Arctonyx hoevenii, (HUBRECHT, 1891)
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2008.00416.x |
|
persistent identifier |
https://treatment.plazi.org/id/9D202A31-FFBB-FFE2-A79E-FB14FC1481AC |
|
treatment provided by |
Felipe |
|
scientific name |
Arctonyx hoevenii |
| status |
|
ARCTONYX HOEVENII ( HUBRECHT, 1891) View in CoL
Taxonomic synonymy (names as originally proposed): Trichomanis hoevenii Hubrecht, 1891 .
Arctonyx collaris hoeveni, Robinson & Kloss, 1918 . (redescription and designation of neotype)
Type material and locality: Hubrecht’s (1891) unfortunate original description of hoevenii was based on a captive specimen that was lost, with only a vague type locality provided (‘in the mountainous districts that separate the Residencies of Palembang and Bencoolen in Sumatra’). Robinson & Kloss (1918: 12) preserved the usage of Hubrecht’s epithet and restricted the type locality by designating a neotype for hoevenii , an adult female (skin and skull) from ‘Sungei Kumbang, Korinchi, West Sumatra’ (= Sungai Kumbang, Gunung Kerinci, West Sumatra Province, Indonesia), at 4700 feet (= 1433 m). This specimen, originally identified as ‘ Federated Malay States Museums No. 440/14’, is now ZRC 4.1143.
Common name: We suggest the common name ‘Sumatran hog-badger’ for this species.
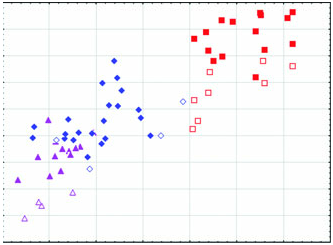
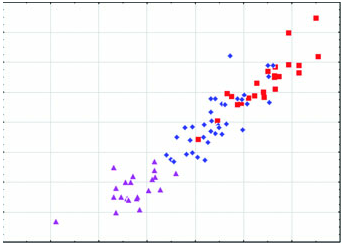
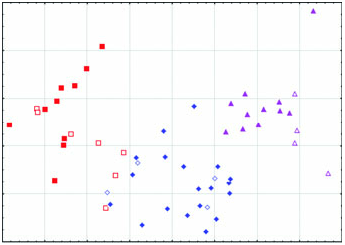
Diagnosis: Arctonyx hoevenii is smaller than other Arctonyx ( Figs 2–6 View Figure 2 View Figure 3 View Figure 4 View Figure 5 View Figure 6 ; Tables 1–4); as Hubrecht (1891) noted, it is ‘an animal of the size of a very large cat’ (apparently meaning a domesticated cat, Felis sylvestris catus ). The skull can be distinguished from other Arctonyx by its small overall size, relatively narrow rostrum, pronounced sagittal crest (an indication of cranial robustness despite its small size) and proportionally small teeth. The skin can be distinguished from other Arctonyx by its sparser fur and darker dorsal pelage.
Distribution: Arctonyx hoevenii occurs in the mountains and adjacent foothills of Sumatra, which extend from north to south across the entire length of the western portion of the island and are collectively known as the Barisan (= Barussan) Range. Corbet & Hill (1992) mapped the distribution of Arctonyx only in the mountains of southern Sumatra, but vouchered records document that it probably occurs essentially everywhere along the length of the Barisan chain, from Aceh Province in the north to South Sumatra Province in the south ( Fig. 11 View Figure 11 ; Appendix 3). The elevational range of A. hoevenii extends from perhaps as low as 700 m in foothill forests ( Holden, 2006) to the very highest point on the island – Robinson & Kloss (1918) reported a skull of A. hoevenii picked up at 3780 m in the alpine zone on the summit of Gunung Kerinci. The core habitat of A. hoevenii would seem to be lower montane and mossy forests and subalpine meadows between 800 and 2600 m, as documented below.
Van Schaik & Griffiths (1996) obtained camera-trap photographs of A. hoevenii at two sites in Gunung Leuser National Park. The lower of these, the upper Bengkung catchment, ‘consists mainly of hilly terrain, with the southern part gently undulating. The area is almost entirely below 800 m altitude, and is clad in mixed lowland dipterocarp forest, interspersed with parts dominated by non-dipterocarps, mainly near rivers’ ( Van Schaik & Griffiths, 1996: 106). In the upper Mamas River catchment they photographed A. hoevenii in a landscape situated in ‘a relatively gently sloping valley surrounded by steep ridges, covered in submontane and montane forests’ between 1200 and 1600 m.
