Triplocania pains, Neto & García Aldrete & Rafael & Ferreira, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4938.5.2 |
|
publication LSID |
lsid:zoobank.org:pub:51D20CB4-97EF-4EE6-9687-8DE1D4D55E1E |
|
DOI |
https://doi.org/10.5281/zenodo.4607807 |
|
persistent identifier |
https://treatment.plazi.org/id/921F7260-FB59-FFE8-FF6D-FDFFFE6FF8AC |
|
treatment provided by |
Plazi |
|
scientific name |
Triplocania pains |
| status |
sp. nov. |
Triplocania pains View in CoL n. sp. Male
( Figs 16–25 View FIGURES 16–22 View FIGURES 23–25 )
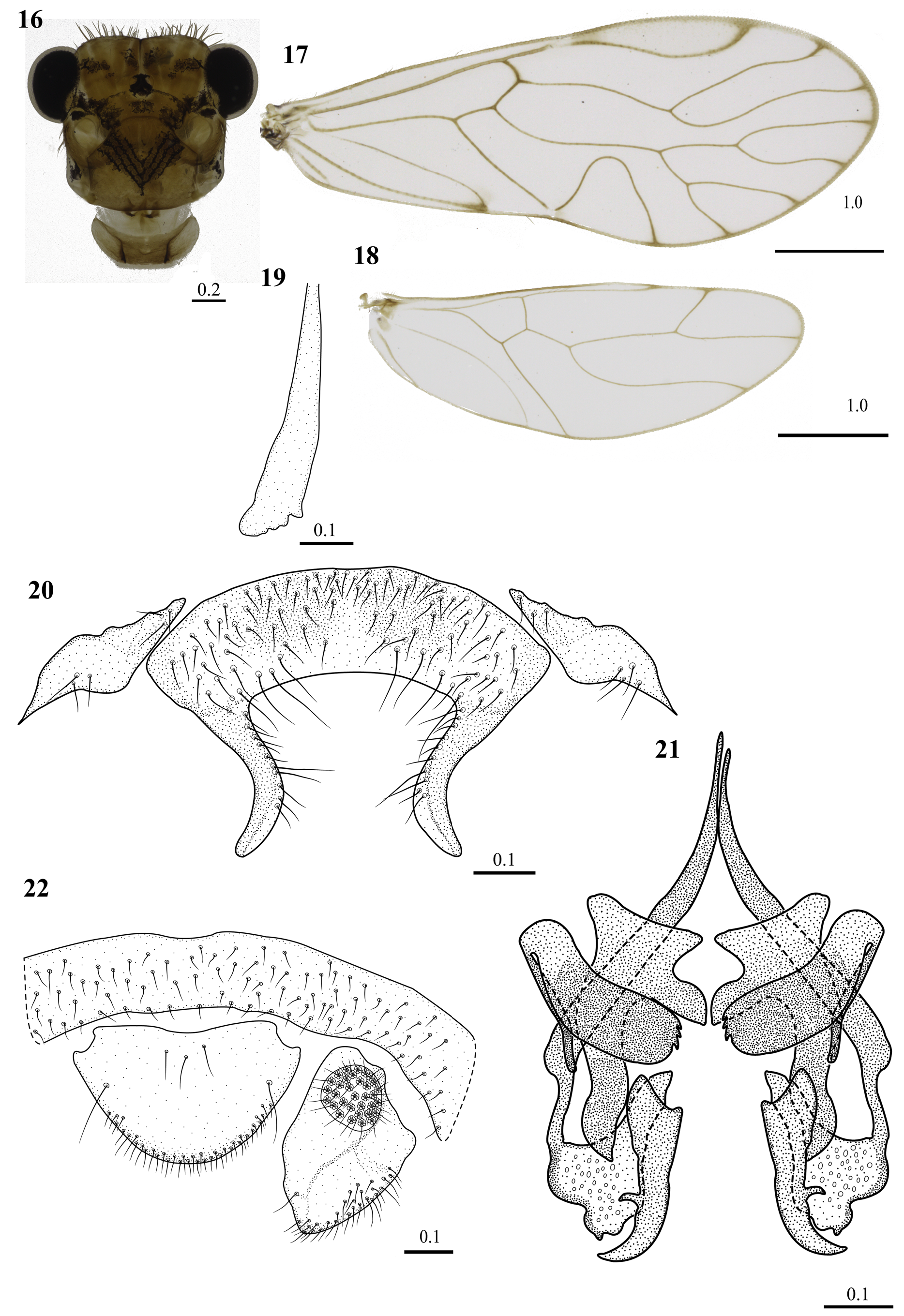
Diagnosis. Forewings almost hyaline, a small brown spot on confluence of CuP–1A; veins brown, with M 3 forked, resulting in M 3a and M 3b; hypandrium of three sclerites, central one large, convex anteriorly, with two postero-lateral processes, elongate, distinctly curved outward, blunt ended, with a row of setae on inner border ( Fig. 20 View FIGURES 16–22 ). Phallosome ( Fig. 21 View FIGURES 16–22 ) with three pairs of endophallic sclerites, anterior pair stout, ax-shaped, anteriorly wide, almost rectangular, with a deeply concavity in the inner margin, with two triangular projections, the “handle” stout, sinuous and distally acuminate; mesal pair sausage-shaped, each arm with an anterior, long, slender process, almost parallel to the main body, which bears a row of three denticles distallypair; posterior pair long, anteriorly wide, diagonal, norrowing posteriorly, bearing a small, subterminal acuminate projection on outer margin, distally curved outward with acuminate apices.
