Hygrobates limnocrenicus Pešić, 2020
|
publication ID |
https://doi.org/ 10.24349/acarologia/20204400 |
|
publication LSID |
lsid:zoobank.org:pub:754BE1B0-A316-409B-8008-556CE54ED5E4 |
|
persistent identifier |
https://treatment.plazi.org/id/F010A08A-BFD9-4E57-9E22-E9CE9CDA740A |
|
taxon LSID |
lsid:zoobank.org:act:F010A08A-BFD9-4E57-9E22-E9CE9CDA740A |
|
treatment provided by |
Marcus |
|
scientific name |
Hygrobates limnocrenicus Pešić |
| status |
sp. nov. |
Hygrobates limnocrenicus Pešić sp. nov.
Zoobank: F010A08A-BFD9-4E57-9E22-E9CE9CDA740A
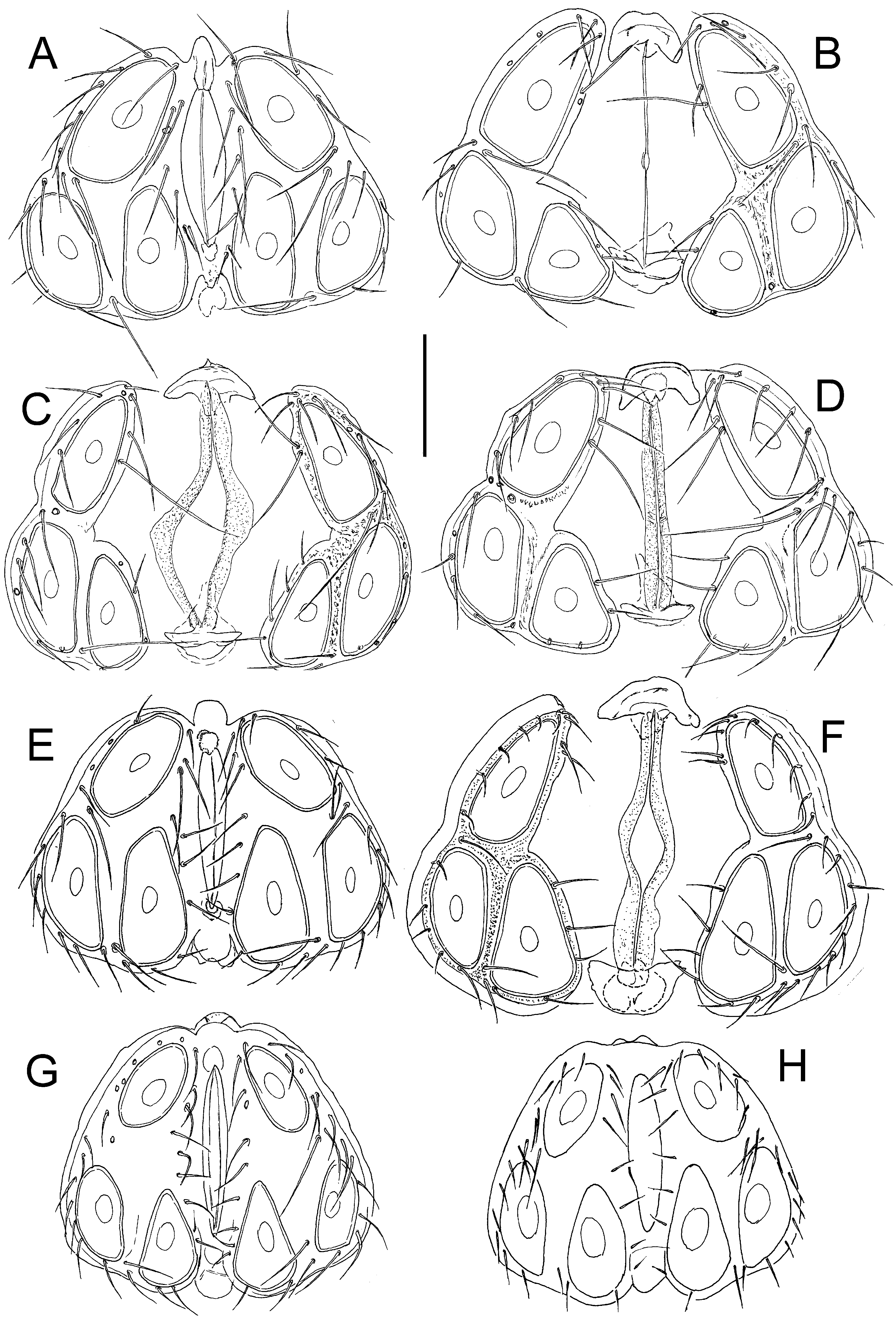
Figs. 5 View Figure 5 C-F, 6, 7
Synonym – Hygrobates setosus sensu Pešić et al. 2018a: 170 ; Bańkowska et al. 2016:
1028; Pešić et al. 2019c: 471.