Augeri (2005) also camera-trapped extensively in Gunung Leuser National Park, at six sites ranging across a broad elevational transect. He obtained camera-trap photographs of A. hoevenii at several montane sites, including Ketambe Atas (1217– 2657 m; 2205 trap-nights, 15 Arctonyx pictures), Gunung Putar (1402–2113 m; 1792 trap-nights, 13 Arctonyx pictures), Ketambe (433–2002 m; 476 trapnights, two Arctonyx pictures), but not at several lowland sites, including Sei Badak (50–210 m, 1848 trap-nights), Sei Birah (211–964 m, 2240 trap-nights) and Sekundur (47–107 m, 1960 trap-nights).
Arctonyx hoevenii View in CoL was ‘photographed regularly’ during 25 000 h of camera trapping at Gunung Tujuh in Kerinci Seblat National Park in central Sumatra by Holden (2006: 36) in forests situated at 2000–2400 m, a habitat he characterized as ‘primary hill forest’. Holden (2006: 35–36) also discovered an Arctonyx View in CoL footprint at Tandai, another site at the park situated in ‘old logged forest in a state of regeneration’ at 700 m, but noted that ‘photo-traps working in this area for 50 000 hours did not record Hog Badger, indicating that it was not a common species in this habitat.’ He further noted that ‘among local informants only those that were familiar with the high altitude forests knew the animal, referring to it as Babi Batang.’
Augeri’s (2005) and Holden’s (2006) observations and impressions indicate that A. hoevenii View in CoL is considerably more common in higher montane forest than in lower elevational foothill forests, a result that echoes the earlier comments of Frederick A. Ulmer, Jr, who collected mammals in Sumatra for ANSP during the Vanderbilt Sumatran Expedition in 1936–1939 (quoted in Miller, 1942: 121–122):
Although two of our specimens [of Arctonyx ] were collected by natives at supposedly low elevations [600 and 2900 ft (i.e. 183 and 884 m, respectively)] I saw definite evidence of hog badgers’ presence in the mountains only. Their diggings were first seen at Bivouac No. 5 (7900 ft [= 2408 m]) where we found the skeleton of an aged animal. Some hair and cartilage were still adhering to the bones. Along a brook flowing through a deep ravine the badgers had dug many funnel-shaped depressions two or three inches deep. A heavy steel trap set there (baited with the carcass of a little jungle squirrel, Tomeutes ) caught one; but the powerful animal pulled free, leaving only a bunch of hair. At Bivouac No. 7 (9300 ft [= 2835 m]) a badger burrow was found in the bank of a stream that flowed through a large ‘blang’ or alpine meadow. This burrow was about 10 inches in diameter; a large heap of debris had accumulated in front of it. In this area we saw the characteristic ‘grubbings’ of the badgers. Again, in a ‘blang’ between Bivouacs 8 and 9 the small funnel-shaped holes were so numerous as to pock the ground in all directions. Members of the expedition who kept the young badger ([ANSP] no. 20231) alive for a few days were in accord concerning the animal’s foetid odor.
As Ulmer noted, the lowest elevational record, 600 feet, must be doubted. It is based on a mounted skin and skull apparently received from locals at Kuta Tjane ( Miller, 1942: 121). Apart from this specimen, the lowest record of occurrence for A. hoevenii is Holden’s (2006) footprint from secondary forest situated at 700 m, where his camera-trapping suggested it was extremely uncommon. Setting the dubious Kuta Tjane record aside, the mean elevation of 25 vouchered sites of occurrence with recorded elevational data – a compilation drawing from museum specimen data and from other elevational records discussed by Robinson & Kloss (1918, 1919), Miller (1942), Van Schaik & Griffiths (1996) and Holden (2006) – is 1748 m (SD 800 m, median 1600 m). Judging from all available museum specimens and from records and discussion in the literature, we suggest that the core elevational and habitat range of A. hoevenii embraces montane forests and adjacent mountain meadows situated between about 800 and 2400 m, if not also extending higher (where little camera-trapping work or other mammal inventory work has commenced). The skull from the summit of Kerinci mentioned by Robinson & Kloss (1918) shows that badgers extend to the very elevational limits of Sumatra, at least on occasion.
