Allobates spumaponens, Kok, Philippe J. R. & Ernst, Raffael, 2007
|
publication ID |
https://doi.org/ 10.5281/zenodo.178208 |
|
DOI |
https://doi.org/10.5281/zenodo.6247769 |
|
persistent identifier |
https://treatment.plazi.org/id/900887B3-B411-FFD9-FF11-FD50FA99CB59 |
|
treatment provided by |
Plazi |
|
scientific name |
Allobates spumaponens |
| status |
sp. nov. |
Allobates spumaponens View in CoL sp. nov.
Figs 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6
Holotype. SMNS 12511, an adult male collected by Raffael Ernst, 23/04/2003, Mabura Hill Forest Reserve, Upper Demerara-Berbice Region, Guyana (5°09'N, 58°41'W, elevation ca. 100 m).
Paratypes (N=6). SMNS 12510, an adult male collected 15 November 2002, SMNS 12512, an adult male collected 15 November 2002, SMNS 12513, an adult male collected 20 November 2002, SMNS 12514, an adult male collected 11 December 2002, SMNS 12515, an adult male collected 3 June 2003, SMNS 12516, an adult female collected 15 December 2002. All specimens with same locality data as holotype.
Etymology. The specific epithet is derived from the Latin word spuma, meaning “foam” and the Latin verb ponere, meaning “to place” in reference to the atypical tadpole deposition site observed in the new species.
Adult definition and diagnosis. A very small species of Allobates (males 13.2–17.0 mm SVL, females 17.0–19.0 mm SVL); body slender; Finger I longer than II when fingers appressed; tip of Finger IV not reaching distal subarticular tubercle on Finger III when fingers appressed; no distal tubercle on Finger IV; Finger III not distinctly swollen in males; basal webbing only between Toes II–IV; lateral fringes on fingers and toes absent; throat in adult males pale with very discrete dark spotting on chin and laterally (visible under magnification only), throat in female pale, free of melanophores; belly in males hyaline-white, belly in females yellow; diffuse pale dorsolateral stripe present; diffuse, pale, partial oblique lateral stripe present; ventrolateral stripe present, with irregular dark brown blotches present ventrolaterally.
Nine species of Allobates (sensu Grant et al. 2006) are reported from the Guiana Shield: A. brunneus ( Cope, 1887) [although the presence of A. brunneus in the area is improbable (see Morales “2000 ” [2002], La Marca et al. 2004), we include it here to facilitate comparison], A. femoralis (Boulenger, 1884) , A. granti , A. marchesianus (Melin, 1941) [several populations referred to as A. marchesianus represent a number of similar species (see Caldwell et al. 2002) and exact distribution of this species needs further investigation. Although the presence of A. marchesianus outside the type locality is uncertain, we include it here to facilitate comparison], A. myersi (Pyburn, 1981) , A. rufulus (Gorzula, “1988” [1990]), A. sanmartini (Rivero, Langone and Prigioni, 1986) , A. sumtuosus ( Morales, “2000 ” [2002]), and A. undulatus ( Myers and Donnelly, 2001) (Señaris & MacCulloch 2005, Kok et al. 2006). Allobates spumaponens is easily distinguished from the more brightly coloured species of the A. femoralis group ( A. femoralis , A. myersi and A. rufulus ) by its cryptic colouration, smaller size (13.2–19.0 mm in A. spumaponens vs. 20.0– 33.5 mm in A. femoralis , 26.4–32.8 mm in A. myersi and 20.0–23.0 mm in A. rufulus ), pale throat in males (dark grey to black), and absence of mottling on chest or belly (present). Allobates brunneus (fide Cope 1887) differs by darker throat in males (pale in A. spumaponens ), an “hourglass” pattern on the back (absent in A. spumaponens ), Fingers I and II almost equal in length (Finger I much longer than II in A. spumaponens ), incomplete lateral black band (complete in A. spumaponens ), and call parameters. Allobates granti can be distinguished from A. spumaponens by the absence of a diffuse dorsolateral stripe (present in A. spumaponens ), Finger II not reaching distal tubercle on Finger III (reaching in A. spumaponens ), and call parameters. Allobates marchesianus differs in having grey to dark grey throat and chest in males (chest white and throat pale in A. spumaponens ), light grey belly in males (white in A. spumaponens ), wide distinct dorsolateral stripe (present but diffuse in A. spumaponens ), and call parameters. Allobates sanmartini differs by larger size (maximum SVL of 25.4 mm vs. 19.0 mm in A. spumaponens ), tympanum larger than half the size of eye (smaller in A. spumaponens ), and Finger I shorter than second (much larger in A. spumaponens ). Allobates sumtuosus differs in having Finger III swollen in males (not swollen in A. spumaponens ), and lateral fringes on Toe II–IV (absent in A. spumaponens ). Allobates undulatus is distinguished by larger size (maximum SVL 25.0 mm vs. 19.0 mm in A. spumaponens ), swollen supracarpal pad atop wrist in males (absent in A. spumaponens ), diffuse dorsolateral stripe absent (present in A. spumaponens ), wavy-edged dorsal markings (absent in A. spumaponens ), and different call parameters.
