Herpyllobius polynoes ( Krøyer, 1864 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4579.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A4015309-D9B3-4BB7-ABCB-B88A1F8CE5FC |
|
persistent identifier |
https://treatment.plazi.org/id/97720E2D-FFFA-D60F-CBF7-B801056DF67F |
|
treatment provided by |
Plazi |
|
scientific name |
Herpyllobius polynoes ( Krøyer, 1864 ) |
| status |
|
Herpyllobius polynoes ( Krøyer, 1864) View in CoL
Syn: Silenium polynoes Krøyer, 1864
Herpyllobius affinis Hansen, 1887
Norwegian material examined: 3♀♀ dorsally on prostomium of 3 specimens of Harmothoe imbricata , 3566 Nessar , Hopen Bank Top Stn 11 (76° 06.98’N, 23° 52.10’E), depth 56 m, 0 1 October 2009; collected by A. Sikorski; NHMUK Reg. No. 2015.2968-2970 GoogleMaps . 1♀ dorsally on prostomium of Harmothoe cf. imbricata, Barents Sea, Spitsbergen Bank Stn 11-5 (76° 07.00’N, 23° 51.3’E), depth 56 m, 0 8 August 1992; collected by A. Sikorski. NHMUK Reg. No. 2015.2966 GoogleMaps . 1♀ dorsally on prostomium of Harmothoe imbricata, Nessar 2007 , Hopen Bank Top Stn 11 (76° 07.40’N, 23° 53.23’E), depth 60 m, 17 August 2007; collected by A. Sikorski. NHMUK Reg. No. 2017.172 GoogleMaps . 1♀ dorsally on prostomium of H. imbricata , no locality data; collected by A. Sikorski; used for SEM. 1♀ dorsally on prostomium of Harmothoe bifera, Mareano Bomtrål, Stn 821-16, depth unknown, 0 8 May 2012; collected by A. Sikorski; NHMUK Reg. No. 2015.2967 . 1♀ with 2 ♂♂ attached, dorsally on prostomium of Malmgreniella mcintoshi (Tebble & Chambers, 1982) , Mareano 2010110, Stn 624-373, depth unknown, 21 September 2010; collected by A. Sikorski; NHMUK Reg. No. 2015.2971. 1 immature ♀ dorsally on prostomium of M. mcintoshi, Mareano Bomtrål, Stn 870-27, depth unknown, 10 October 2010; collected by A. Sikorski; NHMUK Reg. No. 2015.2972 . 2♀♀ dorsally on prostomium of Eunoe ?barbata, H1- Trål , North Cape Bank, Stn Brønn (72.01°N 25.3°W), depth 245 m; 15 August 2007; collected by A. Sikorski; NHMUK Reg. No. 2015.2973 GoogleMaps . 2♀♀ dorsally on prostomium of 2 specimens of Eunoe nodosa , 3703 Mareano 2013, Stn 1218-471, depth unknown, 17 August 2013; collected by A. Sikorski; NHMUK Reg. No. 2016.543-544 . 2♀♀ dorsally on prostomium of E. nodosa , 7018 Melkøya 2014, Stn 3-2 (70.67405°N 23.60273°E), depth 115 m; 12 August 2014; collected by A. Sikorski; NHMUK Reg. Nos 2017.170-171 GoogleMaps .
British material examined: 1 ovigerous ♀ from Gattyana cirrhosa (also with an ovigerous ♀ of another copepod, Selioides bocqueti , attached dorsally behind the host head), St. Abbs Sludge Disposal Grounds, Stn1 (56° 06.50’N, 02° 07.25’W), depth 51 m, 16 June 1992. 1 ovigerous ♀ from G. cirrhosa , (also with ♀ Selioides bocqueti ) Northumberland coast, Stn 20, 0 5 May 1993 ; collected by P. Garwood (see O’Reilly & Geddes 2000); NHMUK Reg. No. 2017.480. 1 ♀ from G. cirrhosa , Northumberland coast, Stn 47, 0 4 June 1993 ; collected by P. Garwood (see O’Reilly & Geddes 2000). 1 ♀ from Harmothoe antilopes, Firth of Clyde, Garroch Head, SEPA Stn T7.1 (55° 38.82’N, 04° 01.45’W), depth 139 m, 9 November 2000 GoogleMaps . 1 ovigerous ♀ from G. cirrhosa, Firth of Clyde, Cloch Point, Stn CMT7 (55° 56.85’N, 04° 53.65’W), depth 80 m, 13 April 2004 GoogleMaps . 3 ♀♀ (1 ovigerous) from single G. cirrhosa, Loch Striven, Ardyne Point Fish Farm, Stn R 1-1 (55° 53.218’N, 05° 03.336’W), depth 40 m, 24 August 2006; collected by J. Richard. 1♀ (+ 2♂♂), 1 immature ♀ (+ 10♂♂), 1 immature ♀ (+ 2♂♂), from single G. cirrhosa , ATL 1.3 FB (locality data unknown); collected by P. R. Garwood. 1♀ from G. cirrhosa , ATL 1.6 FB (locality data unknown); collected by P. R. Garwood. 1♀ from G. cirrhosa, Blyth , ENTEC, Stn 41 (locality data unknown) , collected by P. R. Garwood. NHMUK Reg. Nos 2018.128-133 .