We detect no pattern of geographical morphological variation in A. hoevenii .
Natural history: Arctonyx hoevenii is a montane badger that lives and forages in the mountain forests and upland meadows of Sumatra. It lives in terrestrial burrows, dug perhaps especially in soft soils along riverbanks ( Miller, 1942). It may be active by night or by day, and has been characterized as cathemeral ( Van Schaik & Griffiths, 1996). Its diet may consist almost entirely of terrestrial invertebrates. Based on observations related by van der Hoeven, Hubrecht (1891) described this badger’s ‘long cylindrical tongue, which thrust out, serves the animal in the collecting of ants, which are its natural food’. Based on specimens they collected and prepared on Gunung Kerinci, Robinson & Kloss (1918) noted that ‘its food... appeared to consist chiefly of earthworms and beetle larvae.’ Jacobsen (in Robinson & Kloss, 1919: 265) wrote of a specimen he collected on Gunung Kaba:
I kept this specimen alive for a day; the only food it would take was earthworms; raw meat and insects were refused. The excrement, consisting chiefly of earth from the worms it had digested, had a very nauseous, sweetish smell, very characteristic and quite different from the pungent smell of Mydaus meliceps [= M. javanensis ].
Like Arctonyx albogularis View in CoL and A. collaris View in CoL , A. hoevenii View in CoL probably also takes other, non-invertebrate food opportunistically; Miller (1942) noted that one animal was taken in a snare baited with a squirrel carcass. However, we find the first-hand observations that A. hoevenii View in CoL consumes primarily earthworms, beetle larvae and ants, provided by van der Hoeven, Robinson and Kloss, and Jacobsen to be compelling. Indeed, as noted above, the insectivorous habits of the first specimen of A. hoevenii View in CoL reported in the scientific literature were so convincing that the animal was initially described within the ‘Edentata’ in the absence of clarifying specimens ( Hubrecht, 1891).
Jacobsen also examined scat suggesting vermivory of Arctonyx View in CoL both on Gunung Saba and on Gunung Dempo ( Robinson & Kloss, 1919). The ‘funnel-shaped depressions two to three inches deep’ dug by badgers along streams on Gunung Leuser, mentioned by Ulmer (above), are likely to be places where badgers shoved their muzzles into the earth to secure earthworms with their tongues – sign and behaviour reminiscent of New Guinea’s long-beaked echidna Zaglossus bartoni ( Opiang, 2004) View in CoL , an animal of similar size and habitat requirements that is a committed earthworm-eating specialist ( Griffiths, 1978; Flannery, 1995). Jacobsen, Ulmer, and Robinson and Kloss all described abundant Arctonyx View in CoL diggings in Sumatran mountain meadows, probably reflecting the species’ searches for earthworms ( Robinson & Kloss, 1918, 1919; Miller, 1942). A greater commitment to vermivory/insectivory may offer an explanation for the relatively reduced size of its cheekteeth, even compared with A. albogularis View in CoL , to which it is similar in cranial size, and the great local variability in size of the molars, unusual for a carnivore species with such a restricted distribution. Although more intensive study is desired to clarify the proportion of earthworms, other invertebrates and other elements in the diet of A. hoevenii View in CoL , we suggest that Arctonyx hoevenii View in CoL can join the distinctive Southeast Asian hemigaline civets ( Chrotogale View in CoL , Hemigalus View in CoL , Diplogale View in CoL ), the Malagasy endemic Eupleres View in CoL , the hyaenid aardwolf ( Proteles cristatus View in CoL ), and perhaps the Sloth Bear ( Melursus ursinus View in CoL ) and Bat-Eared Fox ( Otocyon megalotis View in CoL ) on the short list of carnivorans for which invertebrate prey constitute the dominant part of the diet ( Davis, 1962; Von Ketelhodt, 1966; Albignac, 1974; Payne & Francis, 1985; Richardson, 1987a, b; Dang, Anh & Huynh, 1992; Joshi, Garshelis & Smith, 1997; plus data from museum specimens). As in A. hoevenii View in CoL , the molars in these other invertebrate-eating carnivorans are reduced in size and complexity compared with their nearest, less-specialized phylogenetic counterparts (e.g. Gregory & Hellman, 1939; Ewer, 1973; Popowics, 2003; Sacco & Van Valkenburgh, 2004; Friscia, Van Valkenburgh & Biknevicius, 2007).