Material examined — Holotype ♂ [19. M19_20_5_E4], sequenced, dissected and slide mounted, Montenegro, Podgorica , Mareza, outflow of limnocrene spring, 42°28 ′ 44.01 ″
N, 19°10 ′ 52.50 ″ E, 24.6.2019, leg. Pešić & Zawal. Paratypes: 1♀ (dissected and slide mounted), Podgorica, Daljam, limnocrene spring “Kraljičino Oko”, springbrook, 42°29 ′ 9.61 ″
N, 19°10 ′ 25.28 ″ E, 31.5.2018, leg. Pešić & Zawal; North Macedonia, Struga, Crni Drim River-outlet of Ohrid Lake , 41°11 ′ 3.39 ″ N, 20°40 ′ 40.88 ″ E, 12.9.2019, leg. Pešić, 1♀ [19. MECD2019 _3.2_C2], sequenced, conserved in Koenike fluid GoogleMaps .
Other material — Montenegro: Podgorica, Vitoja springs near Skadar Lake, 42°19 ′ 31.25 ″
N, 19°21 ′ 45.93 ″ E, 5.8.2017, leg. Pešić, 6♂♂, 8♀♀ (1♂ and 1♀ dissected and slide mounted); ibid., 30.7.2014, leg. Pešić, 1♂, 1♀ ; ibid., 31.5.2018, leg. Pešić & Zawal, 142 ex. (115 ex. in ethanol; 5♂♂, 4♀♀ in Koenike fluid; 2♂♂, 2♀♀ dissected and slide mounted) .
Compared material – Hygrobates setosus , Germany, Farver Au, 54°16 ′ 0.5556 ″ N,
10°48 ′ 9.0396 ″ E, small stream, 5.9.1992, leg. P. Martin, 2♂♂, 3♀♀ (2♂♂, 1♀ dissected and slide mounted).
Diagnosis — Large in size (mL of Cx-I + gnathosoma ˃ 350, L genital plate ˃ 200, P-4 ˃
170 μm); Cx-I+II apodemes moderately protruding, mediocaudal margins of Cx-IV in females without prominent apodemes; anterior margin of male genital field with a knob-shaped medial projection; L of IV-L-6 proximoventral seta ♂ ˃ 40, ♀ ˃ 35; running waters.
Description — General features – Colour yellowish to brown ( Fig. 8 View Figure 8 , inset). Integument finely striated. Posteromedial margin of Cx-I rounded, caudal apodemes of Cx-I+II welldeveloped ( Figs. 6A View Figure 6 , 7A View Figure 7 ); Cx-IV subtriangular. Genital field: Ac in a triangular position. P-2 ventral margin straight, distally forming a right angle, denticles covering distal half of ventral margin; P-3 with denticles covering distal two thirds of ventral margin ( Figs. 6 View Figure 6 B-C, 7C-D); P-4 ventral setae on the same level.
Male – Anterior margin of genital field convex, with a small knob-shaped medial projection, posterior margin indented, with a rounded central projection not extending beyond posterior genital plate margin ( Figs. 5E View Figure 5 ).
Female – Genital plates distinctly longer than gonopore ( Fig. 5 View Figure 5 C-D, F).
Measurements — Female (Holotype; in parentheses measurements of paratype from Crni Drim River, Macedonia; in square brackets measurements of specimens from Vitoja, given as range and mean, n = 3)
Idiosoma – L (1050), W (853) [1080-1210, χ = 1136]; coxal field: L 475 (525) [489-536, χ
= 514]; Cx-II W 453 (484) [406-513, χ = 472]; Cx-III W 634 (627) [689-753, χ = 721]; mL of
Cx-I + gnathosoma L 375 (403) [339-403, χ = 370]; distance between lateralmost ends of Cx-II apodemes, 203 (221) [197-213, χ = 202].
Genital field – L/W 241 (256) [259-281, χ = 271.7], W 353 (334) [356-386, χ = 371.3];
genital plate L 233-238 (236-241) [247-268, χ = 258]; gonopore L 194 (206) [209-238, χ =
224.3]; L gonopore/genital plate ratio 0.82-0.83 (0.85-0.87) [0.85-0.89, χ = 0.87]. L Ac 1-3:
109-116 (103) [109-116, χ = 112.7]; 108 (109-113) [112-117, χ = 114]; 84-86 (94) [106-124, χ
= 112].