Color (in 80% ethanol and in life). Compound eyes reddish in life ( Fig. 25 View FIGURES 23–25 ), but black in ethanol, ocelli hyaline, with ochre centripetal crescents, head pattern ( Fig. 16 View FIGURES 16–22 ). Scape and pedicel brown, f1–f2 pale brown, with apices white. Mx4 brown. Coxae brown, trochanters and femora yellow, tibiae brown, distally dark brown; tarsomere 1 yellow, distally dark brown, tarsomeres 2–3 brown. Forewings almost hyaline, a small brown spot on confluence of CuP–1A; veins brown ( Fig. 17 View FIGURES 16–22 ). Hindwing hyaline throughout, veins brown ( Fig. 18 View FIGURES 16–22 ).
Morphology. Head with vertex concave in the middle, slightly above the level of the upper border of the compound eyes, these without interommatidial setae ( Fig. 16 View FIGURES 16–22 ). Outer cusp of lacinial tips broad, with three denticles ( Fig. 19 View FIGURES 16–22 ). Forewing pterostigma long, narrow basally, widening posteriorly; areola postica tall, wide basally, broadly triangual; M stem concave proximally, then almost straight, M 1 slightly convex proximally, then almost straight, M 2 sinuous, M 3 forked, resulting in M 3a and M 3b ( Fig. 17 View FIGURES 16–22 ). Hindwing Rs straight, R 2+3 and R 4+5 straight, M stem sinuous ( Fig. 18 View FIGURES 16–22 ). Hypandrium of three sclerites, a large central sclerite, flanked by small, rhomboid sclerites; setae as illustrated ( Fig. 20 View FIGURES 16–22 ). Phallosome ( Fig. 21 View FIGURES 16–22 ), with side struts independent, V-shaped, with a narrowing in its connection to external parameres, these stout, almost triangular, bearing pores distally. Three pairs of endophallic sclerites, an anterior pair, stout, ax-shaped, anteriorly wide, almost rectangular, with a deeply concavity in the inner margin, generating two triangular projections on each side, posteriorly narrow, sinuous and distally acuminate; a lateral pair, V-shaped, elbowed in the middle, inner arms stout, with three small acuminated projections on the inner margin, outer arms, small, slender, elongetd, blunt distally; a posterior pair small, anteriorly wide, with two small triangular antero-lateral projections, norrowing posteriorly, bearing a small, subterminal acuminate projection on outer margin, distally curved outward with acuminate apex ( Fig. 21 View FIGURES 16–22 ). Epiproct broad, with one concave area on each antero-lateral corner; sides converging to round posterior border, one macroseta on each side, three setae mesally, other setae as illustrated ( Fig. 22 View FIGURES 16–22 ). Paraprocts broadly triangular, sensory fields with 33 trichobothria on basal rosettes, setae as illustrated ( Fig. 22 View FIGURES 16–22 ).
Measurements (in microns). FW: 6050, HW: 3940, F: 1330, T: 1743, t1: 831, t2: 109, t3: 173, Mx4: 342, f1: 1473, f2: 1097, IO: 756, D: 482, d: 320, PO: 0.66.
Material examined. Holotype male (UFLA). BRAZIL. Minas Gerais. Lagoa da Prata. Gruta Papo Cabeça. (ISLA 10). 20°04’38.92”S: 45°35’03.07”W. 24.V.2003. R. L. Ferreira GoogleMaps . Paratypes: 1 male (UFLA). Same data as the holotype, except Gruta Diaclase V. ( ISLA 55). 25.III.2003 GoogleMaps . 1 male (UFLA). Same data as the holotype, except Gruta do Pasto. 21.V.1999 GoogleMaps . 1 male (INPA). Same data as the holotype, except Gruta Brega. ( ISLA 17). VIII. 2008 GoogleMaps . 1 male (INPA). Same data as the holotype, except Gruta do Pasto. 21.V.1999 GoogleMaps . 1 male (INPA). Same data as the holotype, except Gruta da Passagem. ( ISLA 961). 01.III.2003 GoogleMaps . 1 male (MNRJ). Same data as theholotype, except Gruta Pared„o Descoberto. ( ISLA 5). V.2003 GoogleMaps . 1 male (MNRJ). Same data as the holotype, except Gruta Saldanha. ( ISLA 972). 10.XII.2001 GoogleMaps .
Etymology. The specific epithet refers to the municipality of Pains, where most of the specimens where found.
Taxonomic Comments. The characteristic forewing M 3 forked, resulting in M 3a and M 3b in Triplocania pains n. sp. is shared by thirteen species of Triplocania : T. calcarata New, 1980 ; T. furcata New,1972 ; T. furcatoides González, Carrejo & García Aldrete, 2017 ; T. huilaensis González, Carrejo & García Aldrete, 2017 ; T. lamasi Silva-Neto, Rafael & García Aldrete, 2014; T. lamasoides Silva-Neto, Rafael & García Aldrete, 2015 ; T. lamensuraensis González, Carrejo & García Aldrete, 2017 ; T. leguizamoensis González, Carrejo & García Aldrete, 2017 ; T. mariateresae Silva-Neto, Rafael & García Aldrete, 2014 ; T. newi Silva-Neto, Rafael & García Aldrete, 2014 ; T. palaciosi García Aldrete & Casasola González, 2012 ; T. plaumanni Silva-Neto, Rafael & García Aldrete, 2014 and T. sarriae González, Carrejo & García Aldrete, 2017 . Triplocania huilaensis and T. sarriae are known only from females; they have a different forewing pigmentation pattern than T. pains . T. huilaensis has a forewing marginal homogeneous brown band, from R 2+3 to M 3a (see Fig. 317 in González et al. 2017). Triplocania sarriae has a marginal brown band with a convex hyaline fenestra on the outer border between each intersection of the veins and the edge of the wing, from R 4+5 to areola postica (see Fig. 347 in González et al. 2017). Triplocania pains n. sp. differs from T. calcarata , T. lamasi , T. lamasoides , T. leguizamoensis , T. lamensuraensis and T. newi by having the hypandrium of three sclerites. It differs from T. furcata and T. furcatoides by having the hypandrium with lateral sclerites located in its anterior region posteriorly. It differs from T. palaciosi in lacking a marginal homogeneous brown band in the forewing, from R 4+5 to areola postica and in having the central piece of the hypandrium with two posterior processes. Triplocania pains n. sp. differs from T. mariateresae and T. plaumanni by having the postero-lateral processes of the central sclerite of the hypandrium strongly curved outwards, with the side struts fused to the external parameres and in details of the phallosome (compare Fig. 21 View FIGURES 16–22 with Figs 58 and 59 View FIGURES 56–64 ).
Habitat. Specimens of this new species were found in several limestone caves in the speleological province of Arcos-Pains-Doresópolis ( Figs 23–24 View FIGURES 23–25 ). Despite the fact that only three municipalities are mentioned in the province’s name, it includes other municipalities, as Lagoa da Prata and Iguatama, where specimens were also found. This region has the highest concentration of caves in South America, with more than 2,500 registered caves, although many areas remain unexplored. Most of the caves in this region are small and devoid of perennial water bodies. The caves where specimens were recorded are distinct in regard their dimensions and morphology, indicating that the species does not seem to select caves based on size and water presence. Individuals of this species were always found in well illuminared entrance zones. Adults were infrequent, but nymphs were quite common on walls covered by algal material. Adults were frequently found in twilight zones, including ceilings and spaces between speleothems, and never as aggregations in contrast to the nymphs. No specimen was ever found in aphotic areas. Thus the species is not adapted to typical cave conditions, being effectively restricted to entrance zones. This occurrence at cave entrances of both immatures and adults indicates that the species is not accidental but has a preference for cave entrances. There are several invertebrates that are typically found in cave entrances, being part of the para-epigean communities ( Ferreira & Martins 2001; Prous et al. 2004, 2015). Given the lack of studies on the population ecology of Psocoptera from caves, it is impossible to determine, at the moment, whether the life cycle of Triplocania pains n. sp. occurs entirely inside the caves or if the species also needs other habitats. It is important to note that dispersal capability is not necessarily related to frequent migration movements. In another Brazilian cave psocoptera (Prionoglaridiidae: Neotrogla ) microsatellite markers have shown significant genetic differentiation between two cave populations located less than 1 km apart, indicating that migration between caves is limited ( Kamimura et al. 2019).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Psocomorpha |
|
Family |
|
|
Genus |