Celleporina bitari, Harmelin, Jean-Georges, 2014
|
publication ID |
https://doi.org/10.11646/zootaxa.3893.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:015E59F7-6450-40E4-81C8-B09024D4C7BA |
|
DOI |
https://doi.org/10.5281/zenodo.4929691 |
|
persistent identifier |
https://treatment.plazi.org/id/95255B41-F25D-FFF0-EEE5-E194E1473DEF |
|
treatment provided by |
Plazi |
|
scientific name |
Celleporina bitari |
| status |
sp. nov. |
Celleporina bitari n. sp.
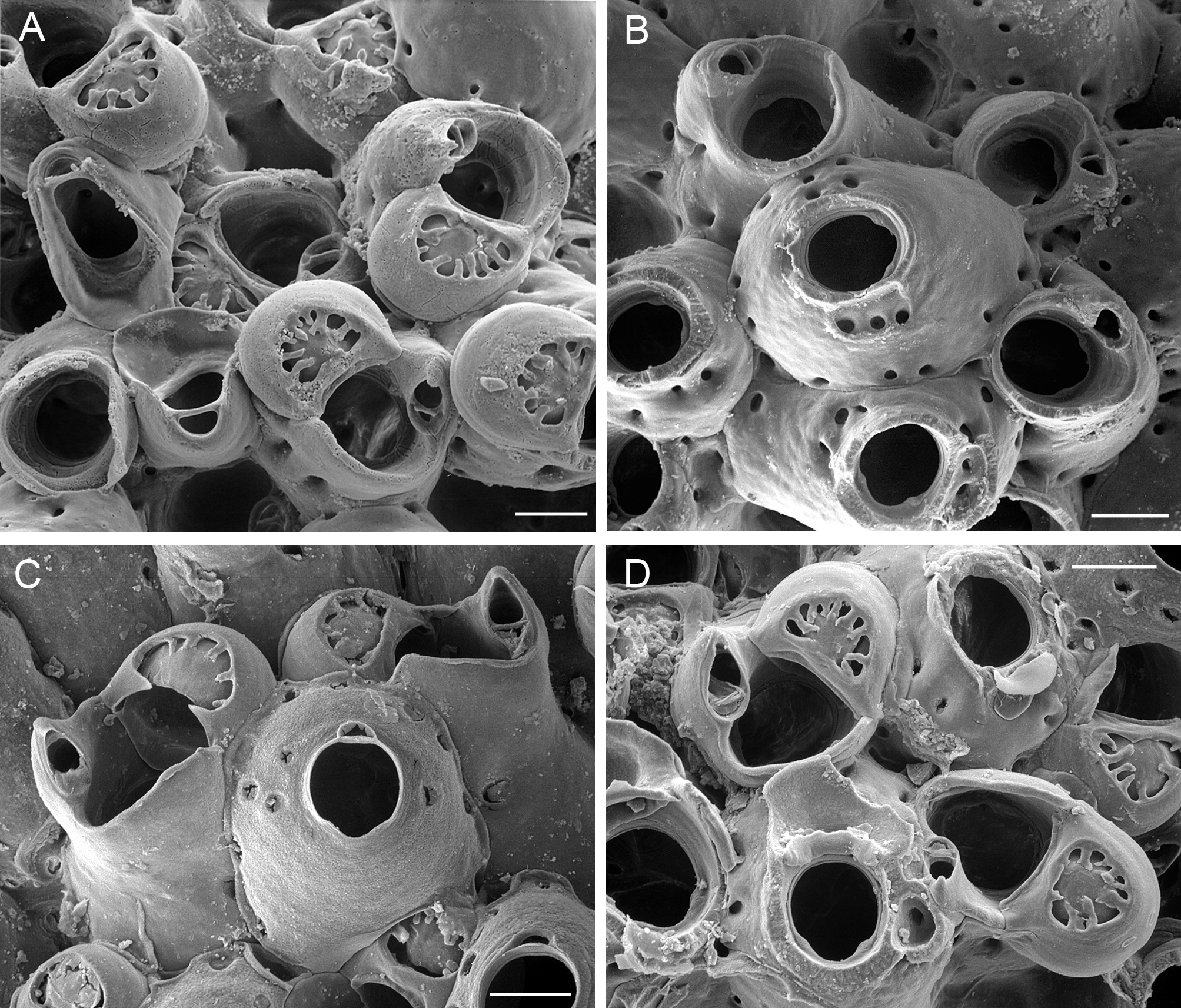
( Fig. 13 View FIGURE 13 A–D; Table 11 View TABLE 11 )
Material examined. Holotype: MNHN-IB-2013-4, ovicellate encrusting colony 7–8 mm diameter, multilamellar in centre, on aluminium plate, Red Sea, Egypt, South Sinai, Ras Mohammed, Yolanda wreck, 18 m, 15 May 1983. Paratypes: 1) MNHN-IB-2013-5, 4 subspherical colonies 1.3–2.2 mm diameter, Eastern Mediterranean, Lebanon, Selaata, 22 m, 14 September 2002, Stn 5C; 2) MNHN-IB-2013-147, 3 encrusting colonies on aluminium plate, same data as holotype; 3) MNHN-IB-2013-148, 4 small, subspherical colonies, Eastern Mediterranean, Lebanon, Selaata, 35 m, 6 July 2003; 4) NHMUK 2014.5.21.1a-h, 8 small encrusting colonies on aluminium plate; 5) NHMUK 2014.5.21.2-7, 6 detached colonies from same substratum, same data as holotype; 6) NHMUK 2014.5.21.8-24, 16 small subspherical colonies 1.8–2.5 mm on Adeonella pallasii or detached, Eastern Mediterranean, Lebanon, Selaata, 35 m, 6 July 2003, Stn 5E. Other material examined: 1) Stn 5D, Selaata, Lebanon, 35 m, coated specimen for SEM; 2) Sinai, Yolanda wreck, on aluminium plate, coated specimen for SEM; 3) SEM images of colonies from northern Bay of Safaga, Egypt, Red Sea, posted by Ostrovsky et al. (2011a).
Etymology. Honorific for Professor Ghazi Bitar, Lebanese University, Beirut, who contributed fundamentally to the collection of bryozoans along the coast of Lebanon.