Palp – total L 537 [568-625, χ = 598]; dL: P-1, 41 [48-45, χ = 47]; P-2, 147 [157-172, χ =
165]; P-3, 94 [109-123, χ = 115]; P-4, 183 [194-209, χ = 203]; P-5, 72 [63-73, χ = 68.3]; H: P-1,
55 [53-57, χ = 55]; P-2, 97 [100-108, χ = 104.3]; P-3, 80 [82-86, χ = 84]; P-4, 52 [50-53, χ =
51]; P-5, 27 [23-25, χ = 23.7]; dL/H ratio: P-1, 0.74 [0.84-0.87, χ = 0.86]; P-2, 1.52 [1.54-1.64,
χ = 1.59]; P-3, 1.18 [1.32-1.42, χ = 1.36]; P-4, 3.54 [3.88-4.15, χ = 3.99]; P-5, 2.7 [2.67-2.94,
χ = 2.85]; P-2/P-4 ratio 0.8 [0.81-0.82, χ = 0.81]. Chelicera total L 434 [403-442, χ = 423.3],
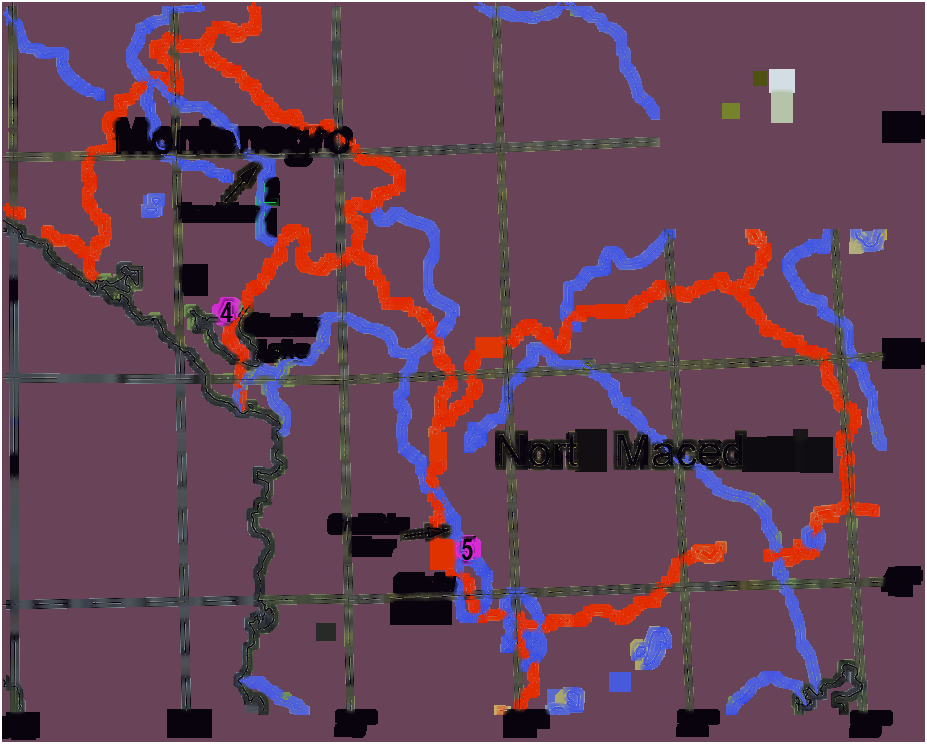
and Kraljičino Oko (Queen’s Eye) springs; 4, Vitoja springs; 5, Crni Drim River-outlet of Lake Ohrid. Inset: Photograph of H. limnocrenicus sp. nov.
L basal segment 278 [269-291, χ = 281], claw 163 [153-172, χ = 160], L basal segment/claw ratio 1.7 [1.65-1.87, χ = 1.76].
Legs – dL of I-L-1-6: 84 (86) [84-89, χ = 86]; 103 (116) [116-136, χ = 126.7]; 144 (169) [160-172, χ = 168]; 203 (244) [225-256, χ = 242.7]; 222 (255) [245-272, χ = 260]; 216 (240)
[238-256, χ = 248]. dL of IV-L-1-6: 163 [172-192, χ = 185], 167 [188-205, χ = 198], 266
[284-325, χ = 304], 353 (411) [413-466, χ = 440], 356 (414) [416-445, χ = 432], 309 (325)
[338-366, χ = 356]. L of IV-L-6 proximoventral seta 41 [37-55, χ = 44].
Male (specimens from Vitoja springs, Montenegro, measurements given as range and mean, n = 3)
Idiosoma – L 1040-1140, χ = 1086; coxal field: L 487-511, χ = 500; Cx-II W 459-500, χ
= 474; Cx-III W 631-700, χ = 656; mL of Cx-I + gnathosoma L 372-386, χ = 381; distance between very lateral ends of Cx-II apodemes, 190-216, χ = 206. Genital field L 227-258, χ =
243, W 300-322, χ = 311.7. L Ac 1-3: 100-122, χ = 110; 113-122, χ = 116; 116-134, χ = 126.
