Moina wierzejskii Richard, 1895
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4820.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:0870E141-C465-4EAB-BCF1-29477B916627 |
|
DOI |
https://doi.org/10.5281/zenodo.4434415 |
|
persistent identifier |
https://treatment.plazi.org/id/9C3DF803-FFC3-FF85-FF7E-F88ADCD03BBA |
|
treatment provided by |
Plazi |
|
scientific name |
Moina wierzejskii Richard, 1895 |
| status |
|
Moina wierzejskii Richard, 1895 View in CoL
( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 )
Moina wierzejskii Richard 1895: p. 195 View in CoL –199, figs. 9–13; Goulden 1968: p. 42–48, figs. 17a–e (reproduced from Richard 1895), 18a–e, 19a–d (original); Smirnov 1976: p. 206–208, figs. 187 (from Goulden 1968), fig. 188 (from Goulden 1968, with an exception of male antenna I).
? Moina wierzejskii in Olivier 1962: p. 217 View in CoL , pl. VIX, figs. 1–3 (reproduced from Richard 1895).
? Moina wierzejskii in Smirnov 1976 View in CoL : fig. 188 (antenna I of male from Birabén 1917).
Etymology. Richard named this species in honor of Dr. A. Wierzejski, a Polish scientist who discovered this species ( Wierzejski 1892), but wrongly identified it as M. brachiata (Jurine, 1820) ( Richard 1895) .
Type locality. “Environs de Port-au Prince” (surroundings of Port-au Prince ), Haiti, Great Antilles Archipelago in the Caribbean Sea, North America ( Richard 1895) .
Type material. Author’s syntypes at the Smithsonian National Museum of Natural History: 5 parthenogenetic females, 5 ephippial females and 5 males from “Mare T. Vienp. et Briqueterie. Port au Prince”, Haiti, coll. 01.05.1885, DGF 0800 ; 3 parthenogenetic females, 2 ephippial females and 1 male from “Carrefour. Port-auPrince ”, Haiti, DGF 0836 ; 10 parthenogenetic females, 6 ephippial females and 3 males from “ Mare Coucher Blain. Port au Prince ”, Haiti, coll. 01.05.1896, DGF 0751 .
Diagnosis. Species of large size for the genus (length of adult parthenogenetic female up to 1.15 mm). Parthenogenetic female with body shape typical of the genus. Surface of head and valves covered with fine hairs. Head without rostrum and dorsal head pore; ocellus absent. On the inner side of the valve, setulae after ventralmost setae not grouped. Preanal and anal margins of postabdomen covered by rows of short setulae. Basal pecten on the dorsal edge of postabdominal claw prominent. Antenna I relatively thick. Antenna II and thoracic limbs as for the genus. Ephippium brownish, containing two resting eggs. Its dorsal part with microsculpture represented by small polygons. In lateral view, macrosculpture of ephippium is represented by polygonal protruding knobs with wavy edges. Male with elongated body. Anteroventral portion of male valve with longer hairs than in female. Gonopore opening on the lateral surface of the postabdomen at some distance from base of postabdominal claw. Antenna I long, terminally with four thick bisegmented hooks of similar size. Thoracic limb I without exopodite. Stiff seta 1 of male limb I thick, with blunt tip.
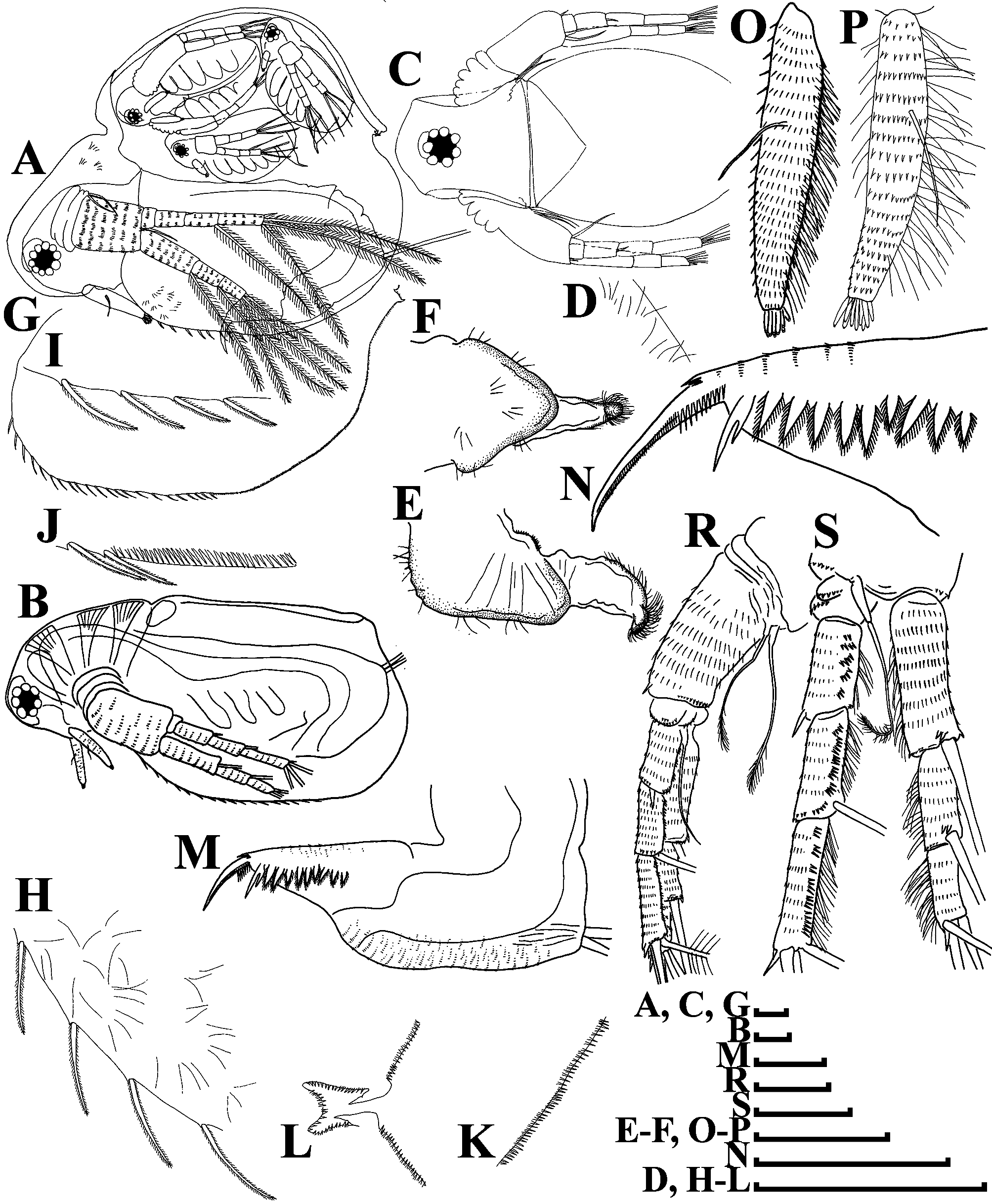