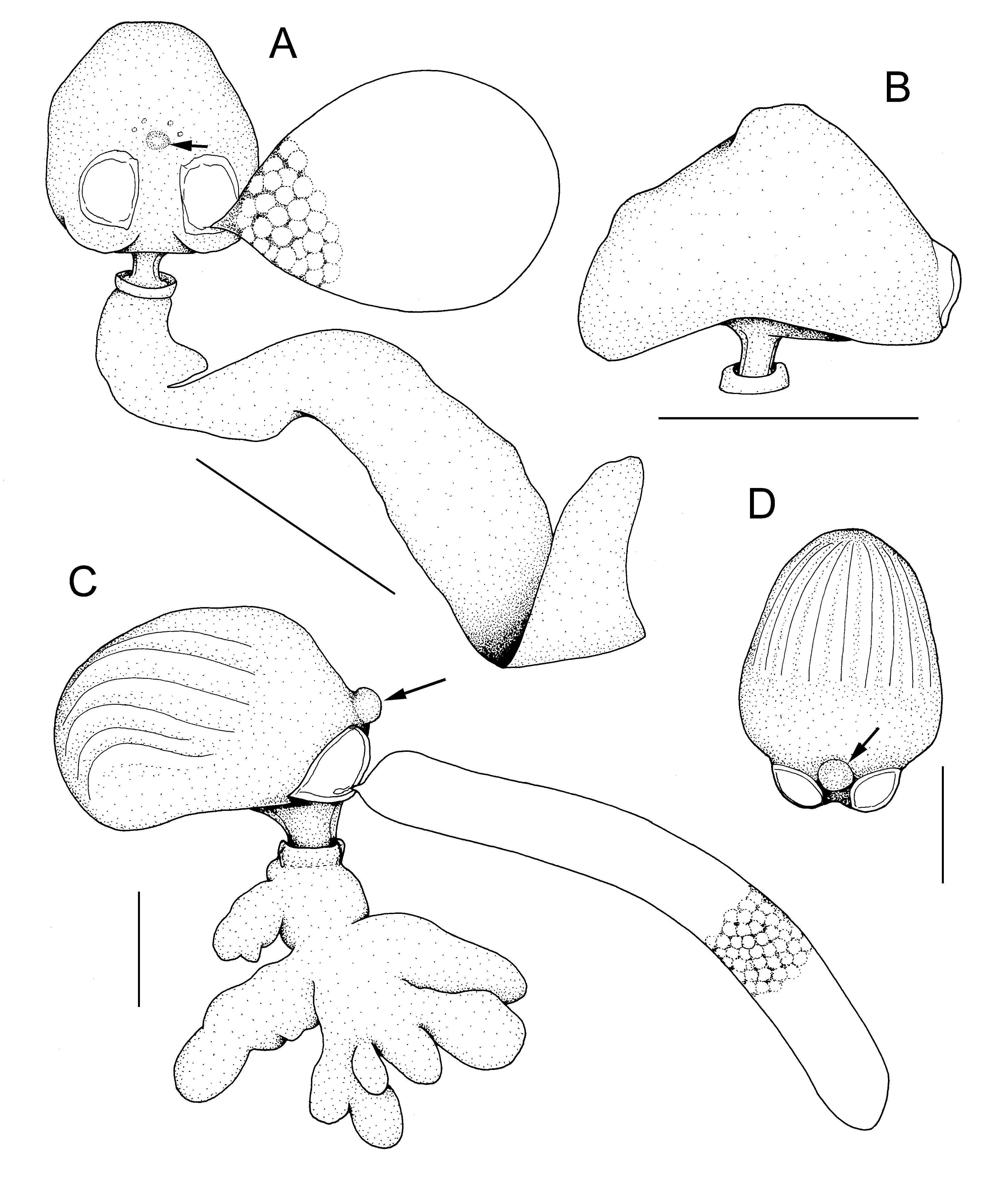
Differential diagnosis. Adult female ectosoma ( Fig. 7A, B View FIGURE 7 ) about 1.5 times longer than wide and often slightly pointed posteriorly; underside flat, or slightly concave; dorsal surface sometimes humped near middle. Genital swellings prominent. Small median swelling located on posterior surface dorsal to genital apertures; four minute sclerotized dots arranged in arc dorsal to median swelling. Stalk short, slender, originating from underside about one third to half of ectosoma length anterior to genital swellings. Endosoma flattened, 2 to 3 times longer than ectosoma (e.g. female with ectosoma 1.86 mm in length, with endosoma 5.8 mm long).