Like most other badgers, Sumatran hog-badgers are undoubtedly preyed upon by humans ( Schneider, 1905) and by large carnivores (perhaps, as in the case of A. collaris , especially by wild cats), and are aggressive when approached. Jacobsen (in Robinson & Kloss, 1919: 265) noted:
The animal is of very savage disposition; if excited it emits a grunting sound, exactly as if somebody was snoring, and not the rumbling or drumming sound which is peculiar to other badgers, as for instance, in Mydaus .
Long & Killingley (1983) recounted a similar anecdote of a nocturnal encounter with A. hoevenii :
In the jungles of Sumatra a group of mountain climbers encountered a hog badger at 2:00 A.M., and they ‘blinded’ it with a flashlight. It did not run, but because of its fierce snarling and growling it could not be approached. Finally it turned away and began to dig itself into the dirt.
Despite its small size, A. hoevenii is tenacious and strong. Ulmer (quoted above) noted how an animal ‘pulled free, leaving only a bunch of hair’ from a heavy steel trap; and Jacobsen (in Robinson & Kloss, 1919: 265) noted that his specimen from Gunung Kaba ‘was captured in a snare; its mate was caught the next day on the same spot but escaped by tearing the string.’
Essentially nothing is known of the social structure or reproduction in this species. Jacobsen’s reference to a mated pair may suggest he observed pair bonds, but nothing definitive is reported in the literature, and most anecdotal accounts of A. hoevenii mention encounters of single animals. Like other Arctonyx , A. hoevenii has three pairs of mammae. Litter size is unknown, but one juvenile was collected in March 1939 ( Miller, 1942).
Arctonyx hoevenii View in CoL is probably common in many areas in the mountains of Sumatra. Van Schaik & Griffiths (1996), Augeri (2005) and Holden (2006) photographed it regularly in appropriate habitat (i.e. forests at and above 800 m) in both northern and central Sumatra. As noted above, Ulmer saw many badger ‘grubbings’ between 2600 and 2800 m on Gunung Leuser in northern Sumatra (so numerous as to ‘pock the ground in all directions’), and Jacobsen (as reported by Robinson & Kloss, 1919: 265) made similar observations on Gunung Dempo in southern Sumatra (‘I saw at an altitude of 1800–2600 m numerous traces of this badger; everywhere the soil was turned up for worms and I found there also its not-to-be-mistaken excrement’). Robinson & Kloss (1918) likewise reported that ‘at Sungei Kumbang and as high as Sungei Kring it was very common and traces of its burrowings were to be seen almost everywhere in dampish spots...’ The relatively long museum series taken between 800 and 1400 m on Gunung Merapi (ZRC, BMNH) is another potential indication of local abundance in appropriate habitat.
Holden (2006) noted that there are no specimens of A. hoevenii View in CoL at the Museum Zoologicum Bogoriense (MZB, now based in Cibinong), a fact that we have confirmed. Reference to specimens from ‘the Garo (= Karo) Highlands’ at MZB (then as ‘the Buitenzorg Museum’) by Robinson & Kloss (1918: 12–13) may be based on a mistaken impression that Schneider’s (1905) Karo Highlands specimen was deposited there. In fact, Schneider (1905) mentioned that his specimen, the first of A. hoevenii View in CoL to be deposited in a museum collection, is in the Zoological Museum (today the Musée Zoologique) at Strasbourg. Schneider noted that the local name for A. hoevenii View in CoL in northern Sumatra was Garum View in CoL , that it lived in burrows in the mountains and that its flesh was considered a local delicacy, perhaps because of its thick layer of subcutaneous fat. We do not know if Schneider’s collections are still to be found in Strasbourg today.
| ZRC |
Zoological Reference Collection, National University of Singapore |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Arctonyx hoevenii
| Helgen, Kristofer M., Lim, Norman T-L. & Helgen, Lauren E. 2008 |
Arctonyx collaris hoeveni
| , Robinson & Kloss 1918 |
Chrotogale
| Thomas 1912 |
Diplogale
| Thomas 1912 |
Garum
| Dall 1900 |
Hemigalus
| Jourdan 1837 |
Eupleres
| Doyere 1835 |
Arctonyx
| F. CUVIER 1825 |
A. collaris
| F. Cuvier 1825 |
Arctonyx
| F. CUVIER 1825 |
Arctonyx
| F. CUVIER 1825 |