Redescription. Parthenogenetic female ( Figs. 1–2 View FIGURE 1 View FIGURE 2 , 4 View FIGURE 4 A–C). General. Body ovoid in lateral view, shape typical of the genus (body height/length ratio is about 0.50 – 0.80 for adults, varies significantly due to the extension of brood chamber’s development), maximum height in the middle ( Figs. 1A – B View FIGURE 1 ). Dorsal margin of valve elevated above head level ( Fig. 1A – B View FIGURE 1 ). Posterodorsal angle of carapace expressed, usually acute ( Figs. 1A – B, G View FIGURE 1 ). Posteroventral angle broadly rounded. Ventral margin of valve rounded, anterodorsal angle rounded ( Figs. 1A – B, G View FIGURE 1 ). Sculpture of valves fine, represented by cells elongated in dorsoventral direction (not shown on the figures). Fine hairs present on head and valve surface ( Figs. 1D, H View FIGURE 1 ). In dorsal ( Fig. 1C View FIGURE 1 ) and ventral view, body subovoid, laterally compressed in anterior view.
Head typical of the genus, with a shallow supra-ocular depression ( Figs. 1A – B View FIGURE 1 ). Compound eye large, ocellus absent. Dorsal head pores absent ( Figs. 1C View FIGURE 1 ).
Labrum with a fleshy main body, its ventral margin almost straight, labral plate covered terminally by long setulae ( Figs. 1 View FIGURE 1 E–F).
Valve large, ovoid ( Figs. 1 View FIGURE 1 A–B, G). Anterior portion of ventral margin with relatively long setae, covered by minute setulae ( Figs. 1 View FIGURE 1 H–I, 4A). Ventral margin and posteroventral valve portion with a row of fine ungrouped denticles after the posteriormost seta ( Figs. 1 View FIGURE 1 J–K, 4B–C). Setulated curved hooks in the dorsalmost portion at valve posterior margin ( Figs. 1A, L View FIGURE 1 ).
Thorax relatively long, abdomen short ( Figs. 1 View FIGURE 1 A–B).
Postabdomen elongated, conically narrowing distally ( Figs. 1 View FIGURE 1 M–N). Its shape and armature typical of the genus. Ventral margin of postabdomen straight, with transverse rows of fine setulae ( Fig. 1M View FIGURE 1 ). Preanal portion long, gradually passing to anal margin. Preanal and anal portions covered by rows of fine short setulae ( Fig. 1M View FIGURE 1 ). Postanal portion conical, both distal margin and dorsodistal angles not expressed ( Figs. 1 View FIGURE 1 M–N). Laterally postanal portion of postabdomen bearing a row of 9–10 large, triangular, plumose teeth. Anteriormost tooth bidentate, with branches unequal in length ( Figs. 1 View FIGURE 1 M–N).
Postabdominal setae long according to original description ( Richard 1895: p. 196, fig. 9), slightly shorter than postabdomen and covered by fine setulae throughout. Unfortunately, all specimens in our materials lost distal segments of postabdominal setae, probably, due to an unsuccessful fixation. Fine setulae on both distal and proximal segments of postabdominal setae were later illustrated by Goulden (1968: p. 42, fig. 17b), but not mentioned by Smirnov (1976). In our material we did not find setulae on proximal segments of postabdominal setae (and this observation corresponds well with observations on other species of the Moinidae ).