Description. Colony small, encrusting, multilamellar by frontal budding, mound-shaped, pisiform, or forming a small pillar with rounded tip, light brown to pale yellow when dry. Autozooids prominent, irregularly arranged and separated by deep grooves. Frontal shield smooth, 8–10 pseudopores around margin and 3–4 pseudopores around peristome. Primary orifice slightly longer than broad (ratio 1.1–1.2) with shallow rounded proximal sinus and indistinct condyles. Peristome high, tubular, rising vertically higher than ooecial surface in brooding zooids, with low proximal notch, one side alate when fully developed, the other bulging with cystid chamber bearing single adventitious avicularium. A single adventitious avicularium on lateral edge of peristome, large, oriented upwardly and distolaterally, with long triangular mandible and rostrum hooked at tip, crossbar complete. Vicarious avicularia diversely oriented among autozooids, resting downwardly on erect cystid; rostrum markedly spatulate, with large, rounded, distal opesia, convex mandible, small proximal area, crossbar complete. Ooecia frequent, hyperstomial, recumbent on distal wall of maternal zooid, spherical, with frontal tabula (endooecium) flat, moderately sized, semicircular or roughly triangular, perforated by 7–8 large, marginal, irregularly shaped pseudopores separated by ridges.
Remarks. The Red Sea specimens from south Sinai (pers. colln) and the northern Bay of Safaga (A. Ostrovsky and J.P. Cáceres-Chamizo, pers. comm.) are morphologically similar to those from Lebanon, except for zooidal dimensions, which are on average slightly higher in Red Sea specimens ( Table 11 View TABLE 11 ). The same trend in zooid-size difference between specimens from these two regions was noticed in Microporella genisii ( Audouin, 1826) by Harmelin et al. (2011). As stressed by Ryland et al. (2009), zooidal dimensions are influenced by multiple sources of variation, including genetic and environmental factors, particularly among colonies from distant regions.