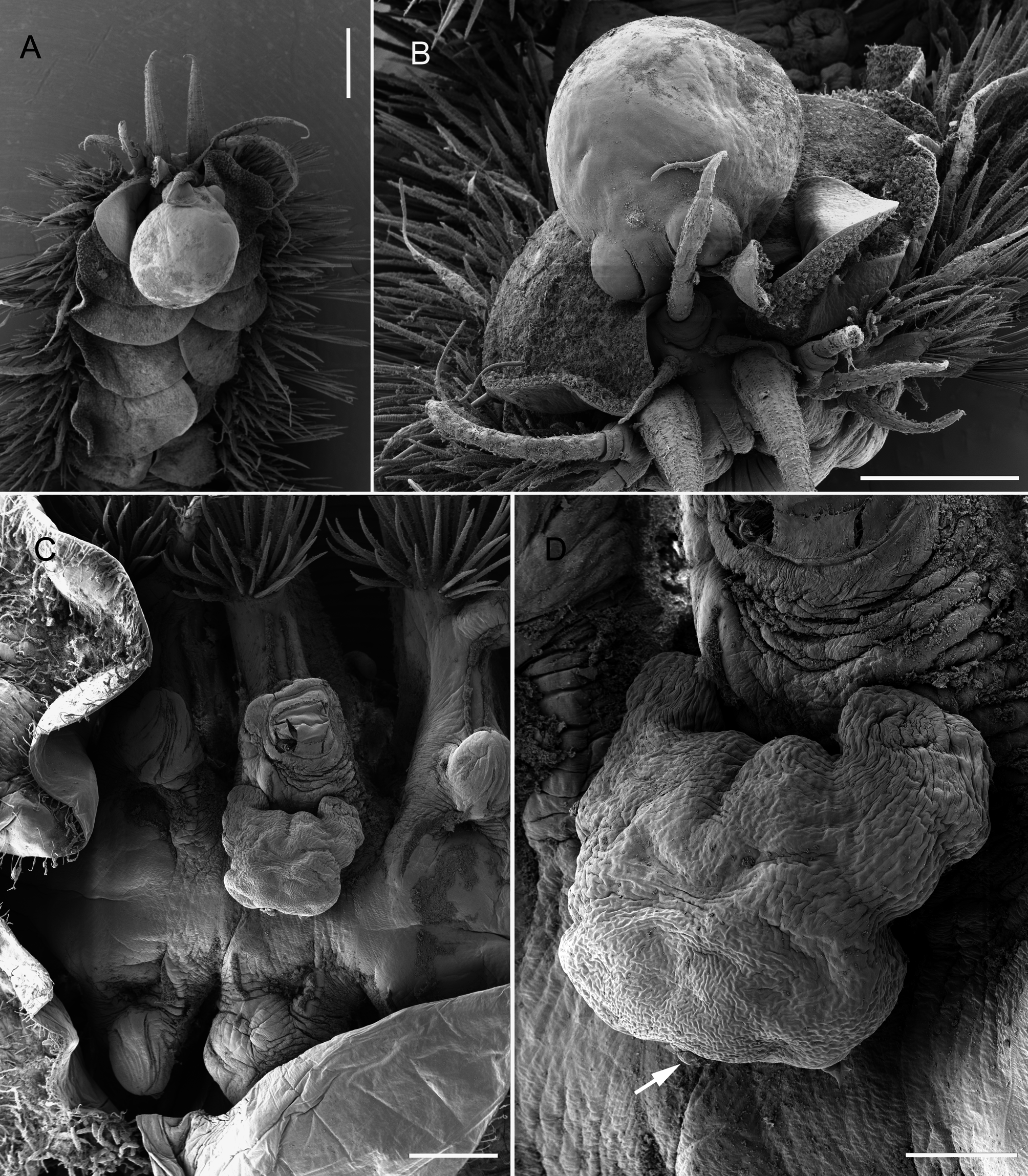
Description of Female from Eunoe ?barbata: Ectosoma of adult female slightly pointed at anterior end and humped in mid-dorsal surface ( Fig. 6A, B View FIGURE 6 ). Ectosomal length 1.50 mm, about 1.7 times longer than maximum width. Genital swellings ( Fig. 6A View FIGURE 6 ) prominent, moderately sclerotized; genital apertures about 310 µm high by 240 µm wide, separated by gap of about 180 µm. Small swelling located in mid-line between and just dorsal to genital swellings (arrowed in Fig. 6A View FIGURE 6 ); four minute sclerotized dots arranged in arc dorsal to median swelling. Stalk short, about 100–105 µm in diameter, originating on underside of ectosoma close to mid-body. Endosoma elongate, in form of flattened lobe 3.27 mm in length and 0.60 mm in maximum width, with simple margins. Egg sacs pearshaped ( Fig. 6A View FIGURE 6 ), up to 1.45 mm long by 1.0 mm wide, eggs multiseriate, about 80 µm in diameter.
Remarks. According to Lützen (1964a) this species always attaches dorsally on the prostomium of its host ( Fig. 5A View FIGURE 5 ). In northern Atlantic waters, its predominant host is Harmothoe imbricata but its known host range includes Austrolaenilla mollis , Bylgides promamme , B. sarsi , Eunoe nodosa , Gattyana amondseni , G. cirrhosa , Gaudichaudius iphionelloides , Harmothoe aspera , H. extenuata , and H. impar (see Boxshall & Halsey 2004). In UK waters the predominant host is G. cirrhosa ( O’Reilly 1999; O’Reilly & Geddes 2000; O’Reilly et al. 2011) but it also been reported on H. antilopes ( O’Reilly et al. 2011) . Three of the hosts recorded here, Harmothoe bifera , Malmgreniella mcintoshi and Eunoe ?barbata are new hosts for H. polynoes . All known hosts of H. polynoes are members of the subfamily Polynoinae . Herpyllobius polynoes may co-occur on the same host specimen with the herpyllobiid copepod Eurysilenium truncatum (see O’Reilly et al., 2011) or with the nereicolid copepod Selioides bocqueti (this paper and O’Reilly & Geddes, 2000).
The sole female from Eunoe ?barbata was attached to the dorsal surface of the prostomium of its host, with its paired genital apertures and eggs sacs directed anteriorly. This is the classic site for H. polynoes according to Lützen (1964a). The endosoma of this female extended anteriorly into the everted proboscis of the host. The characteristic arrangement of four “sclerotized dots” in an arc dorsal to the median swelling, highlighted as a feature of H. polynoes by Lützen (1964a) can be difficult to observe in preserved material. They were visible in the female from Eunoe ?barbata only after it had been cleared in lactic acid and SEM images of a female from Harmothoe imbricata clearly show the median swelling ( Fig. 6B View FIGURE 6 ) but not the sclerotized dots. Lützen (1966) stated that these dots are cuticular thickenings although he noted that, in one large specimen of H. polynoes minute ducts from unicellular subsurface glands opened on them.
The ventral stalk connecting ectosoma and endosoma is located near the middle of the ectosoma in the female from Eunoe ?barbata. This is slightly more anteriorly placed than the typical position, about one third of the ectosoma length anterior to the genital swellings ( Lützen 1964a), but the shared possession of other features discussed above and the location on the prostomium of the host, support the identification of this parasite as H. polynoes .
| NHMUK |
Natural History Museum, London |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |