some other Bibionidae ( Leppla et al. 1975) .
Larvae are known for only two species of Penthetria . Aspects of the larva of the Palearctic species P. funebris have been illustrated in various papers—mandible ( Hennig 1948), habitus and posterior spiracle ( Krivosheina 1962), habitus, posterior spiracle, mandible, and labium ( Krivosheina & Mamaev 1967), and labium and maxilla (Fitzgerald 2004). The egg, larva, and pupal stages of P. japonica Wiedemann were described and illustrated by Yuan et al. (2015). The larval description below is based on the study of these two species.
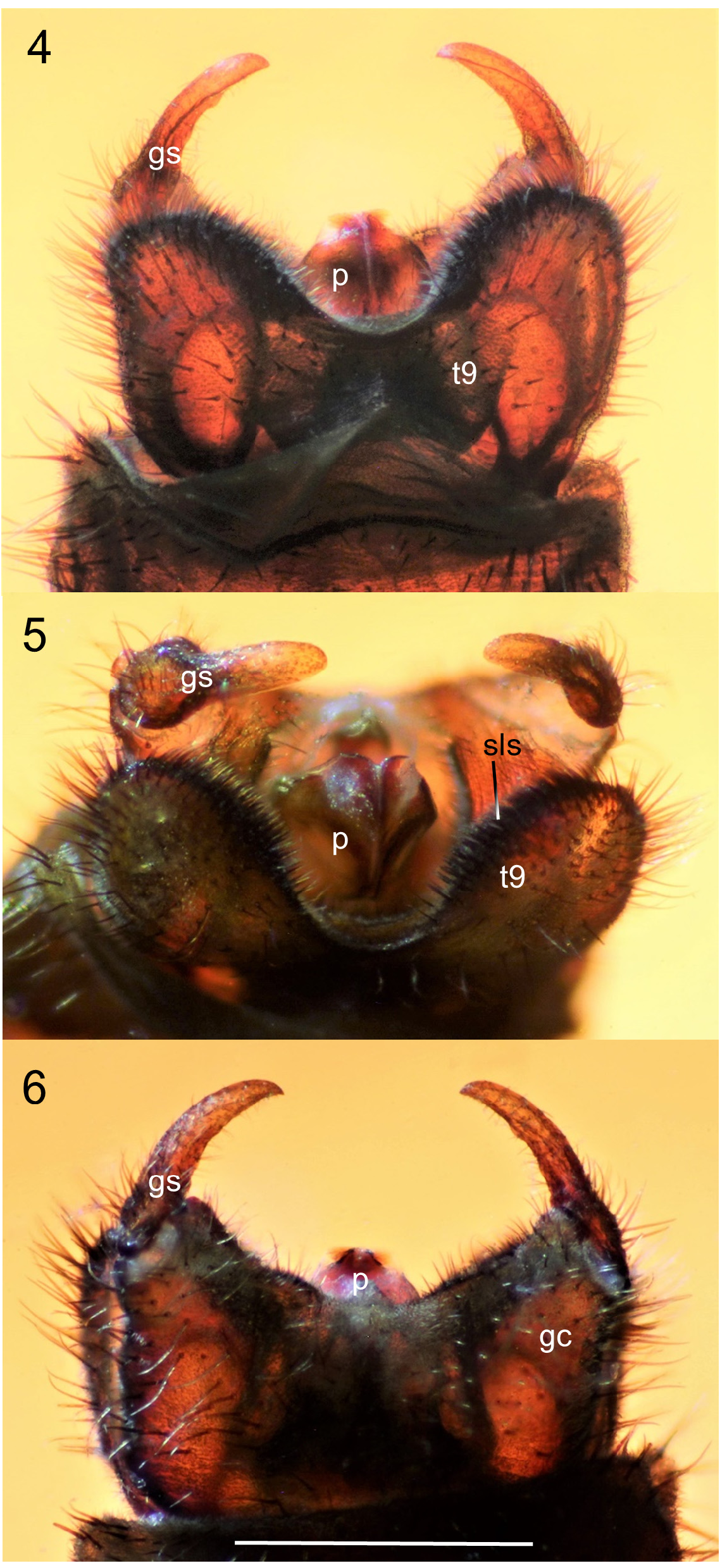
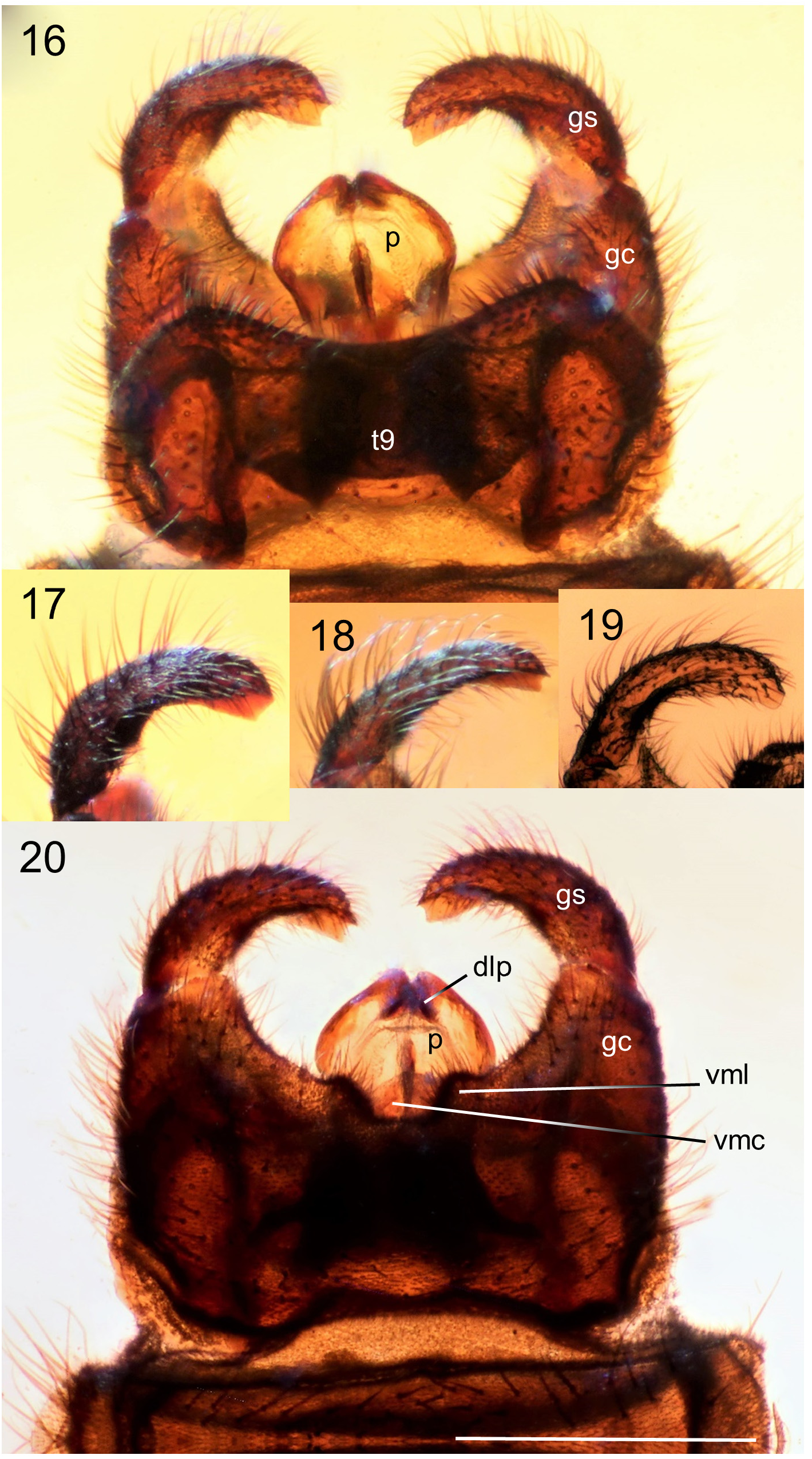
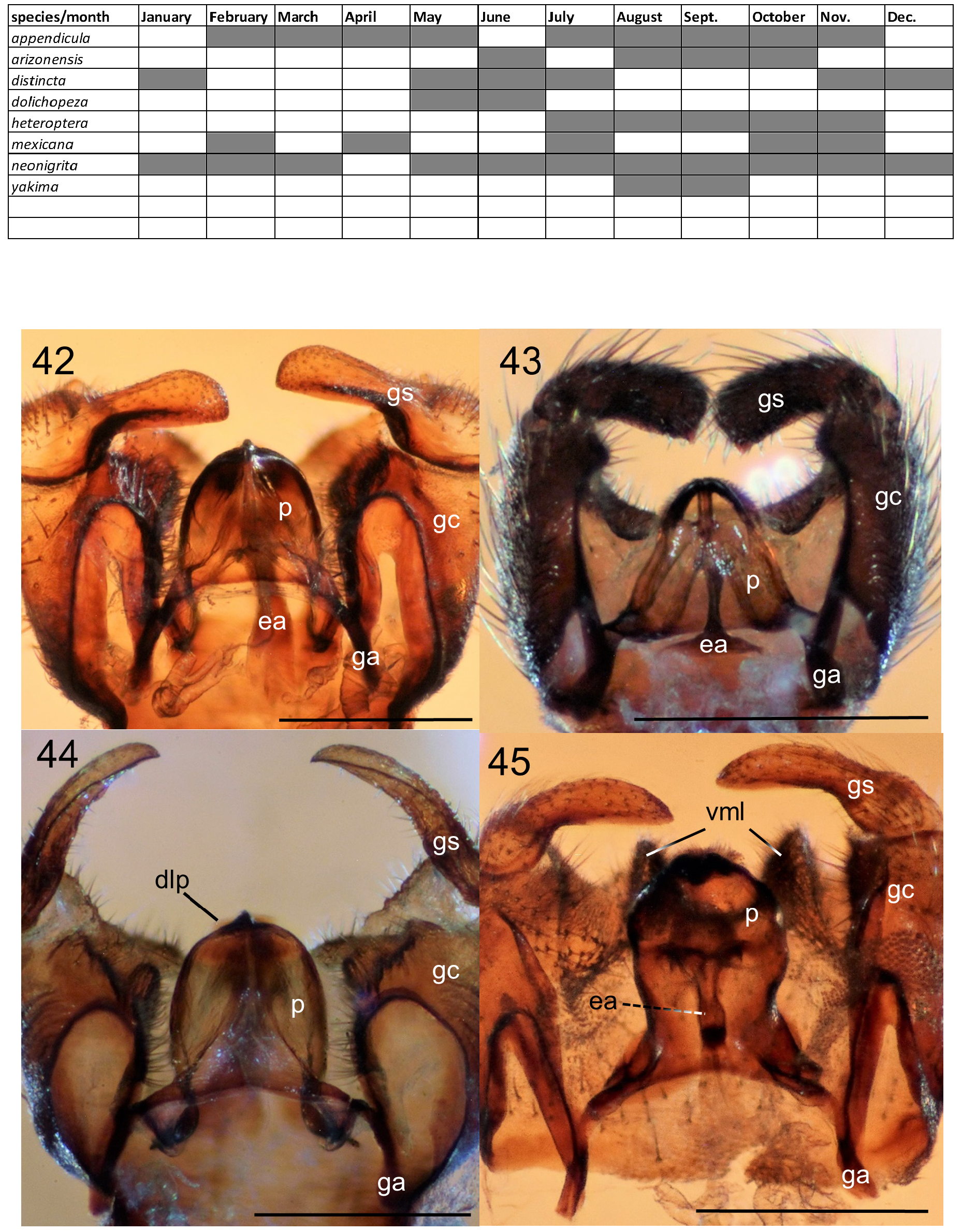
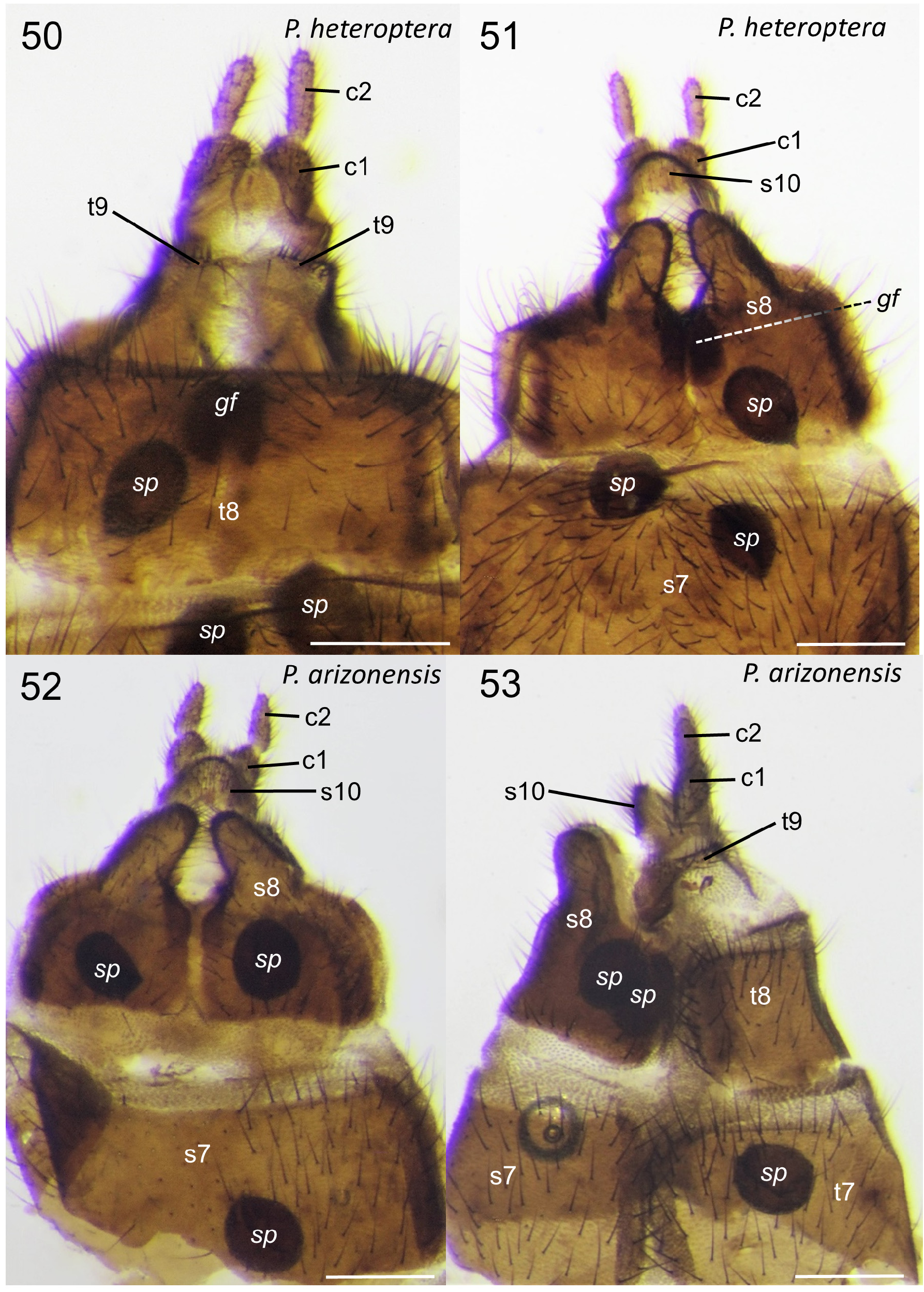
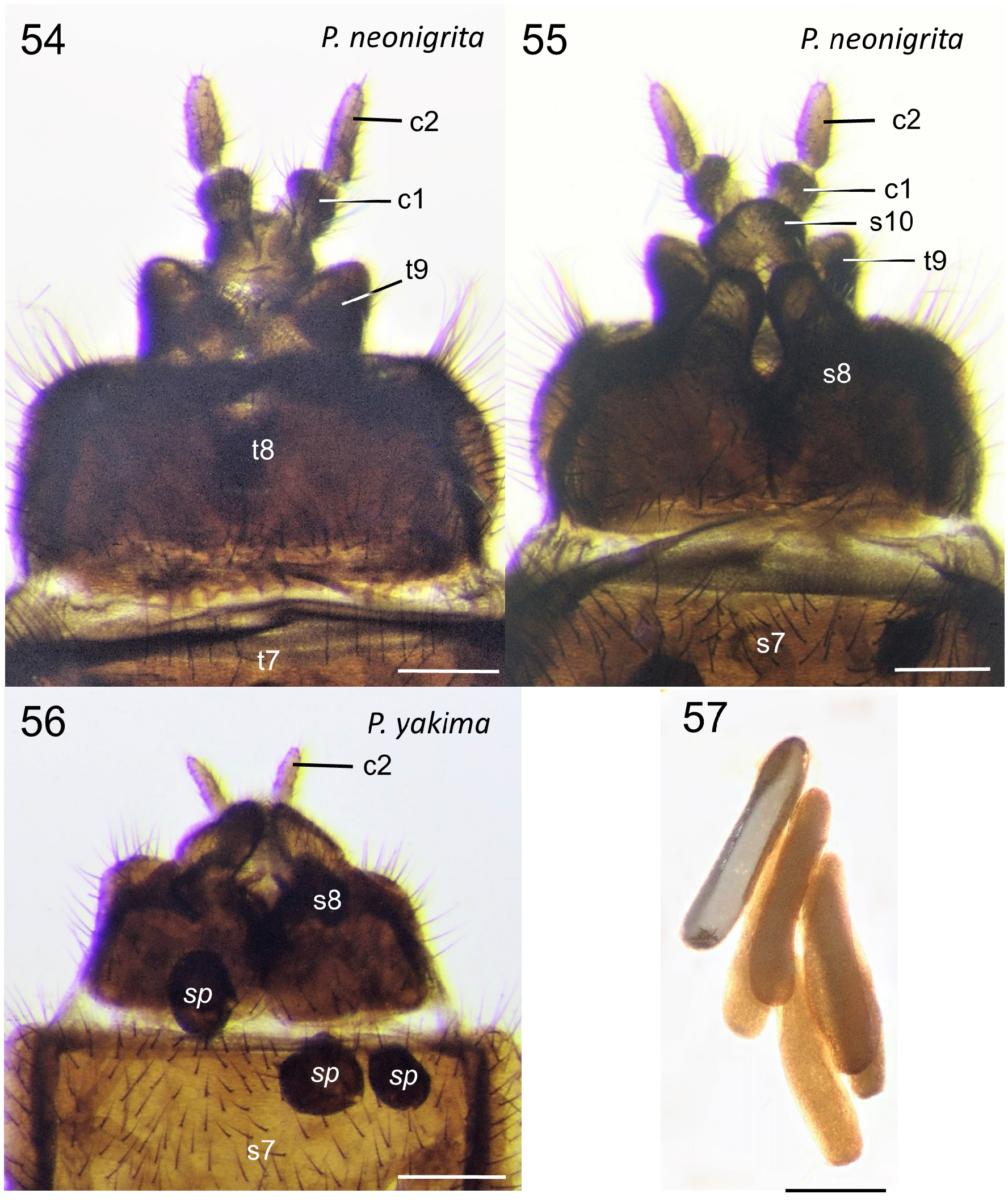
Generic description. Adult: Small to moderately large flies 4.0-11.0 mm. Head: Male head dorsoventrally compressed; in dorsal view, slightly wider than long, oval-shaped. Head and antennae black to brown. Male with almost entire dorsal surface of head occupied by broadly holoptic compound eye ( Fig. 1 View FIGURE 1 ), except P. funebris , with compound eyes narrowly separated dorsally by longitudinal strip of frons approximately subequal in width to antennal flagellum. Male compound eye strongly divided into larger dorsal region and smaller ventral region; dorsal region usually lighter in color than ventral region and with larger ommatidia. Division between dorsal and ventral regions of compound eye often distinguished by distinct narrow longitudinal step (the dorsal region of the eye is often taller than the ventral region of the eye; where the dorsal and ventral regions meet laterally, a short step, curb, or groove is often visible as in Fig. 12 View FIGURES 12–13 ). Division not marked by narrow, longitudinal, shining, sclerotized band; smooth, triangular area devoid of ommatidia also absent. Female head dorsoventrally compressed, slightly wider than long. Female compound eye round or oval in shape, convex, dichoptic ( Fig. 1 View FIGURE 1 ), broadly separated by frons. Female compound eye with inconspicuous, slight longitudinal depression dividing compound eye, but lacking any difference in size of ommatidia or color between dorsal and ventral regions. Compound eye of both sexes bare or clothed with minute, stiff, or very elongate hairs. Ocellar tubercle well developed and prominent in males, moderately to weakly developed in females. Both sexes with three ocelli arranged in small, equilateral triangle. Female with small tubercle or longitudinal ridge just posterior to antennal sockets. Male frons reduced to small, triangular region between anterior margin of compound eye, posterior margin of antennal sockets bearing minute tubercle (or a minute pit in P. funebris ). Sclerotized rostrum not produced, oral margin somewhat ventral in position. Antenna located anteriorly along oral margin, with 7–10 short, stout flagellomeres. Flagellomeres slightly broader than long, except most basal flagellomere, about two times as long as subsequent flagellomere. Pedicel and flagellomeres with subapical ring of short setae and numerous dense, minute, appressed setae. Apical flagellomere slightly more elongate than preceding flagellomere, with rounded point apically. Maxillary palps with five segments; basal segment minute, third segment thickened with dorsal, diagonal sensory pit with dense, minute, specialized setae; apical palpomere slender, more elongate than previous palpomeres. Clypeus broader than long, never elongate. Head entirely sclerotized ventrally. Thorax ( Fig. 3 View FIGURES 2–3 ): Precoxal-bridge complete. Basisternum present, presternum absent. Dorsum of thorax smooth, matte, often with thick dark pruinosity, usually black, dark brown, anteriorly black and posteriorly orange, or rarely entirely orange, as in P. indica (Brunetti) . Parapsidal sutures subtle and not distinct as in some Plecia . Dorsum of thorax with dense, short, stiff to very elongate hairs, laterally, anteriorly, and in dorsocentral rows posteriorly. Thoracic pleura black to dark brown. Males with minute to moderately long hairs on metakatepisternum, and dorsal half of katepisternum. Anepisternum bare or with cluster of hairs at posterior margin. Meron, laterotergite, and mediotergite bare. Female thoracic pleura generally less hairy than male. Legs: Legs black to dark brown. All legs with five tarsomeres, simple tarsal claws, pulvilli and pulvilliform empodia. Anterior coxa short and not reaching ventral edge of katepisternum; reaching about half length of katepisternum in lateral view, usually with elongate hair. Fore femur relatively slender and more elongate; not short and swollen. Fore tibia elongate, slender, apex unmodified, outer edge not developed into a mucron. Fore tibia distally with a single, short spur. Middle legs unremarkable; with two apical tibial spurs. Hind legs with hind femur swollen apically to more slender and only slightly enlarged apically. Hind tibia slender or swollen apically. Inner surface of hind tibia without elongate field of small, round, black, specialized sensilla. Spurs of hind tibia slender, apically acute, subequal in length, or ventral spur slightly more elongate than dorsal spur. In males, hind tarsomeres vary in shape, from slender, elongate, parallel-sided to slightly swollen and sausage-shaped. Female tarsomeres never swollen; slender to robust. Halter black. Wing ( Figs. 2 View FIGURES 2–3 , 13 View FIGURES 12–13 ): Length, 4.5-12.5 mm, elongate, reaching tip of abdomen, except brachypterous and distinctly shortened in males of P. funebris . Wing brown to blackish fumose. Costal cell often darker than remainder of wing in taxa with fumose wings. Wing color solid or with grade in intensity, but without distinct markings. Pterostigma oval, usually brown to black. Anterior wing veins typically darker than posterior veins. Wing without macrotrichia, with microtrichia. Anal lobe well developed. Costa ends at or just beyond R 4+5 (not shortened or thickened as Enicoscolus Hardy ). Subcosta long, complete. Radius without horizontal microstriations. Rs furcate; R 2+3 present, relatively elongate and subparallel to slightly oblique to R 4+5; base R 2+3 often arising at right angle to R 4+5 then sharply bent in direction of wing tip. Base of R 2+3 sometimes with sub-basal stump (appendix) (e.g., P. appendicula Hardy ; Fig. 2 View FIGURES 2–3 ). R 2+3 branches from R 4+5 from slightly basal to, even with, or distinctly distal to, r-m crossvein. Length of r-m crossvein much shorter than base of Rs. Posterior end of r-m meeting stem of medial fork (M 1+2) or more distally, connecting directly to M 1 (e.g., P. japonica Wiedemann ; Hardy & Takahashi 1960, Fig. 2b View FIGURES 2–3 ). Three branches of M present (M 1, M 2, and M 4). Base of M 4 crossvein-like. CuA and CuP present, reaching wing margin, sometimes meeting distally forming closed cell cua. A 1 short, weak; not extending beyond the small fold at the base of anal lobe. Abdomen: Black to dark brown, with short to elongate hairs. Male abdomen elongate, gradually tapered posteriorly; female abdomen much stouter. Male spiracles 1–7 located laterally on abdominal pleura, except spiracle eight, either absent or, if present, a remnant dorsolaterally at membrane between tergites eight and nine (e.g., P. funebris ). Males with tergites and sternites 1–8 unmodified, females with tergites 1–8 and sternites 1–7 unmodified. Male terminalia ( Figs. 4–11 View FIGURES 4–6 View FIGURES 7–8 View FIGURES 9–11 , 14–20 View FIGURES 14–15 View FIGURES 16–20 , 24–29 View FIGURES 24–29 , 33–45 View FIGURES 33–35 View FIGURES 38–41 View FIGURES 42–45 ): Terminalia slightly dorsoflexed, not rotated. Posterior margin of tergite 9 (epandrium) shallowly to deeply (nearly dividing tergite) emarginated (“epandrial cleft”) medially. Epandrial cleft often U-shaped, with lobes at sides of posterior edge of epandrium (epandrial lobes) typically broad, rounded, sometimes more triangular in shape apically. Anterior edge of epandrium sometimes with a shallow to moderate medial emargination. Gonocoxites fused to sternite 9 (hypandrium) into a continuous genital capsule (synsternogonocoxite). Hypandrium distinguishable only by narrow, strap-like thickening ventrally and sometimes a slight medial seam. Posteromedian margin of synsternogonocoxite sometimes with median hump, a median cleft (ventromedian cleft, Fig. 20 View FIGURES 16–20 , vmc), and/or a pair of weak to strong lobes (ventromedian lobes, Figs. 20 View FIGURES 16–20 & 34 View FIGURES 33–35 , vml) which are membranous to lightly sclerotized. Gonocoxites distally with elongate, tubular pedicel to which gonostyli articulate apically. Gonostylus usually rather simple, short, stout, robust, straight to very strongly arched, gradually tapered to slightly expanded apically, apex rounded, truncate, or with obtuse point, sometimes with a small anterior tooth. Proctiger present ventral to epandrium; cerci normally protruding through epandrial cleft. Tergite and sternite 10 (epiproct and hypoproct respectively) present. Cerci rounded, flap-like, fleshy, with hairs (Figs. 36, 40). Gonocoxal apodeme present, fused to parameres ( Fig. 38 View FIGURES 38–41 ). Ventral to proctiger, parameres have a complex three-dimensional shape; dorsal sclerite and ventrolateral apodemes of paramere indistinguishably fused into a posteriorly broadly rounded, dome-like, hood ( Figs. 38–45 View FIGURES 38–41 View FIGURES 42–45 , p). Ventral to parameres, membranous, sac-like, endophallus present, which is cradled by more ventral ejaculatory apodeme ( Figs. 42–43 View FIGURES 42–45 , ea). Ejaculatory apodeme, simple, dorsoventrally flattened. A sclerotized aedeagus absent. Female terminalia ( Figs. 46–56 View FIGURES 46–49 View FIGURES 50–53 View FIGURES 54–57 ): Tergite 9 present as narrow, transverse strap, or subdivided into two sclerites; often more strongly produced ventrolaterally. Tergite 10 minute, longitudinally elongate. Cerci two-segmented. Subgenital plate (sternite eight) large, divided longitudinally, with posterior margin lobate; inner margins of longitudinal cleft sometimes with minute, posteromedially-directed tubercles. Y-shaped genital fork (= sternite 9) present ( Fig. 51 View FIGURES 50–53 , gf). In addition to genital fork, a minute pair of sclerites often present between the posterior margin of the subgenital plate and the anterior margin of sternite ten. Sternite 10 present. Three rounded, sclerotized, balloon-like, spermathecae present.
Immature stages: Egg ( Fig. 57 View FIGURES 54–57 ): Elongate, sausage-shaped, cream-colored.
Larva ( Fig. 58 View FIGURES 58–60 ): Body light brownish to gray, elongate, slightly dorsoventrally flattened, leathery, slightly curved downwards in lateral view, with three thoracic and nine abdominal segments. Thorax and abdomen with transverse rows of elongate, fleshy tubercles on each segment both dorsally and ventrally. Number of tubercles in each row varies depending on the species, though thoracic segments tend to have fewer tubercles. Laterally, two tubercles present near each abdominal spiracle except posterior spiracle. Cuticle with dark brown to black, minute, sclerotized, spine-like scales. Thorax without lightly sclerotized plates and without characteristic stair-step-like swollen developments observed in Pachyneuridae . Ventral flap-like tubercle on prothorax present. Anus terminal, anal papillae apparently absent. Intersegmental fissures unaligned between meso- and metathorax between abdominal segments 1 and 2 and between abdominal segments 7 and 8. Dorsally, prothorax with two pseudosegments, mesothorax two pseudosegments, and most abdominal segments three pseudosegments. Prothoracic and metathoracic spiracles present. Abdominal segments 1–8 with spiracles; spiracles 1–7 lateral in position, spiracle 8 dorsolateral, larger than previous ones. All spiracles slightly protuberant, especially posterior spiracles. Posterior spiracle on posterior border of segment 8, round, with single, central, ecdysial scar. Head strongly sclerotized, black to dark brown, rounded, somewhat dorsoventrally flattened, with setae. Head capable of being completely withdrawn into anterior portion of thorax. Ecdysial lines meeting in form of Y anterior to postoccipital carina. Anterolateral margin of frontoclypeus developed into strong, anteroventrally-directed spine. Labrum subrectangular, without