Celleporina bitari n. sp. is closely related to Celleporina sinica Liu View in CoL in Liu, Yin & Ma, 2001, a fouling species from Chinese seas, particularly in having a primary orifice with a shallow proximal sinus, a salient tubular peristome bearing a single large adventitious avicularium with a triangular rostrum directed upwardly, and numerous globose ovicells not immersed between adjacent zooids. However, according to Liu's description, C. sinica View in CoL lacks vicarious avicularia and the tabular endooecial area of the ooecium is larger and includes more pseudopores. According to Tilbrook (2006), C. sinica View in CoL and Celleporina hainanica Liu View in CoL in Liu, Yin & Ma, 2001 are both junior synonyms of Celleporina granum ( Hincks, 1881) View in CoL . The latter has been revised by Brown (1952), who selected a neotype (BMNH 99.5.1.1296) from Curtis Island, Bass Strait. SEM photos of this type specimen illustrated by Bock (2000) show that C. granum View in CoL differs from C. bitari n. sp. in having orifices with distinct condyles and a clearly narrower sinus. Also, the vicarious avicularia, noted as ‘sometimes present’ by Brown (1952) in the diagnosis, are not visible in these pictures while it is constant in C. bitari n. sp. These morphological differences are clear enough to justify the erection of a new Celleporina View in CoL species based on specimens from both the Red Sea and the eastern Mediterranean.
The occurrence of highly frequent ovicells in small-sized colonies of C. bitari n. sp. suggests precocious reproduction and abundant release of larvae. These features reveal a particular aptitude for colonizing ephemeral or newly immersed substrata. This interpretation is supported by the occurrence of C. bitari n. sp. on debris from the wreck of the Yolanda , a vessel sunk at Ras Mohammed in 1981, two years before the collection of the studied material, and on skeletons of erect Adeonella (Selaata, Lebanon). Early high fecundity should also have facilitated dispersal from the Red Sea to the Eastern Mediterranean either by colonizing successive relays along the Suez Canal (including working barges) and/or by fouled ships transiting across the canal.
Two native Celleporina View in CoL species were present in the Lebanese samples— Celleporina canariensis Aristegui, 1989 View in CoL , which was the most frequent, and Celleporina mangnevillana ( Lamouroux, 1816) View in CoL [= Celleporina caminata ( Waters, 1879) View in CoL ].
TABLE 11. Celleporina bitari n. sp., morphometrics of colonies from the Mediterranean (three colonies from Lebanese Stns 5 C and 5 E) and the Red Sea (two colonies from Ras Mohammed, south Sinai). AV-V, vicarious avicularium; AV-A, adventitious avicularium.
| C. bitari | E Mediterranean | Red Sea | ||||||
|---|---|---|---|---|---|---|---|---|
| X | SD | Range | N | X | SD | Range | N | |
| Or L | 127 | 7 | 120–135 | 11 | 133 | 8 | 120–145 | 8 |
| Or W | 113 | 7 | 100–120 | 11 | 118 | 6 | 110–125 | 8 |
| Ov L | 171 | 13 | 145–185 | 8 | 173 | 14 | 150–205 | 11 |
| Ov W | 199 | 7 | 185–205 | 8 | 208 | 11 | 180–220 | 11 |
| Av-V L | 289 | 24 | 265–330 | 7 | 326 | 28 | 305–365 | 4 |
| Av-V W | 186 | 34 | 160–255 | 7 | 200 | 17 | 180–220 | 4 |
| Av-A L | 123 | 14 | 110–135 | 4 | 142 | 18 | 120–170 | 10 |
| Av-A W | 63 | 13 | 50–80 | 4 | 67 | 4 | 60–70 | 10 |
| NHMUK |
Natural History Museum, London |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Neocheilostomina |
|
Family |
|
|
Genus |
Celleporina bitari
| Harmelin, Jean-Georges 2014 |
Celleporina canariensis
| Aristegui 1989 |
Celleporina granum (
| Hincks 1881 |
Celleporina caminata (
| Waters 1879 |
Microporella genisii (
| Audouin 1826 |
Celleporina mangnevillana (
| Lamouroux 1816 |