Postabdominal claw large, slightly curved, with pointed tip ( Figs. 1 View FIGURE 1 M–N). Its outer lateral side with two pec-tens; basal pecten consists of strong teeth; distal pecten consists of fine setulae ( Fig. 1N View FIGURE 1 ). Ventral margin of claw with several denticles on its basal portion ( Figs. 1 View FIGURE 1 M–N).
Antenna I rod-like, elongated (its length is approximately equal to six diameters of the antennular body base), slightly curved ( Figs. 1 View FIGURE 1 A–B, O–P). Antennular body covered by numerous fine long hairs and transverse rows of minute denticles. Antennular sensory seta slender, arising almost at middle of antennular body. Nine short aesthetascs subequal in size ( Figs. 1 View FIGURE 1 O–P).
Antenna II large, typical of moinids ( Figs. 1 View FIGURE 1 A–B, R–S). Its coxal part with two setulated sensory setae unequal in length ( Fig. 1R View FIGURE 1 ). Basal segment robust, with short distal spine on outer surface between exopod and endopod, and with a long seta on inner surface ( Figs. 1A View FIGURE 1 , R–S). Basal segment covered by numerous transverse rows of fine denticles and hairs. Antennal branches elongated ( Figs. 1 View FIGURE 1 R–S). Exopod four-segmented, slightly longer that threesegmented endopod, all segments cylindrical, covered by transverse rows of denticles and hairs. Antennal formula: setae 0-0-1-3/1-1-3, spines 0-1-0-1/0-0-1. Lateral and apical swimming setae of both antennal branches covered by long, fine setulae. Spine on second exopod segment short, comparable in length with both apical exopod and endopod spines.
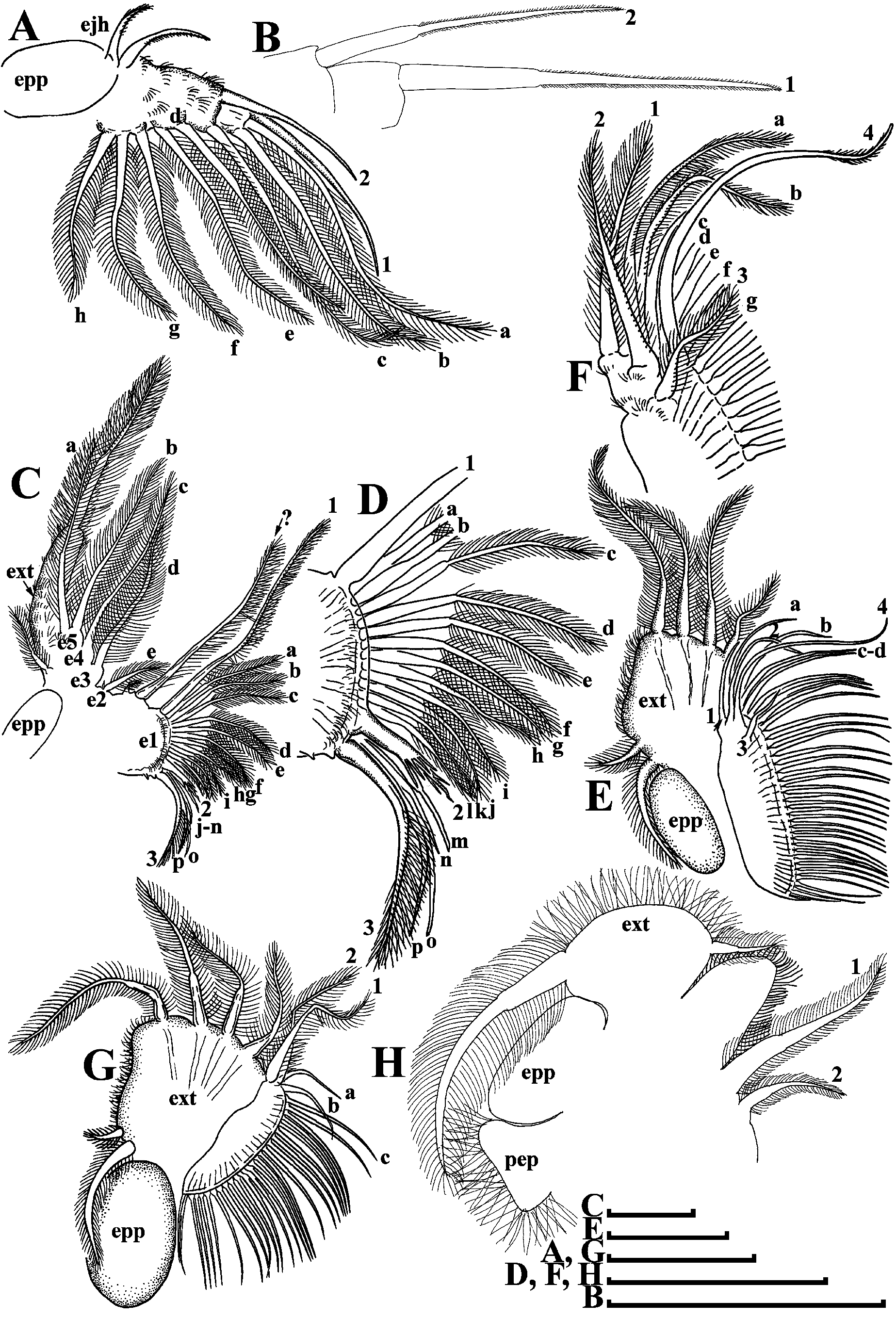
Thoracic limbs: five pairs ( Figs. 2 View FIGURE 2 A–H).
Limb I with elongate setulated corm and ovoid epipodite ( Fig. 2A View FIGURE 2 : epp). Inner distal lobe, or endite 5 (in terms of Kotov 2013) with a single anterior seta ( Figs. 2 View FIGURE 2 A–B: 1) covered by short setulae, and two posterior soft setae ( Fig. 2A View FIGURE 2 : a–b). All posterior soft setae in the limb I bisegmented and densely covered by long setulae. Endite 4 with a single anterior seta ( Figs. 2 View FIGURE 2 A–B: 2) and a single posterior soft seta ( Fig. 2A View FIGURE 2 : c). Endite 3 without anterior setae and with two posterior setae ( Fig. 2A View FIGURE 2 : d–e). Endite 2 with three posterior soft setae ( Fig. 2A View FIGURE 2 : f–h). Two ejector hooks of remarkably different size ( Fig. 2A View FIGURE 2 : ejh).
Limb II with ovoid large epipodite ( Fig. 2C View FIGURE 2 : epp). Exopodite ( Fig. 2C View FIGURE 2 : ext) as elongated, densely setulated lobe bearing a single soft seta and a small setulated soft lateral seta of unknown homology. Endites e5–e2 form a corm of limb II. Endite 5 with two posterior soft setae ( Fig. 2C View FIGURE 2 : a–b); endite 4 with a single posterior soft seta ( Fig. 2C View FIGURE 2 : c); endite 3 also with a single posterior soft seta ( Fig. 2C View FIGURE 2 : d). The homology of the setae located on the edge of the gnathobase (endite 1) is unclear. It seems that endite 2 itself includes only a small posterior soft seta with small sensillum near its base ( Fig. 2C View FIGURE 2 : e). So-called “beating seta” (in terms of Kotov et al. 2005) ( Fig. 2C View FIGURE 2 :?) and a small seta near its base are separated via a shallow incision ( Fig. 2C View FIGURE 2 ). Also, they are separated by an incision from gnathobase body. Gnathobase (endite 1) with two clear rows of setae ( Figs. 2 View FIGURE 2 C–D); there are three anterior setae ( Figs. 2 View FIGURE 2 C–D: 1–3) and 16 posterior setae ( Figs. 2 View FIGURE 2 C–D: a–p) that form the gnathobase filter plate. Seta 1 (located near “beating seta”) long, mostly unilaterally covered by long setulae. Setae 2 and 3 significantly shorter, located in the basal corner of the gnathobase; seta 2 armed by robust short setulae, and seta 3 with relatively long setulae ( Figs. 2 View FIGURE 2 C–D).
Limb III with large ovoid epipodite ( Fig. 2E View FIGURE 2 ). Exopodite almost rectangular, bearing four distal and two lateral setae ( Fig. 2E View FIGURE 2 ). Inner distal portion of limb with three endites: endite 5 with a single posterior ( Figs. 2 View FIGURE 2 E–F: 1) and a single anterior ( Figs. 2 View FIGURE 2 E–F: a) setae; endite 4 with two posterior setae ( Figs. 2 View FIGURE 2 E–F: b–c) and with single anterior seta ( Figs. 2 View FIGURE 2 E–F: 2), endite 3 with a single anterior ( Figs. 2 View FIGURE 2 E–F: 3) and four posterior setae ( Figs. 2 View FIGURE 2 E–F: d–g). All other parts of limb inner margin as a single large lobe (gnathobase), bearing numerous setae ( Figs. 2 View FIGURE 2 E–F). A single anterior seta ( Figs. 2 View FIGURE 2 E–F: 4) in its distal corner.
Limb IV with large ovoid epipodite ( Fig. 2G View FIGURE 2 ). Exopodite, similarly to that in limb III, with four distal and two lateral setae. Inner distal portion of this limb with two endites: distal endite with an anterior seta ( Fig. 2G View FIGURE 2 : 1) and a posterior seta ( Fig. 2G View FIGURE 2 : a); and next endite with an anterior seta ( Fig. 2G View FIGURE 2 : 2) and two posterior setae ( Fig. 2G View FIGURE 2 : b–c). Most part of the limb inner portion is represented by the gnathobasic filter plate, consisting of numerous setae.
Limb V with an elongated pre-epipodite ( Fig. 2H View FIGURE 2 : pep) and a large ovoid epipodite ( Fig. 2H View FIGURE 2 : epp). Large ovoid exopodite ( Fig. 2H View FIGURE 2 : ext) provided with a large distal, and a small apical setae. Inner limb portion as a flat setulated ovoid lobe and two unequal setae ( Fig. 2H View FIGURE 2 : 1–2) of unclear homology.
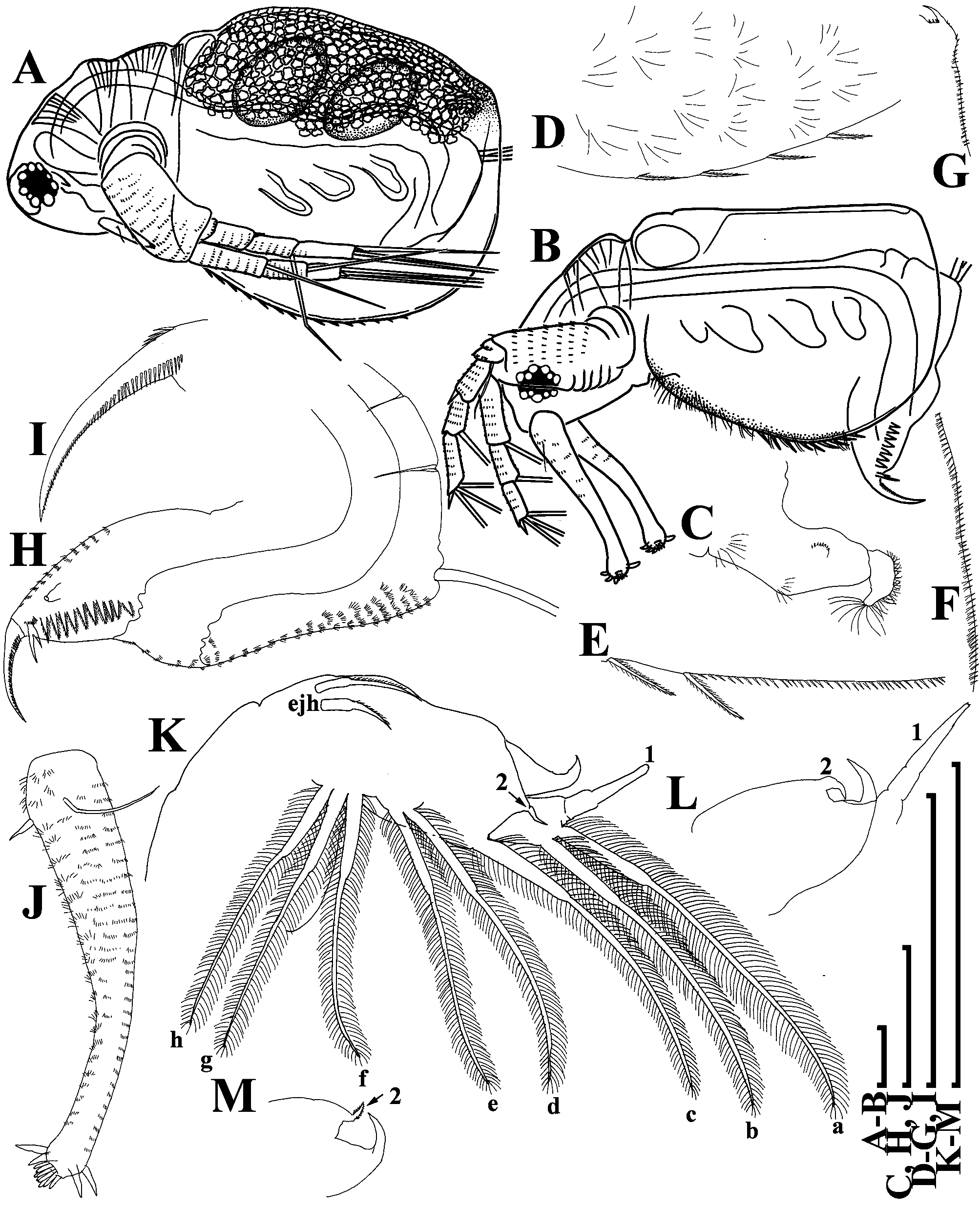
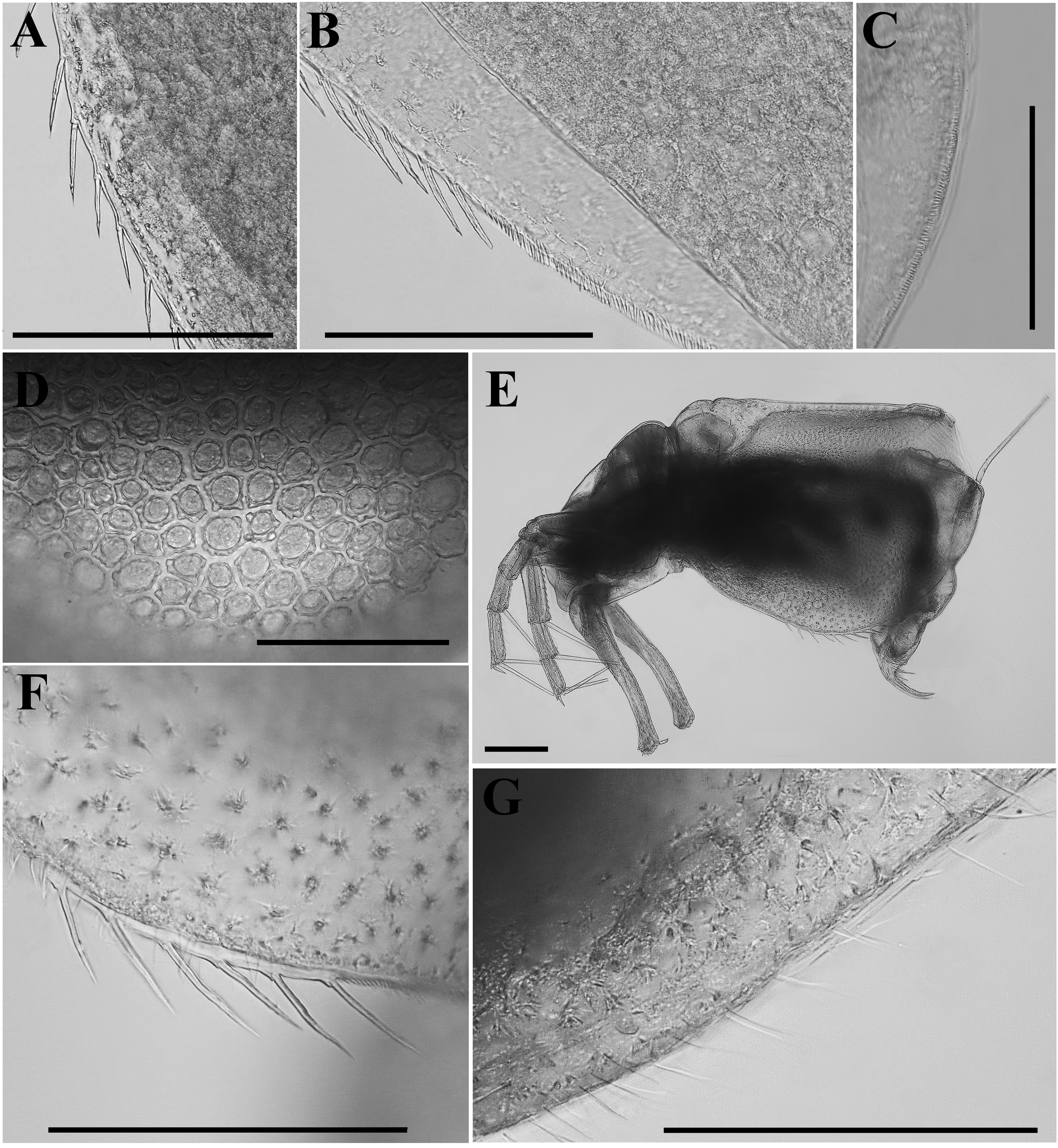
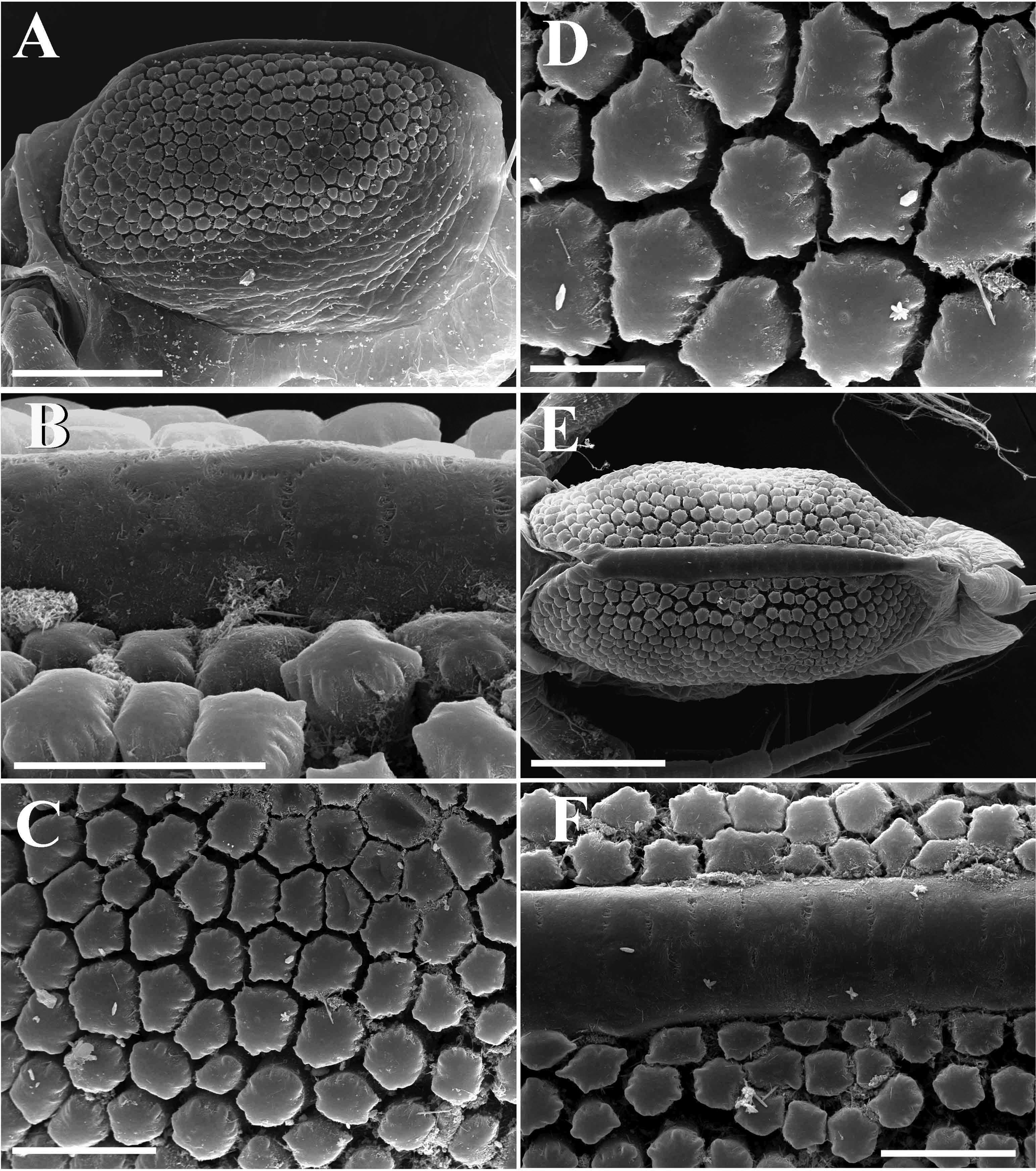
Ephippial female ( Figs. 3A View FIGURE 3 , 4D View FIGURE 4 , 5 View FIGURE 5 A–F). Characters of ephippial female as in parthenogenetic female except for the dorsal portion of valves which is modified into a dark brown ephippium, containing two resting eggs ( Fig. 3A View FIGURE 3 , 5A View FIGURE 5 ). Dorsal part of valves with a reinforced dorsal wrinkled, chitinous plate ( Figs. 5 View FIGURE 5 A–B, E–F). Micro sculpture of this plate is visible only under SEM and represented by small polygons ( Figs. 5B, F View FIGURE 5 ). In lateral view, macro sculpture of anterior portion of ephippium and eggs chamber consists of polygonal protruding knobs with wavy edges; they are well visible also under light microscope ( Figs. 4D View FIGURE 4 , 5A View FIGURE 5 , C–F). In posterior and ventral portion of ephippium, macro sculpture represented by elongated protuberances with smooth edges or anastomosing lines ( Fig. 5A View FIGURE 5 ).
Male ( Figs. 3 View FIGURE 3 B–M, 4E–G). In lateral view, body ovoid, more elongated as compared to female (body height/ length about 0.55). Dorsal margin of valve slightly elevated above head; posteroventral angle distinct ( Figs. 3B View FIGURE 3 , 4E View FIGURE 4 ).
Head more elongated than in female, also covered by fine hairs ( Figs. 3B View FIGURE 3 , 4E View FIGURE 4 ). Dorsal head pores absent. Compound eye large, ocellus absent.
Labrum as in females ( Fig. 3C View FIGURE 3 ).
Valve ovoid, more elongated than in female ( Figs. 3B View FIGURE 3 , 4E View FIGURE 4 ). Its anterior surface also covered by fine hairs (longer than hairs in females) ( Figs. 3B, D View FIGURE 3 , 4 View FIGURE 4 E–G).Armature of ventral margin of valve as in female ( Figs. 3 View FIGURE 3 D–F). Setulated curved hooks located in the dorsal most portion of posterior margin of valve ( Fig. 3G View FIGURE 3 ).
Thorax relatively long, abdomen short ( Fig. 3B View FIGURE 3 ).
Postabdomen in general as in female ( Fig. 3H View FIGURE 3 ), but denticles on lateral sides near base of claws are not grouped as in basal pecten of the female ( Figs. 3 View FIGURE 3 H–I). Gonopore opens on lateral surface of postabdomen at some distance from base of postabdominal claws ( Fig. 3H View FIGURE 3 ).
Antenna I significantly longer than in female ( Fig. 3B, J View FIGURE 3 ), curved, covered by tiny hairs and transverse rows of minute denticles. Long antennular sensory seta, arising from the proximal quarter of the antennula ( Fig. 3J View FIGURE 3 ). Male seta more robust, smaller and located near sensory seta ( Fig. 3J View FIGURE 3 ). Apical tip with two parts: first one with nine short aesthetascs, and the second one with four thick hooks ( Fig. 3J View FIGURE 3 ).
Limb I in general as in female, but with a large, curved copulatory hook ( Figs. 3 View FIGURE 3 K–M). Exopodite absent ( Fig. 3K View FIGURE 3 ). Stiff seta 1 of male limb I thick, with blunt tip ( Figs. 3 View FIGURE 3 K–L).
Size. Adult parthenogenetic females up to 1.15 mm in length; ephippial females up to 1.10 mm in length; adult males up to 0.70 mm in length.
Variability. In the specimens from Haiti investigated by us, no significant variability was found except for the size of the brood pouch in the parthenogenetic females. But Goulden (1968) reported a significant variability in the armature of the ventral side of the postabdominal claw. According to him, individuals from Kansas and Haiti (from original Richard’s type material (see Goulden 1968, p. 45, figs. b, d–e)) have thin and small denticles on the ventral side of the proximal portion of postabdominal claw. Goulden also found an individual from Kansas with a pecten of large denticles on one side of postabdominal claw, and only small denticles on the other side. It could be a sign that the size of denticles in the basal pecten of postabdominal claw may be subject to variability.
Also, according to Goulden (1968), a significant variability affects the development of tiny hairs on head and valves and on male antenna I. Hairs covered only a posterodorsal portion of the head and the anteroventral portion of valves in parthenogenetic females from Haiti, Texas, and Central California, however the head and valves of parthenogenetic females from Arizona, and Oregon were completely covered by hairs, and finally, he found tiny hairs on the dorsal portion of the postabdomen in a population from Kansas ( Goulden 1968, p. 45, figs. 18b, d). Richard did not illustrate hairs on head and valves of M. wierzejskii from Haiti ( Richard 1895: p. 196, figs. 9–10), and armature of dorsal portion of postabdomen seems to be represented by fine setulae rather than long hairs ( Richard 1895: p. 197, fig. 11). But Richard could overlook hairs on head and valves in parthenogenetic females, as the resolution of tools used at his time were significantly lower that recently.
We found parthenogenetic females with hairs on head and valves in Richard’s type specimens. According to our observation, armature of dorsal portion of postabdomen in parthenogenetic females from type material is represented by short setulae rather than long prominent hairs. All males in our material have antenna I with short hairs (see above). There is a chance that Goulden was dealing with males of another taxon, as usually several species of the Moinidae could coincide in the same water bodies and produce males simultaneously. Even in Richard’s material (tubes DGF 0800 and DGF 0806) we found specimens belonging to Moina micrura complex (parthenogenetic females, males, and ephippial females). In fact, it is not easy to discriminate males of M. wierzejskii and M. micrura complex under stereoscopic microscope before dissection. Males of M. wierzejskii may be easily distinguished from the males of M. micrura complex under a light microscope by presence of fine hairs on outer surface of valves and ungrouped denticles on posterior portion of ventral margin. Males of M. micrura complex have no hairs on valves, and denticles on posterior portion of ventral margin are grouped ( Elías-Gutiérrez et al. 2019). At the same time, discrimination of males of these two taxa based on armature of postabdominal claw maybe problematic due to its variability.
Taxonomic remarks. In contrast to Goulden (1968), Smirnov (1976) and Kotov & Ferrari (2010), we do not consider Moina platensis Birabén, 1917 as a junior synonym of M. wierzejskii . Birabén (1917: p. 266 fig. 7) illustrated the antenna I of male bearing very long hairs (comparable in length or even longer than the diameter of the antennular body), while in specimens from Richard’s material, hairs on male’s antenna I are very short ( Fig. 3J View FIGURE 3 ). At the same time, a parthenogenetic female, illustrated by Birabén (1917: p. 265 fig. 4–6), had an ovoid (almost spherical) shape of body, and a postabdomen with prominent long hairs on its dorsal edge. M. platensis share these features with M. affinis Birge, 1893 , or even with M. micrura var. ciliata Daday, 1905 ( Goulden 1968; Smirnov 1976). Both these taxa are known from the New World but, in contrast to M. wierzejskii , their gamogenetic females bear a single egg in the ephippium, and they belong to the small-sized moinids.According to Birabén (1917: p. 264), “adult female length of M. platensis is between 1.75 mm and 1.20 mm; body width varies according to greater or less extension of embryo’s development”, so, based on original measurements, this taxon belong to the large-bodied moinids. At this stage, we offer to consider M. platensis as “species inquirenda ”.
Distribution and ecology. Illustrated records of M. wierzejskii are known from Haiti ( Richard 1895; Goulden 1968) and Arizona and Kansas ( Goulden 1968). M. wierzejskii was also found in Mexico ( Elías-Gutiérrez et al. 1999), but these records were not supported by illustrations. Records from Argentina (e.g. Olivier 1962) were not supported by original illustrations. Olivier (1962) just reproduced figures of Richard (1895). The accepted distribution range of M. wierzejskii includes South America ( Goulden 1968; Smirnov 1976; Paggi 1997; Echaniz & Vignatti 2010; Battauz et al. 2013), but this idea must be specially checked from an integrative taxonomy approach due to the high cryptic diversity showed for the genus ( Bekker et al. 2016; Elías-Gutiérrez et al. 2019; Montoliu-Elena et al. 2019).
Preliminarily, we may conclude, that M. wierzejskii inhabits temporary pools, roadside ditches and ponds in tropical and subtropical regions of the New World.
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Moina wierzejskii Richard, 1895
| Neretina, Anna N., Kirdyasheva, Anna G. & Kotov, Alexey A. 2020 |
Moina wierzejskii
| in Smirnov 1976 |
Moina wierzejskii
| in Olivier 1962: 217 |
Moina wierzejskii
| Richard 1895: 195 |