A few extralimital species could be confused with Allobates spumaponens . These species are Allobates caeruleodactylus (Lima and Caldwell, 2001) , A. crombiei ( Morales, “2000 ” [2002]), A. masniger ( Morales, “2000 ” [2002]), A. nidicola (Caldwell and Lima, 2003) , and A. pittieri (La Marca, Manzanilla and Mijares- Urrutia, 2004). Allobates caeruleodactylus is distinguished from A. spumaponens by blue digits and blue discs on toes in males (never blue in A. spumaponens ), tympanum about 50% of eye length (less than 40% in A. spumaponens ), and different advertisement call. Allobates crombiei differs by larger size in males (17.4–19.0 mm vs. 13.2–17.0 mm in A. spumaponens ), wavy-edged dorsal markings (absent in A. spumaponens ), and presence of lateral fringes on toes (absent in A. spumaponens ). Allobates masniger is distinguished in having a larger size in males (17.9–19.5 mm vs. 13.2–17.0 mm in A. spumaponens ), lateral fringes on toes (absent in A. spumaponens ), and dark grey belly in males (white in A. spumaponens ). Allobates nidicola differs by a larger size in males (18.5–20.5 mm vs. 13.2–17.0 mm in A. spumaponens ), a light grey to solid black throat and chest in males (chest white and throat pale in A. spumaponens ), diffuse dorsolateral stripe absent (present in A. spumaponens ), and different advertisement call. Allobates pittieri is distinguished from A. spumaponens by a larger size in males (16.3–18.3 mm vs. 13.2–17.0 mm in A. spumaponens ), and black throat and grey belly in males (belly white, throat pale in A. spumaponens ).
Measurements of the holotype (in mm). SVL 15.7, HL 5.0, HW 5.0, SL 2.8, EN 1.4, IN 2.1, EL 2.4, IO 2.0, TYM 0.7, FAL 3.5, HAND 3.4, WFD 0.5, TL 7.5, FL 6.4, WTD 0.6.
Description of the holotype. An adult male ( Figs 1–2 View FIGURE 1 View FIGURE 2 ); body slender; head as wide as long; head length 31.8% SVL; snout broadly rounded in dorsal view, acutely rounded in lateral view, extending past lower jaw, snout 56.0% head length. Nares located laterally, opening posterolaterally; canthus rostralis rounded, loreal region slightly concave, flaring slightly at upper lip; internarial distance 42.0% head width; eye-naris distance 28.0% head length, 58.3% eye length. Tympanum subcircular, directed posterolaterally, 29.2% of eye length; separated from eye by a distance equal to 25.7% of tympanum diameter; supratympanic fold absent; tympanic annulus visible anteroventrally; posterodorsal aspect of tympanum barely visible; anteroventral margin of tympanum distinct. Tongue attached anteriorly, broadly rounded posteriorly, median lingual process absent. Choanae small, circular, lateral. Vocal slits long, lateral. Small teeth present on maxillary and premaxillary, dentigerous process of vomers absent. Cloacal tubercles absent; vent at level of upper thighs; a small anal flap above vent. Dorsum shagreen with some larger granules, more granular posteriorly; belly smooth.
Forelimb slender, skin smooth; metacarpal ridge absent; ulnar fold absent; hand length 21.7% SVL; Finger I longer than Finger II when fingers appressed; fingers unwebbed, lateral fringes absent; Finger III not distinctly swollen; tip of Finger IV not reaching distal subarticular tubercle on Finger III when fingers appressed; tip of Finger II reaching distal subarticular tubercle on Finger III when fingers appressed; terminal discs slightly expanded, slightly wider than long, equal to or slightly larger than the width of digit; width of disc on Finger III 0.50 mm; discs with distinct dorsal scutes. Relative lengths of appressed fingers III> I> II> IV; palmar tubercle large, ovoid, 0.60 mm in diameter, 17.6% hand length, anterior third pigmented; thenar tubercle protuberant, ovoid, anterior quarter pigmented, about half the size of the palmar tubercle and narrowly separated from it. One subarticular tubercle on Fingers II and IV; two subarticular tubercles on Finger I and III; basal subarticular tubercle on Finger I largest, subarticular tubercle on Finger II and basal subarticular tubercle on Finger III subequal; basal subarticular tubercle on Finger IV and distal subarticular tubercle on Finger III smaller, distal subarticular tubercle on Finger III smallest ( Fig. 3 View FIGURE 3 ).
Hindlimb robust, skin granular; tibia length 47.8% SVL; heels overlapping when hindlimbs are flexed at right angles to sagittal plane of body; foot length 40.8% SVL; relative length of appressed toes IV> III> V> II> I; Toe I very short, its tip reaching the base of subarticular tubercle on Toe II when toes appressed on left foot, not reaching the base of subarticular tubercle on Toe II when toes appressed on right foot; discs on Toes II, III, IV, and V larger than width of toes; disc on Toe I equal to width of digit. Width of disc on Toe IV 0.60 mm; rudimentary webbing present only between Toes II–IV; webbing slightly pigmented; lateral fringes absent. Toe webbing formula II 2 +-3¾ III 3 - - 4 IV. Inner metatarsal tubercle oval, 0.50 mm in length, distal portion pigmented, outer metatarsal tubercle round, 0.26 mm in diameter, entirely pigmented; medial metatarsal tubercle present on both feet, unpigmented, subequal to inner metatarsal tubercle. Two subarticular tubercles present on Toes III and V, three on Toe IV and one on Toes I and II on the right foot; distal subarticular tubercle on Toe III unpigmented and barely visible on the left foot. Subarticular tubercles on Toes I and II largest; basal subarticular tubercle on Toe IV very small on both feet. Metatarsal fold absent. Tarsal keel well defined, short, tuberclelike, directed transversely across tarsus, located 1.41 mm from proximal edge of inner metatarsal tubercle, not extending from metatarsal tubercle ( Fig. 3 View FIGURE 3 ).
Colour of Holotype in life. Dorsal ground colour medium brown with several dark brown flecks on dorsum; dorsal surface of head slightly darker than dorsum. Wide lateral black band from tip of snout to vent, containing 2/3 of tympanum and not tapering posteriorly from axilla. Pale, narrow, diffuse dorsolateral stripe above the band, extending from tip of snout to vent. Diffuse, pale, partial oblique lateral stripe imbedded in the black band, extending from groin to about one-quarter of the distance to arm insertion. Flanks white with a few irregular brownish grey blotches; ventrolateral stripe present. Upper lip white, suffused with barely visible tiny melanophores. Throat hyaline white with barely visible tiny melanophores laterally and on the chin. Belly immaculate hyaline white. Upper surfaces of thighs and shanks brown with dark brown blotches, no crossbars discernible. Pale paracloacal mark present. Pale yellow spot on groin. Forelimbs light brown with a dark brown line posteriorly from elbow to wrist and a short dark brown line anteriorly discontinuous from arm insertion to elbow. Toes greyish with some light grey blotches. Fingers light brown with some pale blotches. Palm and sole dark brown. Iris golden bronze.
Colour of Holotype in preservative. Dorsum light brown, with two diffuse whitish dorsolateral stripes suffused with melanophores (melanophores visible under magnification), extending from snout to vent. Some small, irregular, dark brown spots are visible on the dorsum (these spots correspond to darker granules). Dorsal surface of head slightly darker than body. Lateral band blackish brown extending from tip of snout to vent. Diffuse, pale, partial oblique lateral stripe visible. Belly white, flanks white with a few patches of melanophores, upper lip white suffused with melanophores (visible under magnification). Throat white with a few melanophores located on the chin and laterally (visible under magnification only). Upper surfaces of thighs and shanks same colour as dorsum with some dark brown spots. Pale paracloacal mark present. Forelimbs cream dorsally, brown with pale spots ventrally; the brown lines are still visible. Fingers cream, digits dark brown; palm and sole brown.
Male secondary sexual characters. Males are smaller than females (mean SVL in males 16.16 mm; females 17.95 mm) and have a pale throat with a few melanophores located on the chin and laterally (melanophores visible under magnification only, throat immaculate in female). Under magnification melanophores are visible on lips in males, these melanophores are absent in females. Male ventral colouration is hyaline-white, that of adult females yellowish.
Variation among type specimens. Descriptive statistics of the type series are given in Table 1 View TABLE 1 . Excluding the sexual dimorphism, all of the paratypes conform to the description of the holotype. Two paratypes ( SMNS 12515 and SMNS 12516) have a dark brown dorsum and are much darker than the other paratypes. The pale diffuse dorsolateral stripe is darker in SMNS 12515 and may sometimes be difficult to distinguish in other specimens (under magnification the stripe is always visible). In some specimens it is unclear whether the dorsolateral stripe originates from the tip of the snout or from the eye. The medial metatarsal tubercle occurs in four specimens (57%). SMNS 12515 has a truncate snout in dorsal view, while it is broadly rounded in other type specimens. The number of small spots on the dorsum is variable ( Fig. 4 View FIGURE 4 ).
Additional data taken during transect sampling. During visual transect sampling we measured the SVL of 82 individuals; 51 adult females, 21 adult males, and 10 juveniles. Average SVL in females was: 17.95 mm (SD = 0.54 mm, range = 17.0–19.0 mm, N = 51), in males: 16.16 mm (SD = 0.93 mm, range = 15.5–17.0 mm, N = 21) and juveniles ranged between 6.5–12.0 mm (mean = 10.10 mm, SD = 1.43 mm, N = 10).
Tadpole description. Fourteen tadpoles were collected (SMNS 12608, subnumbered 1–14). The following description is of an individual in Stage 40, reared in the field (SMNS 12608-1, Fig. 5 View FIGURE 5 ): Type 4 tadpole ( Orton 1953); exotrophic; body skin with small pustules; total length 18.1 mm; body length 6.3 mm, 34.8 % of total length, 1.4 X body width, 2.3 X body height; body width 1.9 X height; body depressed, oval in dorsal view; snout round in dorsal and lateral view; eyes positioned and directed dorsolaterally; eye diameter 1.0 mm, equal to interorbital distance; interorbital distance less than internarial distance; nares positioned and directed anterodorsally; narial opening reniform in dorsal view; eye-naris distance 0.7 mm, equal to narissnout distance. Spiracular tube sinistral, short, projecting posterodorsally, its base located 54 % of body length from snout and 21 % of body height from venter. Vent tube not visible. Lateral-line system barely visible (see below). Caudal musculature highest at body-tail junction, tapering posteriorly, terminating anterior to tail tip; tail tip tapered, rounded; upper fin originating slightly posterior to junction of body and tail, gradually increasing in height to about midlength of tail, maintaining that height nearly to terminus; height of upper fin at midlength of tail 82 % of caudal musculature; comparable height of lower fin 27 % of caudal musculature; lower fin gradually increasing in height to just anterior to terminus; tail height 24 % tail length.
Mouth ventral, oral disc not emarginate, laterally folded, width 1.4 mm. Teeth long, in a single row, each tooth with 15–17 cusps ( Fig. 6 View FIGURE 6 ), LTRF 2(2)/3(1). Tooth row A-1 complete, A-2 consisting of two short, widely separated rows at level of upper jaw sheath, A-1 slightly longer than posterior rows; P-1 slightly shorter than P-2, both medially interrupted by a small gap (unnatural in P-2); tooth row P-3 shorter than P-2 or P-1. Marginal papillae moderately long, tapered, blunt-tipped, in a single row; broad median gap on upper labium approximately equal to the length of A-1; submarginal papillae absent; jaw sheaths typical, thin, serrated, lower jaw sheath broadly V-shaped, upper jaw sheath with long slender lateral processes ( Fig. 6 View FIGURE 6 ).
Variation of 14 meristic characters from tadpoles in stage 28–40 is given in Table 2 View TABLE 2 . The only obvious variation is in the P-1 gap, which is fairly variable in size and is absent in some specimens; apparently this variation is unrelated to tadpole stage. Lateral-line system evident in a few specimens, barely distinct in most, with a line originating above anterior labium, extending posteriorly above snout, passing above the eye and extending along the upper part of the body to upper tail musculature; left and right lines slightly converging between nares. A second line originates approximately above posterior labium, extending to anterior corner of eye, or stopping at mid-distance between labium and eye. In one specimen a short line is visible on the left middle part of the body.
In life, tadpoles have a greyish brown ground colour with irregular dark brown mottling on body, tail musculature and fin; a broken black stripe extends from naris to eye, followed by a short black stripe posterior to eye, another black stripe extends from mouth to eye; iris silvery gold. In preservative, upper surface of body grey-brown, sides of body with grey-brown mottling; an ill-defined dark brown stripe extends from naris to eye, followed by a short dark brown stripe posterior to eye; another dark brown stripe extends from oral disc to eye. Tail musculature and fin with grey-brown mottling. Venter translucent white, suffused with brown anteriorly, intestine visible.
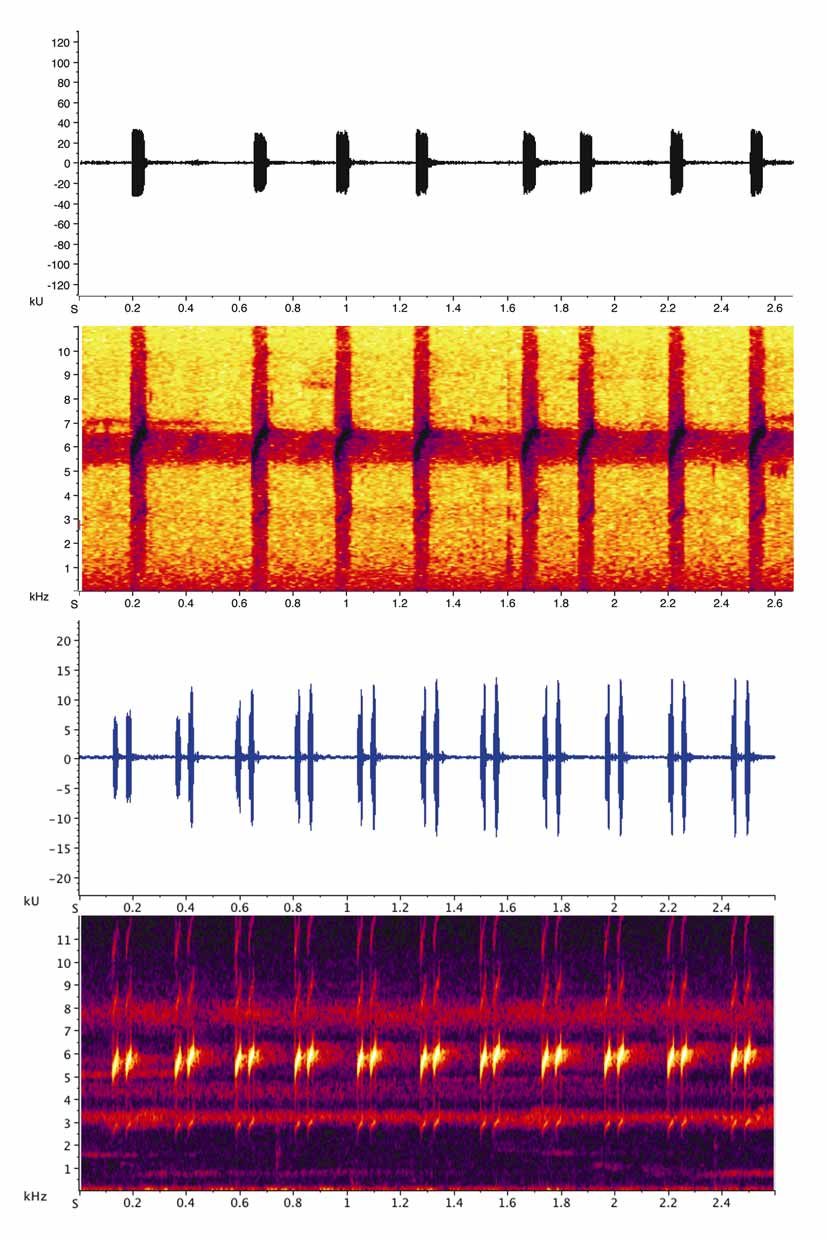
Advertisement call. The advertisement call of Allobates spumaponens comprises a series of short-pulsed high-pitched chirps ( Myers & Daly 1976); there is only one pulse per note ( Fig. 7 View FIGURE 7 ). The call consists of two harmonics, the main harmonic (dominant frequency) at about 6500 Hz and an additional harmonic (fundamental frequency) at about 3100 Hz. Duration of a regular call sequence is difficult to determine as call sequences are usually relatively long continuous periods, i.e. a call sequence may last well over four minutes in which notes are emitted with varying frequencies. The number of notes per second ranges between two and four. After the emission of 2–4 calls, a somewhat longer break of 0.49 s (mean, SD = 0.11, range = 0.277– 0.727 based on two sequences) may occur. Table 3 View TABLE 3 summarizes measurements for the main harmonic exclusively. Likewise, Fig. 7 View FIGURE 7 illustrates the main harmonic only.
Natural history. The first Allobates spumaponens (SMNS 12510) was detected on November 11th 2002 at around 12:30h in a large depression, next to a buttress root of a large Mora tree ( Mora excelsa ). The frog was partly concealed under a thick leaf layer that covered the entire depression. The depression regularly fills up during the rainy season forming a pond. The pond is used as reproductive site by several sympatric frog species, including members of the genera Leptodactylus , Osteocephalus , Phyllomedusa, Hypsiboas, Dendropsophus , and Pipa . There was no standing water in the depression at the time of collection of the voucher. Two additional males were heard calling only a few meters from the locality in which SMNS 12510 was collected. Additional specimens were collected at this site the same day (SMNS 12512) and on November 20th, respectively (SMNS 12513). Allobates spumaponens is a diurnal terrestrial species. Even though the first collections were made on a dry and sunny midday, calling males are most active shortly after rains and calling activity peaks in the morning. Males appeared to be territorial although we did not observe direct competitive interactions between individuals. Individuals usually call completely exposed on leaf litter or small rotten branches. Individuals are extremely vigilant and immediately hide under dead leaves when approached. We have not been able to observe courtship or the deposition of clutches. The species was encountered both in primary and secondary forest covering a broad range of habitats from well drained mixed forest on white sand (albic Arenosols) to mixed forest on gravely clay laterite (Leptosols), but it appeared to be most abundant in the latter. We recorded specimens in all but one transect. The latter was located in a secondary forest patch of an old recovery stage (i.e. logged in 1988). Another transect situated in a similar patch exhibited the lowest density of the species of all sites studied.
With a total of 1,025 individuals detected, Allobates spumaponens was the fourth most abundant amphibian species recorded during 393.5 hours of transect sampling (= 787 transect walks) equalling 2.6 individuals per transect hour (ind/th). However, the abundance of the species differed between primary and secondary forest sites and between wet and dry seasons, respectively. Even though A. spumaponens was one of the most abundant anuran species in secondary forest (1.7 ind/th), it was even more abundant in primary forest (3.3 ind/ th). Generally we recorded more individuals during the wet season (2.7 ind/th) than during the dry season (2.4 ind/th). This was true for both habitat complexes (primary forest: 5.1 ind/th, wet season; 3.3 ind/th, dry season; secondary forest: 1.9 ind/th, wet season; 1.2 ind/th dry season).
Despite these differences in abundance between habitat complexes, Allobates spumaponens appeared to cope comparatively well with the more restrictive conditions within secondary forests. This may partly be due to its reproductive biology, which is relatively independent of open water.
Reproductive biology. As in other species of the genus, Allobates spumaponens deposits its eggs (5–11) in moist leaf litter. We did not observe clutch deposition, but found two clutches (seven and nine eggs) attached to dead leaves that were deposited by individuals kept in an enclosure. Subsequent to hatching, tadpoles are usually carried by the male (total of 27 tadpole-carrying males recorded throughout the study period), although we recorded two cases in which tadpoles were carried by the female. Eventually tadpoles are deposited in small lentic pools and continue their development within these aquatic habitats. We did not observe any form of parental care after deposition of tadpoles. The sites chosen for tadpole deposition, even though comparatively small, are usually too large for the deposition of nutritious eggs, although this has not been tested systematically. We frequently found tadpoles in artificial pools (5,000 ml plastic bowls) that were set up for experiments of in situ tadpole development of three sympatric Leptodactylus species ( Ernst et al. 2007). Usually the pools were used within five to seven days after installation and given the average clutch size, contained more than a single clutch. The highest number of A. spumaponens tadpoles recorded in any of the artificial pools was 29 with a mean of five (SD = 3.3; N = 11) tadpoles per bowl.
* Lötters and colleagues indicated 2 pulses per note followed by a question mark for Allobates brunneus (under the name Colostethus brunneus ) in their comparative table [ Lötters et al. (2003: 1901)]. We cannot distinguish any modulation in the notes in the oscillogram they provided [ Lötters et al. (2003: 1900)] and we suspect that there is only one pulse/note in A. brunneus .
On two occasions we observed a very peculiar behaviour that needs further examination and testing. On 27th April 2003 we collected a foam nest of a yet undescribed species of Physalaemus (compare Ernst et al. 2005). Upon transferring the nest to a plastic container filled with ca. 1 cm of rainwater, seven tadpoles that were later identified as Allobates spumaponens emerged from the nest. The second observation, on June 6th 2003, was made during a regular transect walk in one of the secondary forest transects (Transect S3 segment 6). There had been no rain for several days and the leaf litter had dried up completely. We observed a male A. spumaponens , carrying tadpoles and approaching a large foam nest of Leptodactylus rhodomystax that had been deposited in a small depression. At the time of observation, the depression was not filled with water. The male immediately climbed the nest and dropped all of the tadpoles into the foam. Tadpoles (N=6) were retrieved from the nest, counted, and subsequently returned to the foam. At the time the nest contained L. rhodomystax tadpoles at Gosner stage 24. The foam nest was not collected and unfortunately was preyed upon by a freshwater crab (family Pseudothelphusidae ) only four days later.
These observations prompted us to set up a series of experiments in which tadpole-carrying males were transferred to plastic containers (L x W x H = 33 cm x 22 cm x 19 cm) and given the choice between water, moist leaves and foam for deposition of tadpoles. However, due to limited availability of individuals that were carrying tadpoles, experiments could not be conducted systematically. Results are therefore not conclusive and cannot be tested statistically. We tested a total of nine males. For the first setup we used three small plastic dishes that were equipped with foam, taken from a Leptodactylus rhodomystax foam nest, wet leaves, and rainwater, respectively. These dishes were placed in a plastic container and tadpole-carrying males were introduced. The number of tadpoles in each dish was counted after 12 hours and again after 24 hours. By this time the males had dropped all tadpoles. We tested three males independently: the first carrying eight, the second six, and the third three tadpoles. In all cases, tadpoles were deposited after 12 hours. None of the males chose the foam for deposition and only one tadpole was deposited in the water. The remaining tadpoles were deposited in the wet leaves. The second setup comprised only two dishes equipped with either foam or dry leaves that were placed in the plastic containers. We only tested two males, the first carrying eight, the second 11 tadpoles. After 12 hours, Male 1 had dropped one tadpole in the foam and two in the leaves. Twelve hours later, the remaining five tadpoles had been deposited in the foam. After 24 hours, Male 2 had dropped all but three tadpoles in the foam; the remaining tadpoles were found on the bottom of the plastic container. In the third setup we tested two males, the first carrying nine and the second carrying seven tadpoles. In this setup we provided a choice between rainwater and wet leaves. In both cases tadpoles were dropped within 12 hours. Male 1 had dropped all tadpoles in the wet leaves and Male 2 all but one, which was deposited in rainwater. Finally we tested two additional males, Male 1 carrying 11 and Male 2 carrying eight tadpoles. Plastic containers were equipped with two dishes containing either wet or dry leaves. Once more, all tadpoles had been dropped after 12 hours. Male 1 deposited nine tadpoles in wet leaves and the remaining two in dry leaves. Male 2 deposited seven tadpoles in wet leaves and only one in dry leaves.
Distribution. The new taxon is currently known only from the type locality in central Guyana: the Mabura Hill Forest Reserve ( Fig. 8 View FIGURE 8 ). The MHFR is characterized by a gently undulating terrain with low elevations ranging from 90–100 m asl. Specimens of Allobates spumaponens were found throughout the forest in low-lying as well as slope habitats, e.g. on laterite hill slopes. The species was not recorded on top of a rock plateau that supports dry evergreen low forest. See Ernst et al. (2005) for a detailed description of MHFR.
TABLE 1. Morphometric measurements (in mm) of type specimens of Allobates spumaponens sp. nov. Abbreviations are defined in the text. Mean ± SD are followed by the range in parentheses.
| Character | Males (N = 6) | Female (N = 1) |
|---|---|---|
| SVL | 14.97 ± 1.04 (13.2–16.0) | 18.1 |
| HL | 4.80 ± 0.24 (4.3–5.0) | 5.8 |
| HW | 4.82 ± 0.34 (4.3–5.4) | 6.0 |
| SL | 2.52 ± 0.18 (2.3–2.8) | 3.0 |
| EN | 1.40 ± 0.10 (1.2–1.5) | 1.7 |
| IN | 1.92 ± 0.13 (1.7–2.1) | 2.2 |
| EL | 2.28 ± 0.16 (2.0–2.5) | 2.7 |
| IO | 1.98 ± 0.13 (1.8–2.2) | 2.4 |
| TYM | 0.75 ± 0.10 (0.6–0.9) | 0.9 |
| FAL | 3.43 ± 0.24 (3.0–3.8) | 3.9 |
| HAND | 3.37 ± 0.21 (3.0–3.6) | 4.0 |
| WFD | 0.45 ± 0.05 (0.4–0.5) | 0.4 |
| TL | 7.33 ± 0.45 (6.6–7.8) | 8.5 |
| FL | 6.12 ± 0.43 (5.3–6.6) | 6.9 |
| WTD | 0.55 ± 0.05 (0.5–0.6) | 0.6 |
TABLE 2. Morphometric measurements (in mm) of tadpoles of Allobates spumaponens sp. nov. Abbreviations are defined in the text. Mean ± SD are followed by the range in parentheses.
| Character | Stage 28 (N=4) | Stage 34 (N=1) | Stage 35 (N=2) | Stage 39 (N=1) | Stage 40 (N=1) |
|---|---|---|---|---|---|
| TL | 11.98 ± 1.62 (10.4–14.4) | 17.50 | 18.45 ± 0.85 (17.7–19.3) | 18.80 | 18.10 |
| BL | 3.65 ± 0.65 (2.8–4.5) | 6.30 | 6.45 ± 0.15 (6.3–6.6) | 6.40 | 6.30 |
| TAL | 8.36 ± 0.97 (7.4–9.9) | 11.30 | 12.00 ± 0.60 (11.4–12.6) | 12.40 | 11.90 |
| BH | 2.05 ± 0.71 (1.3–3.1) | 2.10 | 2.70 ± 0.30 (2.4–3.0) | 2.30 | 2.80 |
| TMH | 0.98 ± 0.13 (0.9–1.2) | 1.30 | 1.40 | 1.40 | 1.40 |
| MTH | 1.68 ± 0.20 (1.5–2.0) | 2.30 | 2.70 ± 0.20 (2.5–2.9) | 2.80 | 2.90 |
| HW | 2.18 ± 0.41 (1.8–2.8) | 3.50 | 3.55 ± 0.05 (3.5–3.6) | 3.60 | 3.70 |
| BW | 2.55 ± 0.56 (2.0–3.4) | 4.20 | 4.65 ± 0.15 (4.5–4.8) | 4.60 | 4.70 |
| TMW | 0.95 ± 0.15 (0.8–1.1) | 1.50 | 1.65 ± 0.05 (1.6–1.7) | 1.80 | 1.80 |
| END | 0.38 ± 0.08 (0.3–0.5) | 0.60 | 0.60 | 0.70 | 0.70 |
| IOD | 0.70 | 0.90 | 1.00 | 1.00 | 1.10 |
| ED | 0.65 ± 0.05 (0.6–0.7) | 0.90 | 0.85 ± 0.05 (0.8–0.9) | 0.90 | 1.00 |
| IND | 0.93 ± 0.18 (0.8–1.1) | 1.30 | 1.35 ± 0.05 (1.3–1.4) | 1.40 | 1.50 |
| NSD | 0.43 ± 0.08 (0.4–0.5) | 0.60 | 0.60 ± 0.10 (0.50–0.70) | 0.60 | 0.70 |
TABLE 3. Acoustic parameters for two recorded males of Allobates spumaponens sp. nov. Measurements are for the main harmonic, exclusively.
| Acoustic parameter | Mean | SD Range |
|---|---|---|
| Internote duration (s) | 0.234 | 0.093 0.125–0.418 |
| Note duration (s) | 0.066 | 0.003 0.06–0.07 |
| Lower frequency (Hz) | 5339.175 | 83.934 5186.44–5472.82 |
| Upper frequency (Hz) | 6802.886 | 62.456 6713.79–6920.62 |
| Delta frequency (Hz) | 1463.711 | 105.912 1320.52–1686.45 |
| Max power (db) | 127.691 | 0.851 126.24–126.40 |
| SMNS |
Staatliches Museum fuer Naturkund Stuttgart |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.