Thysanozoon brocchii (Risso 1818)
|
publication ID |
https://doi.org/ 10.5281/zenodo.202319 |
|
DOI |
https://doi.org/10.5281/zenodo.6183439 |
|
persistent identifier |
https://treatment.plazi.org/id/8C5F87DF-8C6C-FFF2-FF67-FD538373FF04 |
|
treatment provided by |
Plazi |
|
scientific name |
Thysanozoon brocchii (Risso 1818) |
| status |
|
Thysanozoon brocchii (Risso 1818)
( Figures 4–6 View FIGURE 4 View FIGURE 5 View FIGURE 6 )
Examined material. Voucher: 8.0 μm sagittally sectioned specimen, mounted on 81 slides. Collected by M.J. Albano, 9 May 2008, North Breakwater of the Mar del Plata harbour. On a mussel and ascidians community, 1–2 m depth. MACN-In 38206.
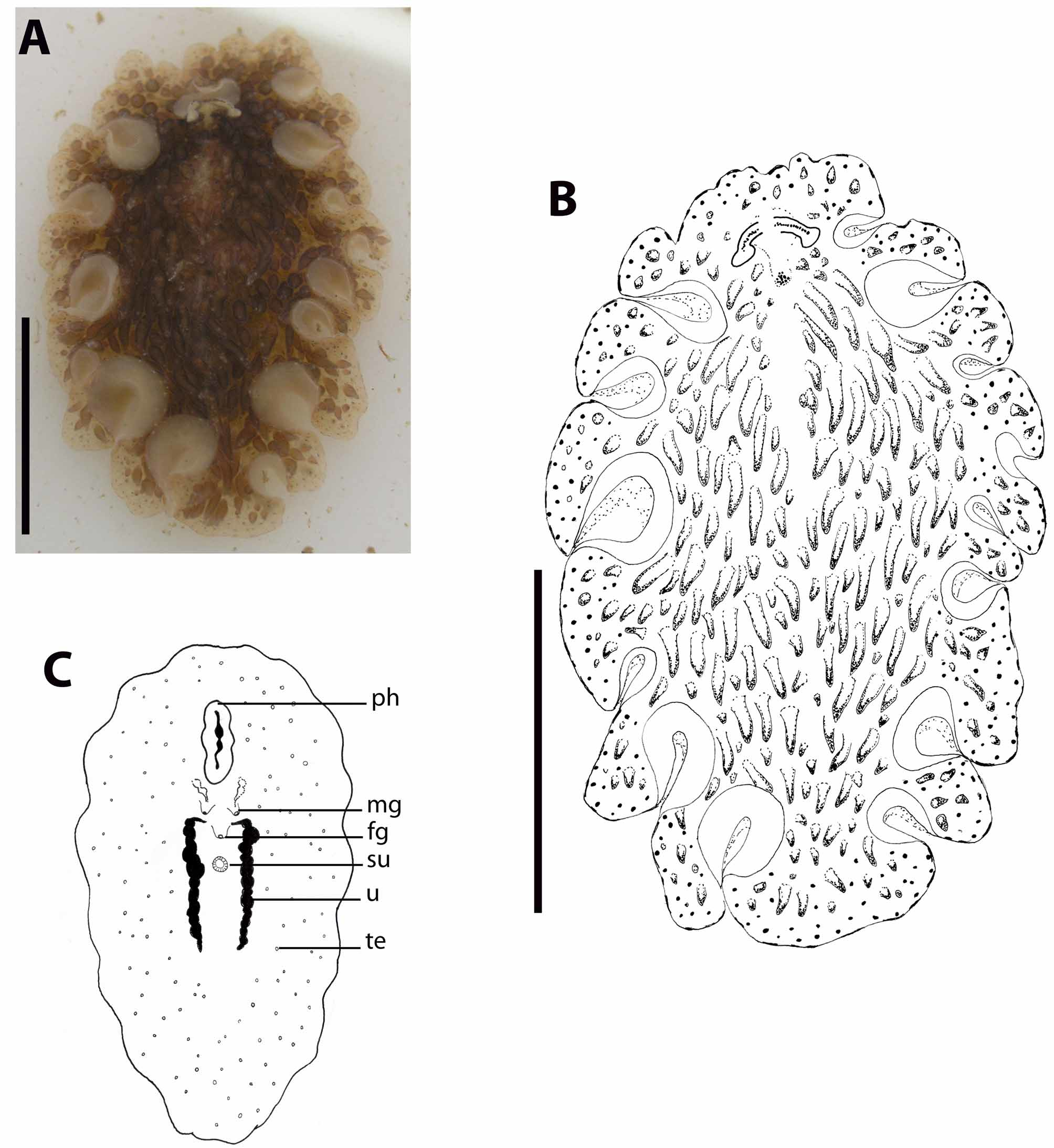
Morphology. Large, oval, 26 mm long by 15 mm wide alive, 23 mm by 14 mm preserved. Marginal tentacles formed by foldings of the fore margin, conspicuous, oriented dorsally and directed backwards, distally enlarged, with no pigmentation. Ground colour is light brown, delicately translucent in the margins, centrally yellowish brown. Round black spots cover the dorsal surface, especially in a wide marginal area. Dorsal surface covered with big bulky conical dark brown papillae, which are longer and more densely distributed to the sides of the dorsal bulge, but scattered on the bulge itself, and declining in length and number to the margins, leaving the margin free of papillae.
A second area free of papillae extends from the cerebral eyes up to between the tentacle bases (figures 4A–4B). Though the presence of blunt dorsal papillae gives the animal a solid appearance, the body itself is very delicate and frail. The dorsal tentacular eyespots are numerous on the dorsal surface, distributed in a single row; they are scattered ventrally. The cerebral eyespots form two separated triangular clusters.
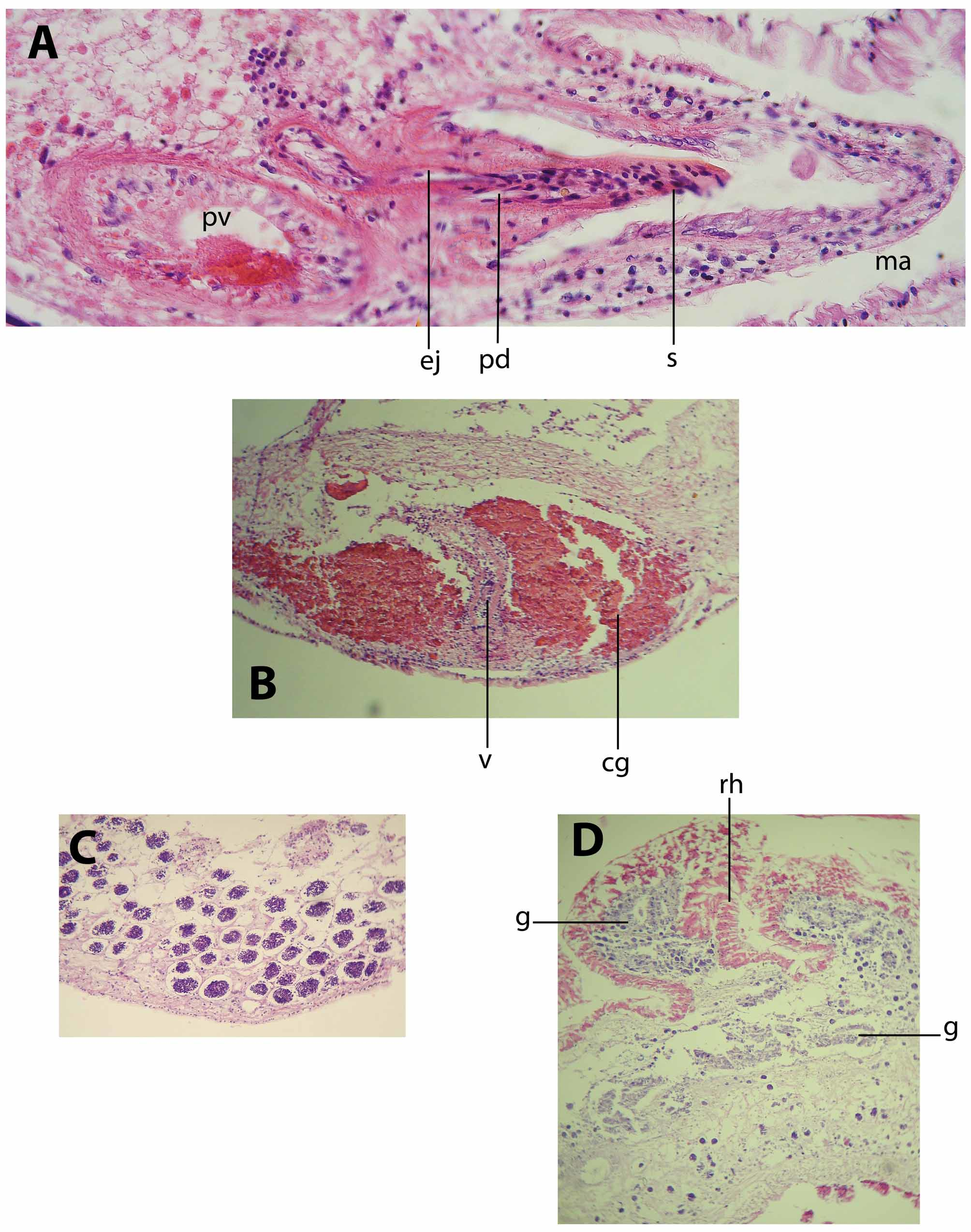
Pharynx ruffled, 320 µm long. The mouth opens at 7.2 mm from anterior margin, in the middle of the pharynx cavity. The main gut opens in the middle of the pharynx cavity roof and runs distally up to short behind the female system. Intestinal ramifications are conspicuous and numerous, running parallel to the body surface and giving out extensions into every dorsal papilla (figure 6D).
Ventral sucker at 13 mm from the fore margin of the body (figure 4C).
Dorsal body wall 50 µm high. The ciliated cellular epidermis has rhabdites and granular pigmentation. The cilia are very short, and the rhabdites and pigmentation are abundant and more densely packed on the papillae, but they are scattered on the dorsal epidermis. Beneath the epidermis a frail layer of circular muscle fibres is present, followed by a longitudinal muscle layer. The granular pigmentation is present in the epidermis and the circular muscle layer.
Ventral body wall 70 µm high. The cilia are numerous and longer than those on the dorsal epidermis. Ventral surface with scarce rhabdites and no granular pigmentation. Beneath a well defined basement membrane, a thin layer of circular muscle fibres is underlain by a layer of loose longitudinal muscle fibres.
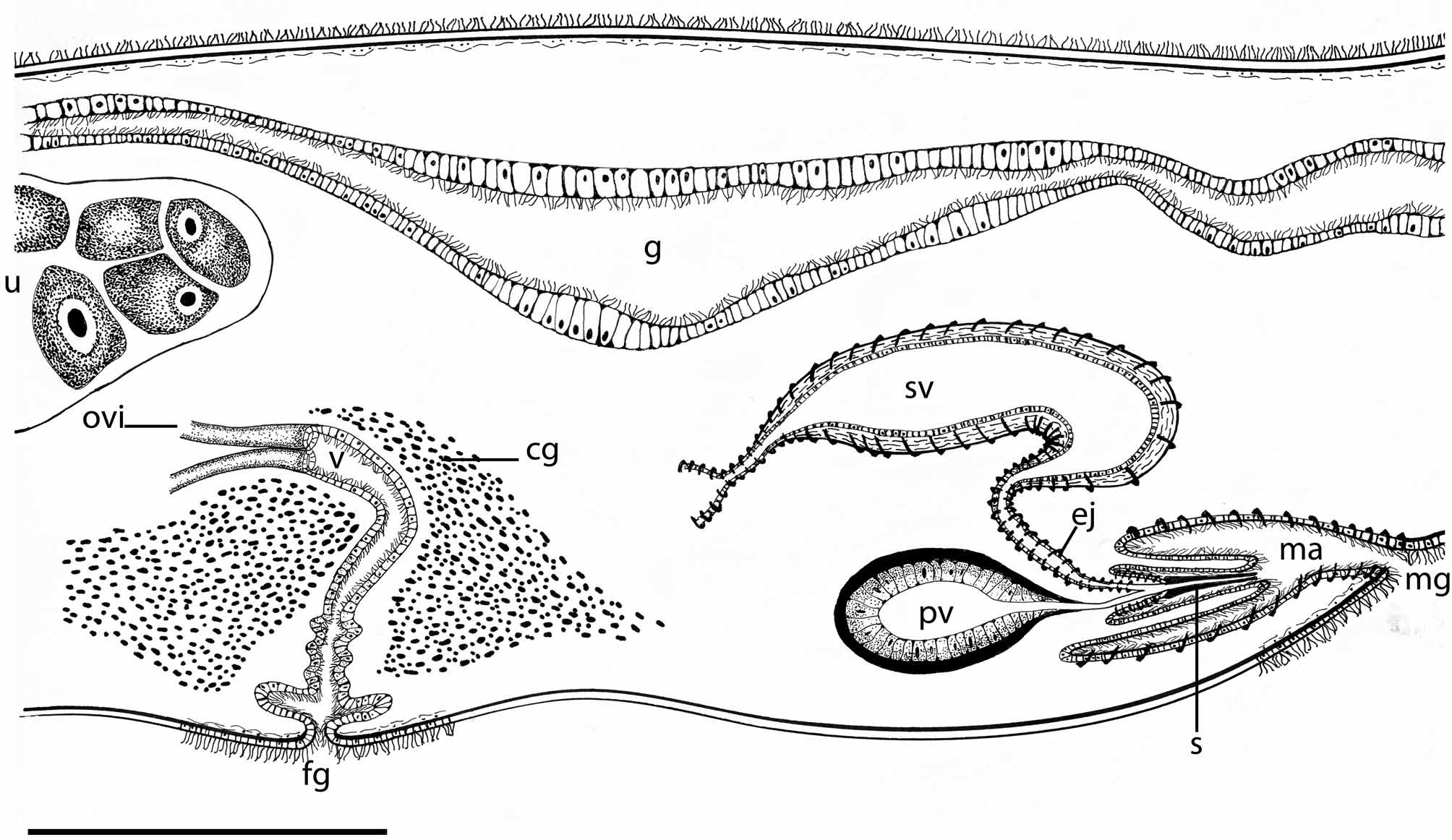
The testes are latero-ventrally distributed but especially greatly accumulated in the rear part of the body (figure 4C, 6C), beyond the uteri. The ovaries are dorsal; the uteri are short, filled with small and few mature cells.
The male reproductive system is double. Each male copulatory organ consists of a true seminal vesicle, free prostatic vesicle and penis papilla armed with a stylet (figure 5). The swollen vas deferens runs ventrally before entering the elongated seminal vesicle.
The seminal vesicle is curved and dorsal to the male prostatic vesicle and stylet; it has a well-developed muscular wall and its lumen is narrow. The rounded prostatic vesicle has a muscular wall and a very high, smooth glandular inner lining. The ciliated ejaculatory duct describes a tortuous trajectory up to reach the penis papilla, having a well-developed muscular layer. The prostatic duct is short and straight, joining the ejaculatory duct beyond the point where the stylet is attached. The stylet is conical and elongated, 330 µm long and slightly curved. The penis sheath is ciliated, short and narrow (figure 6A). The spacious male atrium is ciliated and directed forwards, opening to a male gonopore located 7.6 mm from the fore end. The second male copulatory organ is similar to the one already described.
The female reproductive system is single (figure 5). The oviducts enter the vagina separately from the rear end. The canal is proximally swollen, later narrowing and turning ventrally, to finally enlarge distally. The fore and middle tract of the vagina is immersed in an eosinophilic glandular mass (figure 6B). There are no cement glands either distally to the vagina or surrounding the elongated female atrium. Without an apparent cement pouch, the vagina and atrium are completely ciliated, underlain by a frail layer of circular muscle fibres. The female atrium opens to a female gonopore ventrally in the midline of body.
Taxonomical discussion. Species in genus Thysanozoon are identifiable by their papillated dorsal epidermis, each species showing a definite body shape, a particular distribution of the papillae and a specific colour pattern. The studied specimen of T. brocchii from Mar del Plata matched the detailed descriptions of this species made by Lang (1884) and Stummer-Traunfels (1895).
Under the current frame of morphological identification, the crucial characters that discriminate T. brocchii from its relatives are the dorsal surface covered with large bulky conical dark brown papillae, with the margin and cerebral areas free of papillae; its ground colour light brown, delicate translucent in the margins, and the internal anatomy being consistent with the descriptions of the T. brocchii specimens described for Brazil, Curaçao and Patagonia ( Marcus 1949, 1952; Marcus & Marcus1968; Brusa et al. 2009). Marcus (1949) described T. lagidium from the coast of Brazil; later on, after comparing the anatomy with the specimen from Curaçao and Europe, he synonymised T. lagidium with T. brocchii ( Marcus & Marcus 1968) . These descriptions were accurate enough for Brusa et al. (2009) to identify the specimen from Patagonia as T. brocchii and the specimen from Mar del Plata as well.
Thysanozoon brocchii has been described inhabiting Indopacific waters, the Mediterranean, and the Atlantic coast of South America, from the Caribbean to Patagonia. Such a worldwide distribution is curious, especially since water temperature has been considered a limiting factor for the distribution of polyclad species.
Prudhoe (1985) proposed that the presence of dorsal papillae in the genera Acanthozoon and Thysanozoon could be related with their flotation capacity as a mean of dispersion, but from personal observation, T. brocchii possess a benthonic life habit, crawling over rocks and into cracks between short swimming periods.
Faubel (1984) published a list of pelagic polyclad species collected during different expeditions to South and Central Atlantic Ocean and Sargasso Sea. The unpapillate polyclads collected by these expeditions, were not in accordance to Prudhoe’s (1985) hypothesis. We agree with Brusa et al. (2009) in that the presence of the papillae should be associated with increasing the surface of gas exchange and digestion. The cosmopolitan dispersion of T. brocchii over the South American Atlantic coasts could be associated with the Brazilian ocean current, especially at larval stage.
However, because the traditional taxonomy of the Pseudocerotidae is mostly based on external morphological characters, it does not necessarily reflect their phylogenetic relationships. There is still no molecular evidence to discuss if the South American T. brocchii is genetically identical with the morphologically similar specimens collected from the Indopacific waters and Mediterranean coasts.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.