strong spines at apex of labrum/epipharynx. Anteroventral stemmata present, dorsoposterior stemmata absent. Antennae short, sensory cone in form of ovoid plate positioned within larger, round, antennal socket. Postgenal bridge complete. Ventral prothoracic sclerites in cuticle at posterior, ventral margin of head capsule present. Anterior tentorial arm present, weakly developed, connected at anterior margin of head near mandibular articulation. Posterior tentorial bridge absent. Submentum apparently absent or indistinguishably fused to ventral head capsule. Anterior mentum (hypostoma) absent, posterior mentum present as a narrow, longitudinal sclerite, not fused to anterior margin of cranium or to posterior margin of labium. Labial synsclerite present, as an inverted U-shape; each anterolateral margin with small knob homologous to glossae. Posterior labial sclerite present, large, in same plane as and smoothly fused to labial synsclerite, forming sclerotized frame completely enclosing membranous labial area. Tubercle of labial palps absent, membranous labial area bearing field of papillae. Cibarial bar absent. Membranous, hypopharynx with patches of minute spines, supported by two pairs of hypopharyngeal sclerites: each pair with one large and one minute sclerite. Pharyngeal filter absent. Cardo large, transverse, not closely appressed to anteroventral margin of cranium, T-shaped, inner apex of sclerite with an anteriorly directed lobe and a posteriorly directed lobe. Cardo with four setae (some represented only by alveoli); one seta at inner apex and a group of three at outer (lateral) apex. Galeolacinia adjacent, but not closely appressed to palpifer. Galeolacinia sclerotized ventrally, with numerous teeth and spines on inner edge apically and on dorsal surface. Laterobasal sclerite of maxillary palpifer present. Palpifer sclerotized, tubular, bearing one-segmented cylindrical palpus. Palpus with single sensory region apically, bearing numerous short, stout setae. Mandible heavily sclerotized, subtriangular, with small number of short, stout, apical teeth. Mandible operating in horizontal plane, without line of weakness separating apical and basal portions, and lacking basal thumb of teeth. Prostheca present. Mandibular comb absent. Epipharynx dorsoventrally flattened, slightly bilobate, with numerous small inwardly directed spines and small number of peg-like setae. Torma wrapped dorsolaterally, fused and continuous with dorsal labrum. Premandible absent.
Pupa ( Figs. 59–60 View FIGURES 58–60 ): Brown to greyish in color, leathery, sometimes enclosed within last larval skin. Head and thorax without distinct setae or spines. Respiratory horn absent; anterior thoracic spiracle on short tubercle. Abdomen with fleshy tubercles, without sclerotized spines or setae. Abdominal tergites without transverse rows of minute spinules. Leg sheaths superimposed.
Geographic Distribution and Diversity. Penthetria presently includes 36 extant species distributed mainly in the Holarctic and Oriental regions, but also with species in the Neotropics. The greatest diversity is north of the equator, particularly in the Oriental and eastern Palearctic regions (28 species), with only eight species described from the Nearctic and Neotropical regions.
Fossil Record. Compression fossils are known from the Nearctic and Palearctic regions; most of these are Oligocene and Miocene in age, with a few from the Eocene and Pliocene/Pleistocene ( Evenhuis 2014). Penthetria dubia Geinitz was described from the Lower Jurassic ( Germany), but based on the illustration of the wing of this species ( Geinitz 1884, Plate XIII, Fig. 26 View FIGURES 24–29 ) it is not a Penthetria nor a Bibionidae and should be removed from the family, thus making the oldest compression fossils of this genus Eocene in age (e.g., Rice 1959).Additionally, Skartveit (2009) described two species from Baltic amber, which are also Eocene in age. One of these species is quite peculiar as vein R 2+3 is absent, but based on other features it was included in Penthetria , where it seems to fit better than anywhere else in Bibionidae . It is possible that this species is based on a specimen with a teratological wing, as in cases known in extant species of Plecia (pers. obs.) and Hesperinus ( Paramonov 2005) where R 2+3 is absent. It is noteworthy that some of the Mesozoic stem-bibionids currently placed in Protopleciidae have wing venation identical to Penthetria (e.g., Epimesoplecia Zhang, 2007 and Lichnoplecia Ren, 1995 ).
There are nine fossil species from the Nearctic region currently placed in Penthetria ( Evenhuis 1994, Fitzgerald 1999, Skartveit 2009). However, as noted by Gentilini (1991), many of the Nearctic fossils described by Rice (1959) in Plecia may actually belong to Penthetria and need to be re-evaluated. The number of fossil species from the Palearctic (about 35) is also in a state of flux, as there has been significant recent work re-evaluating the generic placement of old species of Penthetria and Plecia and adding new species (e.g., Skartveit 2009, Skartveit & Nel 2012, Skartveit & Pica 2014, Skartveit & Nel 2017, Skartveit & Krizmanic 2020).
Bionomics. Little is known about the biology of the adults of this genus, but it is assumed to be similar to other members of the family (summaries of biology can be found, e.g., in Hardy 1981, Skartveit 1997, Fitzgerald 2009). However, while there are numerous records of other bibionid genera (especially Bibioninae ) being collected on flowers, the label data of none of the New World Penthetria specimens indicate that any were collected on flowers, which seems unusual (the author is unaware of any records of adult feeding in this genus). Seasonal activity varies between species and region (e.g., Table 1). Pecina (1965) states that adults of the Palearctic P. funebris emerge in mid-April and are gone by mid-May, though “they may be found singly later.” Curiously, Frouz et al. (2015), working on the same species in the same country but 50 years later, found peak emergence was in late May and early June, though this disparity may be due to differences in altitude or annual seasonal variables. At a similar latitude in the Nearctic region, P. heteroptera (Say) is fall-emerging, rather than spring-emerging, but has a similarly narrow duration of adult activity, with most adult records from a two-month window of time. In contrast to these temperate species with narrow windows of adult activity, the Neotropical species P. neonigrita Fitzgerald n. sp., has been recorded every month of the year except April. Yuan et al. (2015) reported that lab reared P. japonica had two adult emergence peaks (late August to early September and late March to early April) and that the female could lay “hundreds” of eggs. Frouz et al. (2015) found the adult life span of P. funebris was relatively short (less than one month) and that females laid about 130 eggs, which hatched within a few days. Unlike Bibioninae , which have the fore tibia modified with apical mucron/spines which females use to dig a burrow for egg laying, Penthetria and Plecia have the fore tibia unmodified; based on this, it is assumed that Penthetria females do not burrow, but lay their eggs within small crevices on the surface, as do females of Plecia ( Pinto & Amorim 1996) .
Most of the known biological information on the genus comes from studies of the larvae of P. funebris in Europe, where this species is found to be important in nutrient cycling and the decomposition of forest litter ( Frouz et al. 2002, Šustr & Frouz 2002, Šustr et al. 2014, Frouz et al. 2015). The saprophagous larvae live in the top layers of soil and “feed selectively on partially decomposed leaves, which are densely colonized by bacteria and fungi” and “their coprophagic behavior allows [the larva] to harvest the microbial production in their faeces during a second gut passage, providing bibionid larvae with an important energy and nutrient source” ( Šustr et al. 2014). Based on studies of larval biomass and laboratory consumption of leaf litter, Frouz et al. (2015) determined that the larvae of this species is responsible for consuming roughly 40% of the annual litter fall at the study site. Pecina (1965) reports larvae of P. funebris “inhabiting moist soils with the mouldering remains of vegetables” and that they are “commonly found on the banks of bodies of water where the larvae develop under the litter of deciduous trees, especially of alders and poplars, or in moist meadows.” Pecina (1965) also notes that the larvae live solitarily in the soil in contradistinction to members of the Bibioninae , which are gregarious, but this statement is difficult to interpret as many of the studies appear to find larvae in high numbers. Gemesi & Disney (1991) reported that during the month of September, mature larvae of P. funebris were collected in decaying leaf litter of alder trees ( Alnus glutinosa (L.) Gaertn.) along a river; 8.7% of the individuals were later found to be parasitized in the 2 nd or early 3 rd instar by Borophaga germanica Schmitz ( Diptera : Phoridae ). The later authors also reported that P. funebris overwinters as a mature larva. Based on measurements of larval head capsules, Frouz et al. (2015) determined that P. funebris has seven larval instars and spends most of its developmental time (as well as usually overwinters) in the sixth instar, which takes about two months to reach; the seventh instar and pupa were inferred to have short durations based on the fact that they were infrequently detected. Yuan et al. (2015) found the larvae of P. japonica in the upper layers of cow dung at an earthworm breeding farm; larvae were successfully reared on this medium in the laboratory. It is noteworthy, that while much of what we know of the biology of Penthetria is based on P. funebris , this species is not an average representative of the genus, as it is the only member of the genus to have dichoptic and brachypterous males, which are flightless ( Skartveit & Willassen 1996, Frouz et al. 2015).
The immature stages and biology of the New World species is entirely unknown though it is expected to be similar to what is known for the few Palearctic species in which immature stages and biology have been studied.
Identification. Aside from the New World species (treated in the present study and Hardy 1945), the following papers include keys or other information helpful for identifying Penthetria in the following regions: Europe ( Fitzgerald & Werner 2004), China ( Li & Yang 2010), Japan ( Hardy & Takahashi 1960, Sasakawa 1967), Nepal (Hardy 1965, 1967), and Russia and Mongolia ( Krivosheina & Krivosheina 1998, Krivosheina 1999, Nartshuk 1990, Hardy & Takahashi 1960). Additionally, the catalog of world species (below) references all the primary literature.
Intraspecific Variation. While the general form of Penthetria species is remarkably homogeneous (most species look very similar to Fig. 1 View FIGURE 1 ), there is considerable intraspecific variation that makes identification and species delimitation challenging. Some of the characters that have been previously used to help distinguish species, such as whether vein R 2+3 has an appendix at the base ( Hardy 1945), have been found to be variable (pers. obs.) and not consistently present or absent within a given species (e.g., P. appendicula typically but not always has an appendix ( Fig. 2 View FIGURES 2–3 , ba), P. distincta generally does not but may have an appendix, and P. neonigrita usually does not but rarely does have an appendix). Additionally, some species have a tendency to have CuA and CuP meeting apically near the wing margin forming a closed cell cua ( Fig. 32 View FIGURES 30–32 ), yet this character is not consistent when numerous specimens are studied. In some cases, one wing will have a closed cell cua and the other wing has an open cell (pers. obs). Plasticity in the wing venation (e.g., presence/absence of an appendix on R 2+3, length of stem of M, and position of r-m crossvein) has also been noted in some of the Palearctic species ( Nartshuk 1990, Krivosheina & Krivosheina 1998). Unfortunately, the strong plasticity of the wing venation observed in extant taxa does not bode well for the taxonomy of fossil members of the group, since many species have been delimited largely or solely on differences in wing veins. For example, the slope and length of vein R 2+3 has been used to help delimit both extant and fossil species. The study of the intraspecific variation of this character in extant species indicates that vein R 2+3 can vary drastically in slope and length even between specimens of the same species collected from sites that are relatively near each other (e.g., Figs. 21 View FIGURES 21–22 a–b). Concerns about how the intraspecific variation found in the wing venation of extant species of bibionids translates into the delimitation of fossil species was previously raised by Collomb et al. (2008). His study noted the plasticity of the length and shape of r-m in Bibio Geoffroy , the slope and length of R 2+ 3 in Plecia , and the length and shape of R 2+ 3 in Penthetria and arrived at the conclusion that male terminalia were the best source of information for delimiting fossil species (which mirrors conclusions by those working on extant taxa). It is possible that the number of fossil species of Penthetria (and possibly other genera) have been artificially inflated due to variable wing venation described under several names.Additional examples of intraspecific variation in the genus can also be found under “ P. heteroptera (Say) ” (below).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Penthetria Meigen
| Fitzgerald, Scott J. 2021 |
Amasia
| Meigen, J. W. 1800: 20 |