Palp – total L 546-585, χ = 561; dL: P-1, 46-50, χ = 48; P-2, 144-158, χ = 151.3; P-3, 105-113, χ = 108; P-4, 185-198, χ = 190.3; P-5, 61-66, χ = 63.3; H: P-1, 52-54, χ = 53; P-2,
94-98, χ = 95.3; P-3, 75-78, χ = 76; P-4, 42-50, 46.7; P-5, 23, χ = 23; dL/H ratio: P-1, 0.98-0.93,
χ = 0.91; P-2, 1.53-1.68, χ = 1.58; P-3, 1.4-1.44, χ = 1.42; P-4, 3.96-4.38, χ = 4.1; P-5, 2.68-2.8,
χ = 2.74; P-2/P-4 ratio 0.77-0.82, χ = 0.8. Chelicera total L 391-413, χ = 400.3, L basal segment
254-273, χ = 266, claw 147-153, χ = 150, L basal segment/claw ratio 1.66-1.86, χ = 1.77.
Legs– dL of I-L-1-6: 75-89, χ = 81.7; 113-121, χ = 117; 148-154, χ = 152; 213-228, χ =
220; 228-248, χ = 237; 225-239, χ = 232. dL of IV-L-1-6: 181-203, χ = 190; 181-188, χ =
183; 269-288, χ = 276; 396-411, χ = 404; 398-397, χ = 398; 331-341, χ = 335.3. L of IV-L-6
proximoventral seta 45-48, χ = 46.7.
Etymology — The species is named after its dominant occurrence in limnocrene springs.
Remarks — Due to its larger dimensions (and also regarding habitat preference), the new species is similar to H. lacrima sp. nov. Females of the latter species differ from H.
limnocrenicus sp. nov. in having well-developed apodemes at mediocaudal margins of Cx-IV
(compare Fig. 3A View Figure 3 with Fig. 6A View Figure 6 ), males in the medial projection of the genital field bluntly-
pointed (knob-shaped in H. limnocrenicus sp. nov.; compare Fig. 5A and 5E View Figure 5 ), both sexes bear a shorter proximoventral seta on IV-L-6 (L H. lacrima vs. H. limnocrenicus , ♂♂: ˂ 20 vs. ˃
40, ♀♀: ˂ 35 vs. ˃ 35 μm).
Analysis of COI sequences suggests that H. limnocrenicus sp. nov. is most closely related to H. setosus Besseling, 1942 , which has also a preference for running water habitats. However,
the latter species, as well as H. lacrima sp. nov., was mostly found in pools of running waters, while H. limnocrenicus sp. nov. prefers deeper, fast flowing water, typically being found in the outflow of limnocrenic springs or lake outlets ( Figs. 9 View Figure 9 D-F). The COI divergence between H. setosus and H. limnocrenicus sp. nov. was 12.43% (SD = 1.47). K2P indicating a long independent history of these two species. The studied specimens H of. setosus (in parentheses) differ in both sexes by a shorter proximoventral seta on IV-L-6 (L H. setosus vs. H.
limnocrenicus, ♂♂: ˂ 30 vs. ˃ 40, ♀♀: ˂ 20 vs. ˃ 35 μm) and in males by the comparatively less wide genital field (L/W ratio ˃ 0.8), a smaller dimensions of acetabula (Ac-3 ˂ 105 μm),
and the medial projection of the genital field less pronounced, typically with irregular margin of a secondary sclerotization (see Figs. 5 View Figure 5 G-H).
Biology — Morphological and genetical analysis of populations from the Vitoja springs situated at the northeastern shore of the Lake Skadar indicates that the oviposition data published by Bańkowska et al. (2016) for H. setosus in fact refers to H. limnocrenicus sp. nov. The highest number of females laying eggs was found in springs, with an average number of eggs per female of 58.2±30.6. A lower number of females laying eggs was noted in rivers, but here the average number of eggs per female (80±24.5) was higher ( Bańkowska et al. 2016). A statistically significant difference in number of laid eggs between May and October was found.
The average time of hatching was 14.1 ± 5.29 days.
Distribution — Montenegro and North Macedonia ( Fig. 8 View Figure 8 ). Details of distribution are unknown due to the previous confusion with H. setosus , but the species is obviously widespread in the Balkans. It is likely that the records of the latter species from the limnocrene springs of the Mediterranean region of the Balkan refers to H. limnocrenicus sp. nov. For example,
Pozojević et al. (2019) reported occurence of H. setosus in the limnocrene spring Modro Oko in Southern Dalmatia, in a low abundance not exceeding 4 individuals per square meter.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |