Eliomys melanurus (Wagner, 1840)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6604339 |
|
DOI |
https://doi.org/10.5281/zenodo.6604300 |
|
persistent identifier |
https://treatment.plazi.org/id/9B215C43-FFD2-DD14-C96D-F38AFB15F86A |
|
treatment provided by |
Carolina |
|
scientific name |
Eliomys melanurus |
| status |
|
27. View On
Black-tailed Garden Dormouse
French: Lérot dAsie / German: Loffelbilch / Spanish: Liron careto de cola negra
Other common names: Asian Garden Dormouse, Large-eared Garden Dormouse
Taxonomy. Myoxus melanurus Wagner, 1839 ,
Sinai. Restricted by J. A. Nader and colleagues in 1983 to “vicinity of Mount Sinai,” Egypt.
In 1983, Nader and colleagues clarified the publication date of A. Wagner's original description of genus Eliomys and this species to be 1839, and restricted the type locality to the vicinity of Mt Sinai, Egypt. Skull of the holotype, which was thought to be lost, was located and figured by R. Kraft and B. Krvstufek in 2009. This species has historically been listed as a synonym of E. quercinus as exemplified by J. Niethammer in 1959 and G. B. Corbet in 1978, but it has subsequently been considered a valid species based on morphological, karyological, and genetic studies reviewed by M. E. Holden in 2005 and G. C. L. Perez and colleagues in 2013; recent mtDNA and nDNA analyses by C. Montgelard and colleagues in 2003, and Perez and colleagues in 2013 provide strong support for its recognition as a valid species. Montgelard and colleagues in 2003 estimated that E. melanurus and E. quercinus diverged c.7 million years ago during the early Miocene. Distribution and taxonomy of E. melanurus remains unresolved as discussed by Holden in 2005 and Perez and colleagues in 2013. The western African populations have been included in E. quercinus by researchers such as H. Kahmann and G. Thoms in 1981, Niethammer in 1959, and Krystufek and Kraft in 1997, or M. G. Filippucci and colleagues in 1988, Filippucci & T. Kotsakis in 1994, and Filippucci and E. Capanna in 1996 treated them as populations of E. melanurus . Holden in 2005 agreed with Krystufek & Kraft in 1997 that E. melanurus only occurs in eastern North Africa and the Middle East and suggested, as did M. Delibes and colleagues in 1980, that the western North African populations should be recognized as a separate species, E. munbyanus . Results of mtDNA sequence analysis by Perez and colleagues in 2013 provide some support for recognition of two closely related species in North Africa and the Near East, as opposed to E. quercinus , but also raise additional questions regarding geographical limits and biology of these putative species; chromosomal analysis, however, was contradictory. Further integrative studies are required to resolve the distributional limits and evolutionary relationships of this species. Monotypic.
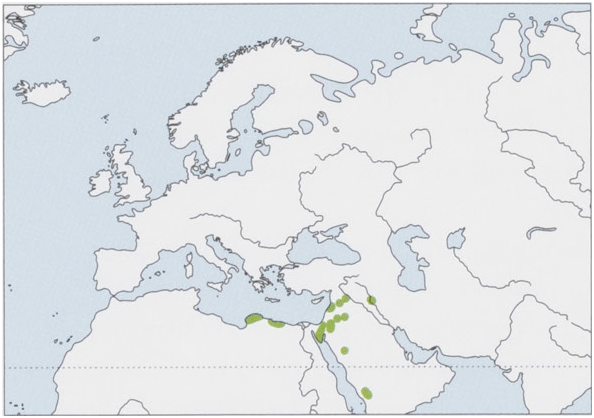
Distribution. Disjunct distribution in the E Mediterranean, the Middle East, and the Arabian Peninsula, from E Libya (Barqah, Cyrenaica), Egypt, and the Sinai Peninsula, S to SW Saudi Arabia, and E to N Syria and N Iraq; also recorded in S Turkey, but the last published record is more than 50 years old. View Figure
Descriptive notes. Head-body 111-144 mm, tail 100-136 mm, ear 26-29 mm, hindfoot 26-27 mm; weight 38-4-63 g. No sexual dimorphism reported. Compared with other members of this genus, Black-tailed Garden Dormice have longer ears and tails, longer tooth rows, and average larger in body sizes. Dorsal pelage ranges from medium grayish brown to pale gray or pale yellowish gray. Pelage is soft and long. Ventral pelage appears predominantly white or cream, and dorsal and ventral pelage colors are well delineated. Head color on crown generally matches that of dorsal pelage but becomes conspicuously paler on top of snout. A thick, conspicuous black eye mask extends from above and below ear pinnae, broadly encircles eyes, but does not extend to roots of vibrissae; nose and lips are thinly haired thus appear almost naked and pink. Ears are brown, very long, and ovate; white preand post-auricular patches are present. Hindfeet are sparsely haired, with white fur and moderately long, c¢.21% of head-body length. Tail is long, ¢.95% of head-body length. Dorsaltail color is black, or black with faint white tip, except color of base of tail is similar to dorsal pelage; ventral tail coloration similar to dorsal surface. Greatest length of skull is 34.2-37 mm, zygomatic breadth is 19-8-22 mm, and upper tooth row length 5-3 mm. External and cranial measurements listed are of eastern Libya specimens. Chromosome numberis 2n = 48. Females have four pairs of nipples (I pectoral + 1 abdominal + 2 inguinal = 8).
Habitat. Rocky areas in coastal dunes and adjacent inland plateaus, Mediterranean scrubland, escarpments, steppe-deserts, rocky areas, sandstone outcrops, alpine, and gardens and human dwellings from sea level to elevations of 2850 m. In northern Africa and the Sinai Peninsula, Black-tailed Garden Dormice were recorded from upper slopes of a large wadi (river bed that are dry for most of the year) near edge of the coastal escarpment, at the base of a large evergreen bush. Individuals have also been captured in limestone cliffs in coastal desert. Black-tailed Garden Dormice have been found in and around human dwellings, in gardens, beside a small mountainside garden at 1700 m, and in Bedouin tents and huts. In 2014, A. N. Alagaili and colleagues captured individuals in the Raidah Protected Area, adjacent to Asir Mountains National Park, Saudi Arabia, where juniper forests cover rocky upper slopes at 2500-3000 m, and deciduous trees and shrubs grow at lower elevations in the wadis; at anothersite in the Asir range, Nader and colleagues in 1983 also collected them among large boulders in sparse Acacia (Fabaceae) scrub forest at 2000 m. In the Middle East, they dwell in rocky terrain, usually in scrub, dense bushes, and forested habitats in wadis, along river banks, in cliffs and limestone hills, on slopes of the Anti-LLebanon Mountains, and on the margin of the lava desert in Jordan; individuals have also been captured in gardens and along stone walls in oases. In 2010, G. Shenbrot and colleagues only collected individuals in deep valleys filled by loess with densely vegetated wadi among rocky hills in the Negev Desert. In Iraq,it is restricted to rocky steppe in the northern part of the country.
Food and Feeding. The Black-tailed Garden Dormouse is omnivorous, predominantly insectivorous and carnivorous. Stomach contents have included insects and other invertebrates such as snails and centipedes; small vertebrates such as small mammals and a fan-fingered gecko (Ptyodactylus) have also been recorded. Two individuals caged together entered torpor or “partial hibernation,” during which time one of the individuals preyed on the other and consumed the entire animal exceptfor tail, hindfeet, and cranium; brain was also consumed.
Breeding. In captivity, mean litter size of the Black-tailed Garden Dormouse was 2-8 young, and gestation lasted c.22 days. Reproductively active females were collected in January and April-May, and males with enlarged testes were captured in January and at the end of April; immature individuals were recorded in May-July. Juveniles raised in captivity had an average body mass of 15 g at 30 days, reached 42 g at 90 days, and stabilized at 65 g by 200 days. Adult dentition was fully erupted by 80 days. It was also observed that young could move with a lateral walking gait by the end of the third post-natal week.
Activity patterns. The Black-tailed Garden Dormouse is nocturnal. Individuals from Saudi Arabia studied under different lighting conditionsin the laboratory at 25°C were active almost exclusively during the night, with an activity peak c.2 hours after onset of darkness. They also showed daily cycles of oxygen consumption and body temperature consistent with that for a nocturnal species, with both measures increasing during the dark period. Following a decrease of 10°C in ambient temperature, they became inactive and were either in daily torpor or hibernation. In the Negev Highlands, individuals are most active in early March to early June, because much fewer individuals are trapped during other months of the year. Individuals captured in the Negev Highlands were found to have a relatively low resting metabolic rate of 0-49 ml O,/g/h, and also entered daily torpor at ambient temperatures of 25°C; It has been hypothesized that low resting metabolic rate and ability to enter facultative daily torpor at comparatively high temperatures might not be characteristic of all populations, but adaptive for coping with less food availability, cooler temperatures, and aridity of the Negev Highlands compared with conditions in other parts ofits distribution. Ambient temperatures in this area ranges from above 30°C to below 0°C during winter; Black-tailed Garden Dormice captured in winter at ambient temperatures close to 0°C were found in traps in torpor with a body temperature of 12°C; torpor bouts can last up to several days.
Movements, Home range and Social organization. The Black-tailed Garden Dormouse is arboreal, terrestrial, and apparently solitary. It is probably uncommon in parts ofits distribution, especially in North Africa, based on low numbers of specimens obtained. In Israel, trap success during one night in spring was 117%. Densities of 0-14 ind/ha have been reported in the Negev Desert.
Status and Conservation. Classified as Least Concern on The IUCN Red List. The Blacktailed Garden Dormouse has a broad distribution and does not face any major identified threats. It tolerates a wide variety of habitats including those close to anthropogenic activity. They occur in several national parks and protected areas. Populations appear to be isolated, although sampling is difficult in many areas due to restricted access and civil and military conflicts.
Bibliography. Al-Sheikhly et al. (2015), Alagaili et al. (2014), Amori, Aulagnier et al. (2008a), Atallah (1978), Corbet (1978), Delibes et al. (1980), Eilam (1997), Filippucci & Capanna (1996), Filippucci & Kotsakis (1994), Filippucci, Catzeflis & Capanna (1990), Filippucci, Rodino et al. (1988), Filippucci, Simson et al. (1988), Flower (1932), Haim & Rubal (1994), Harrison & Bates (1991), Hartert (1923), Holden (2005, 2013), Kahmann (1981, 1987), Kahmann & Thoms (1981), Kraft & Krystufek (2009), Krystufek & Kraft (1997), Krystufek & Vohralik (2005), Montgelard et al. (2003), Nadachowski et al. (1978), Nader et al. (1983), Niethammer (1959, 1987), Obuch (2001), Osborn & Helmy (1980), Perez et al. (2013), Qumsiyeh (1996), Ranck (1968), Sannier et al. (2011), Setzer (1957), Shehab et al. (2009), Shenbrot et al. (2010), Wagner (1839), Wassif & Hoogstraal (1953), Zima et al. (1994).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.