Alpheus buckupi, Almeida, Alexandre O., Terossi, Mariana, Araújo-Silva, Catarina L. & Mantelatto, Fernando L., 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3652.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:355A8986-8CD9-4C71-A709-2E2874DEEA6C |
|
DOI |
https://doi.org/10.5281/zenodo.3508100 |
|
persistent identifier |
https://treatment.plazi.org/id/4657C259-952D-4057-9004-FE5332B63179 |
|
taxon LSID |
lsid:zoobank.org:act:4657C259-952D-4057-9004-FE5332B63179 |
|
treatment provided by |
Plazi |
|
scientific name |
Alpheus buckupi |
| status |
sp. nov. |
Alpheus buckupi View in CoL spec. nov.
( Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 )
Type material: Brazil, state of Pernambuco. Holotype: 1 m (CL = 10.6 mm), Paulista, Timbó River (07º51’38.3”S, 34º50’21.4”W), colls. A.O. Almeida & R.J.C. Paiva, 09.IV.2008, under rocks with Alpheus carlae Anker, 2012 , Salmoneus carvachoi Anker, 2007 , and unidentified gobiid fish, muddy bottom, salinity 30 (MZUSP 27548). Paratypes: 1 ovf (CL = 12.3 mm), same data as holotype (MZUSP 27549); 1 m (CL = 11.6 mm), same data as holotype (UESC 1366) (specimen used in genetic analysis); 1 m (CL = 8.5 mm), 1 ovf (CL = 9.5 mm), Recife, confluence between Pina and Capibaribe rivers (08º04’12.15”S, 34º52’19.11”W), colls. A.O. Almeida, R.J.C. Paiva & F.S. Santana, 19.VIII.2008, under rocks, sandy bottom, salinity 25 (CCDB 3966); 1 m (CL = 8.5 mm), Recife, confluence between Pina and Capibaribe rivers (08º04’12.15”S, 34º52’19.11”W), colls. A.O. Almeida & R.J.C. Paiva, 13.VII.2007, under rocks covered with Mytella sp., dead oysters, and live barnacles, muddy bottom, salinity 21 (UESC 1530).
Additional material: VENEZUELA: 2 m, 1 ovf, Orinoco Delta, XI-5234, coll. G. Pereira, 2003, AA fcn 03-030 (OUMNH.ZC. 2011-06.003); 1 m, Orinoco Delta, XI-5234, coll. G. Pereira, 2003 (OUMNH.ZC. 2011-06.009); 1 m, 1 ovf (in bad condition), Orinoco Delta, XI-5040, coll. G. Pereira, 2003 (OUMNH.ZC. 2011-06.011). BRAZIL: Rio Grande do Norte: 2 m, 3 f (1 ovf), Natal, Ponta Negra, Morro do Careca (05°51.858’S; 35°10.809’W), coll. R. Robles, 07.VI.2011, under rocks (rocky shore) (CCDB 3822). Pernambuco: 8 m, 10 f (6 ovf), Itamaracá, Paripe River near Vila Velha (07º48’38.6”S, 34º51’23.0”W), colls. A.O. Almeida, R.J.C. Paiva & F.S. Santana, 01.IX.2008, in cavities of estuarine sponge, 0–0.3 m depth (UESC 1367); 1 m, Olinda, Casa Caiada Beach (07º59’21.31”S, 34º50’17.58”W), coll. F.S. Santana, 18.VIII.2008, under rocks, sandy bottom (UESC 1368); 2 f (1 ovf), Recife, confluence between Pina and Capibaribe rivers (08º04’12.15”S, 34º52’19.11”W), colls. A.O. Almeida & R.J.C. Paiva, 13.VII.2007, under rocks covered with Mytella sp., dead oysters, and live barnacles, muddy bottom, salinity 21 (UESC 1369). Alagoas: 3 m, Passo de Camaragibe, Morro do Camaragibe, Station E5, littoral, colls. W. Santana, M. Cardoso Jr. & N. Oliveira, 30.V.2011 (MZUSP 25369). Bahia: 1 m, 1 ovf, Todos os Santos Bay, PROAMB, Poço Dom João, Station 12, 07.IX.2002 (UESC 1520); 1 m, 1 f, Cairu, Boipeba Island, Tassimirim Beach (13°34’49.6”S; 38°54’49.4”W), colls. A.O. Almeida, P.S. Santos & G.O. Soledade, 19.V.2011, under rocks, salinity 31 (UESC 1521) (one specimen used in genetic analysis); 1 m, 2 f (1 ovf), Cairu, Boipeba Island, Moreré Beach (13°36’49.5”S; 38°54’16.2”W), colls. A.O. Almeida, P.S. Santos & G.O. Soledade, 20.V.2011, under rocks, salinity 38 (UESC 1522); 1 m, 1 f, Itacaré, Contas River, coll. G.O. Soledade, 14.VIII.2011, under rocks (UESC 1523). São Paulo: 1 ovf, Ubatuba, Grande Beach (23°28'01"S; 45°03'36"W), coll. F.L. Mantelatto, 27.XI.2002, at low tide under rocks in small subtidal pools (CCDB 3494); 3 m, Ilhabela, Engenho d’Água Beach (23°47’43.3”S, 45°21’55.4”W), coll. E.C. Mossolin, between III.2000 and II.2002 (UESC 1524); 1 ovf, São Sebastião, Araçá, 14.I.2004 (CCDB 1205); 1 ovf, São Sebastião, Araçá, coll. F.L. Mantelatto, 04.V.2007, at low tide under rocks (CCDB 1907); 1 m, 1 ovf, Santos, Ponta da Praia, coll. C. Magenta, VIII.2008, under rocks, low tide (MZUSP 18903).
EASTERN ATLANTIC: SÃO TOMÉ AND PRÍNCIPE: 1 f, São Tomé and Príncipe, Lagarto Beach, near Hospital, Station 2A, colls. N. Knowlton & A. Anker, 02.II.2006, sand with Montastrea , zoanthids, and rocks embedded in sand, intertidal (extreme low tide), AA fcn 06-129 (OUMNH.ZC. 2011-06.005) (specimen used in genetic analysis); 1 m, São Tomé and Príncipe, Lagarto Beach, near Hospital, coll. A. Anker, 09.II.2006, upper intertidal under rocks and zoanthid colonies, in burrows, low tide, AA fcn 06-194 (OUMNH.ZC. 2011-06.012).
Comparative material of Alpheus cf. lobidens : 1 m, Low Isles, Queensland, Great Barrier Reef (NMV - J5860); 1 m, 1 f, New South Wales, Lennox Head (NMV - J21615 View Materials ).
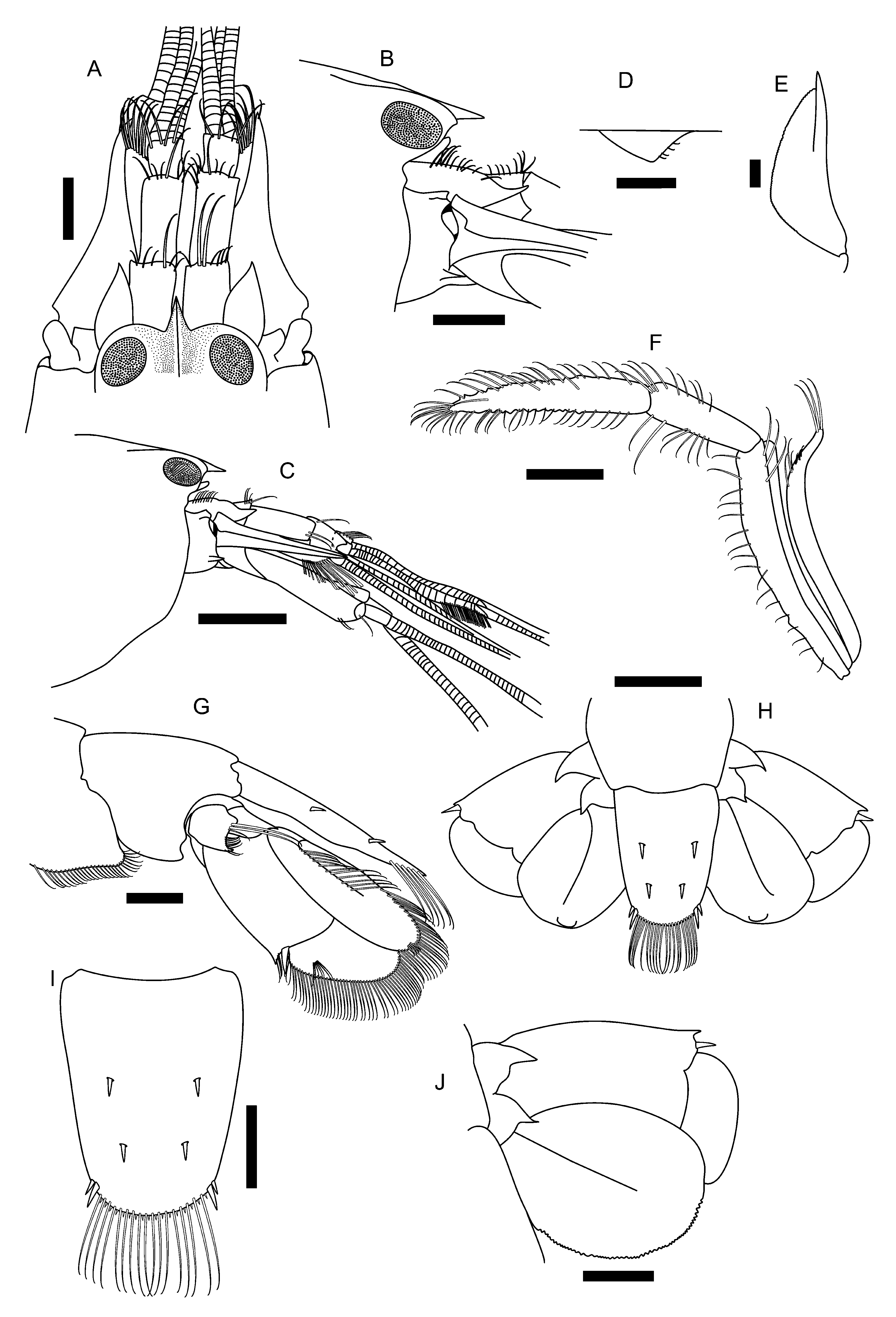
Description: Carapace smooth, glabrous, laterally not compressed; rostrum triangular, with acute tip reaching approximately midlength of first segment of antennular peduncle ( Fig. 1 View FIGURE 1 A); rostral carina sharply delimited between orbital hoods, reaching posterior margin of orbital hoods, not broadening posteriorly ( Fig. 1 View FIGURE 1 A); adrostral furrows moderately deep, not abruptly delimited posteriorly ( Fig. 1 View FIGURE 1 A); orbital hoods inflated dorsally, distally rounded, unarmed ( Fig. 1 View FIGURE 1 A); pterygostomial angle rounded ( Figs. 1 View FIGURE 1 B, C); cardiac notch well developed.
Abdominal somites smooth, glabrous; ventral and posterior margins of pleurae 1–4 broadly rounded and pleura 5 forming angle of approximately 90° with tip rounded; sixth pleura without articulated plate ( Fig. 1 View FIGURE 1 G); protopod pleopods without spines; male pleopod 2 with appendix masculina slightly shorter than appendix interna; preanal plate with rounded tip. Telson broad, tapering distally, approximately 1.2 times as long as wide at base; lateral margins slightly sinuous; dorsal surface slightly convex, without median groove, with two pairs of spiniform setae, inserted at some distance from lateral margins, first pair slightly anterior to midlength, second pair well posterior to telson midlength ( Fig. 1 View FIGURE 1 H, I); posterior margin broadly rounded, fringed with spinules (short spiniform setae) and long setae, posterolateral angle each with two pairs of spiniform setae, lateral seta approximately 1/2 length of mesial seta ( Fig. 1 View FIGURE 1 H, I); anal tubercles well developed.
Eyes totally concealed in lateral, dorsal, and frontal views; cornea well developed, rounded ( Fig. 1 View FIGURE 1 A–C). Ocellar beak protruding dorsally between eyes, apically rounded ( Fig. 1 View FIGURE 1 B).
Antennular peduncle moderately slender ( Fig. 1 View FIGURE 1 A). Stylocerite distally acute, reaching distal margin of first segment of antennular peduncle ( Fig. 1 View FIGURE 1 A); ventromesial carina of first segment with tooth ending rounded, anterior margin slightly concave ( Fig. 1 View FIGURE 1 D); visible part of first segment as long as wide; second segment longest, 2.5 times as long as wide; third segment as long as wide, 0.4 times length of second segment ( Fig. 1 View FIGURE 1 A); lateral antennular flagellum with row of aesthetascs starting at 15th segment. Antenna with basicerite bearing robust acute distolateral tooth ( Fig. 1 View FIGURE 1 B, C); carpocerite stout, reaching distinctly beyond end of antennular peduncle and tip of distolateral tooth of scaphocerite ( Fig. 1 View FIGURE 1 C); scaphocerite with lateral margin slightly concave; blade broad, separated from distolateral tooth by deep cleft running about 1/3 length of blade ( Fig. 1 View FIGURE 1 E); distolateral tooth well developed, distinctly overreaching distal margin of blade and end of antennular peduncle ( Fig. 1 View FIGURE 1 E).
Mouthparts not dissected, appearing typical in external view. Third maxilliped relatively slender, conspicuously longer than antennular peduncle and carpocerite when extended; lateral plate with subacute point; antepenultimate segment not flattened or expanded, mesial margin distinct, distodorsal portion not protruding ( Fig. 1 View FIGURE 1 F); penultimate segment about three times as long as wide, lateral margin smooth, with tufts of setae; last segment tapering distally, smooth, with several bands of setae; exopod reaching slightly beyond distal margin of antepenultimate segment ( Fig. 1 View FIGURE 1 F).
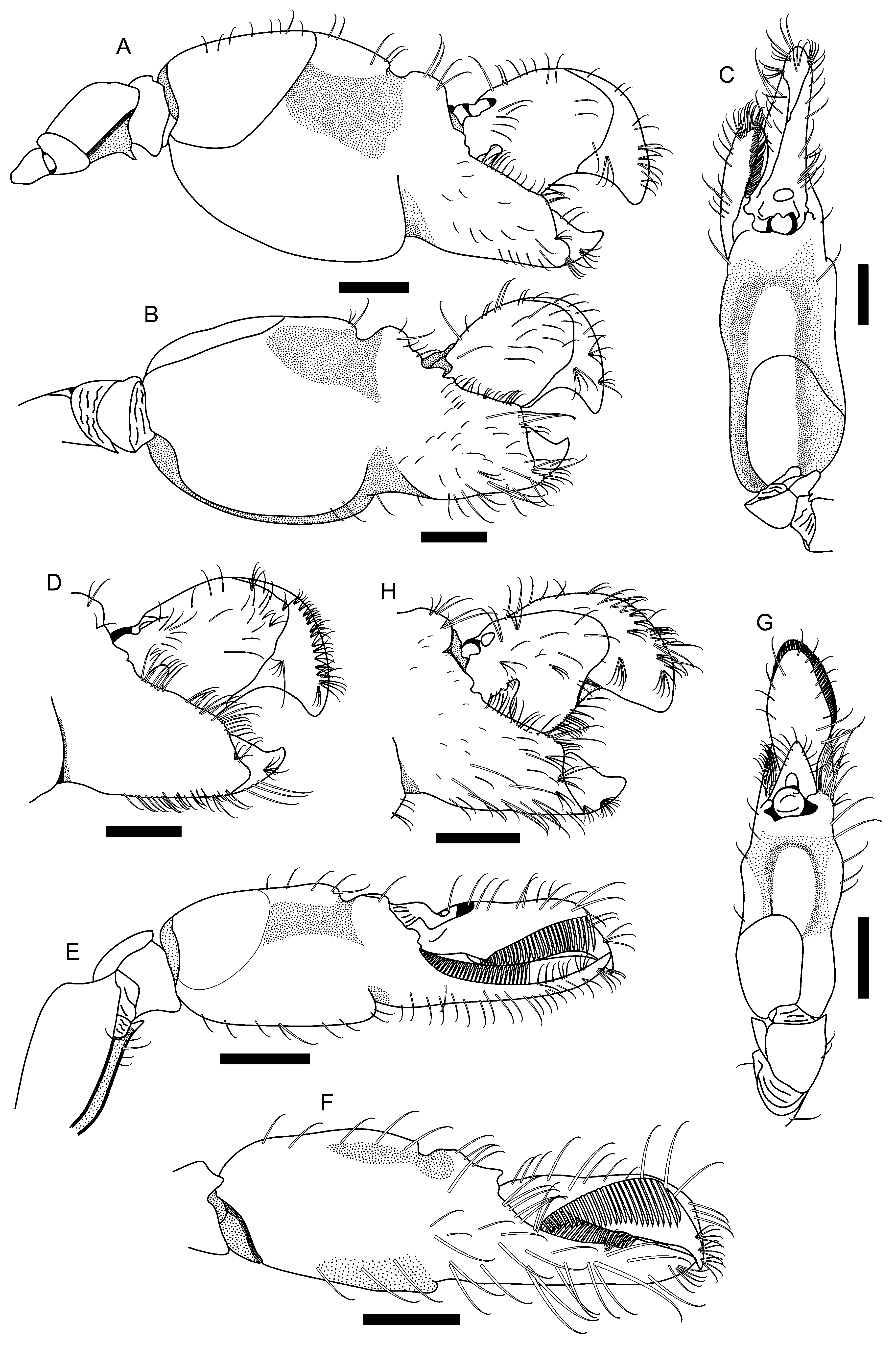
Major male cheliped with short, stout ischium; merus slightly excavated ventrally; ventrolateral margin straight, with blunt distal end; ventromesial margin also straight, ending in strong tooth distally ( Fig. 2 View FIGURE 2 A); carpus short, cup-shaped; chela somewhat compressed; fingers closing in same plane as palm; palm with dorsal and ventral margins convex, with broad transverse grooves; dorsal and ventral grooves extending to mesial and lateral surfaces as deep depressions, latter extending posteriorly; dorsal shoulder rounded, slightly overhanging groove; ventral groove broad, oblique, deep, also extending mesially and laterally as well-delimited deep depressions, latter not extending posteriorly; ventral shoulder rounded, slightly protruding; lateral and mesial surfaces mostly smooth; linea impressa well marked; mesial surface ending bluntly distally ( Figs. 2 View FIGURE 2 A–C); fingers compressed, longer than half palm length ( Figs. 2 View FIGURE 2 A, B); pollex with tip curved upward, with V-shaped notch on cutting edge anterior to deep fossa; proximal mesial surface surrounding fossa forming obtuse angle, fringed with rows of setae; dactylus reaching slightly beyond pollex, with rounded tip, cutting edge with stout plunger ( Fig. 2 View FIGURE 2 D); adhesive disks conspicuous ( Figs. 2 View FIGURE 2 C, D). Female major cheliped similar in shape, but proportionally smaller than that of male.
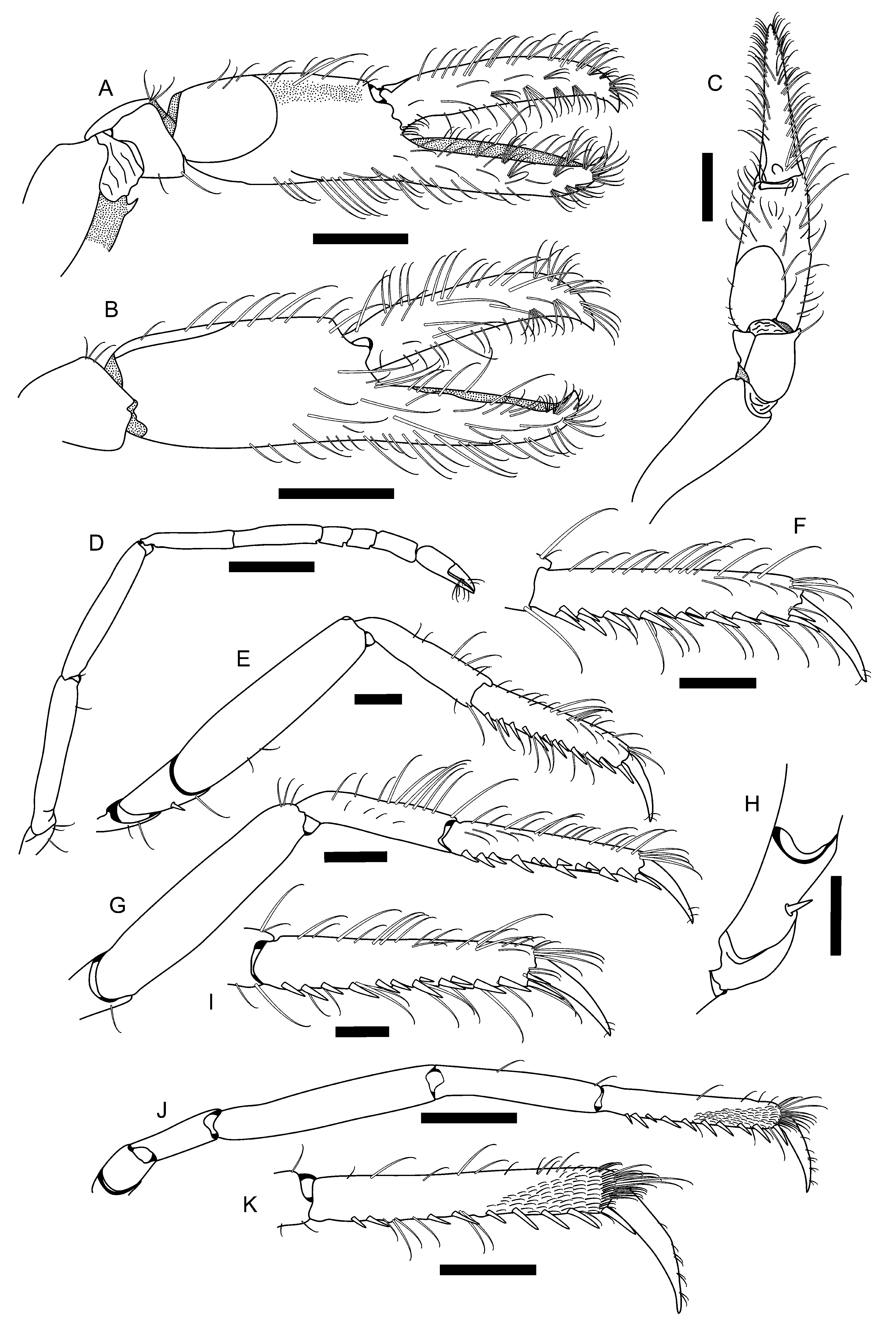
Minor male cheliped with ischium short and stout; merus proportionally longer than that of major cheliped, slightly excavated ventrally; ventrolateral and ventromesial margins as in major cheliped; tooth on distal portion of ventromesial margin generally smaller than that of major cheliped ( Fig. 2 View FIGURE 2 E); carpus short, cup-shaped; chela roughly cylindrical in transverse section; palm sculpturing resembling that of major cheliped, with dorsal and ventral margins slightly convex, with transverse grooves ( Figs. 2 View FIGURE 2 E–G); dorsal and ventral grooves also extend to mesial and lateral surfaces as deep depressions, latter extending posteriorly; dorsal shoulder rounded, not overhanging groove; ventral groove not as broad and deep as that of major cheliped; ventral shoulder rounded, not protruding; lateral and mesial surfaces mostly smooth; linea impressa well marked; dorsomesial angle of palm with blunt tooth ( Fig. 2 View FIGURE 2 F); fingers as long as palm, with conspicuous rows of balaeniceps setae, cutting edges sharp, tip curved ( Figs. 2 View FIGURE 2 E, F); dactylus expanded laterally, with dorsal disk similar to adhesive disk of dactylus of major cheliped, and conspicuous carina on proximal region ( Fig. 2 View FIGURE 2 G). Female minor cheliped more slender than that of males. Palm inconspicuously sculptured; dorsal and ventral grooves absent; linea impressa well marked ( Figs. 3 View FIGURE 3 A– C). Fingers slightly longer than palm, without balaeniceps setae, cutting edges blade-like, tip curved ( Figs. 3 View FIGURE 3 A–C); dactylus not expanded laterally, without proximal carina on dorsal margin, but with inconspicuously marked disk similar to that of male minor chela.
Second pereiopod slender, ischium and merus subequal in length; carpus five-segmented, first segment longest; segment ratio (proximal to distal) subequal to 4: 2.5: 1: 1: 2; chela simple, fingers slightly longer than palm and bearing tufts of curved setae distally ( Fig. 3 View FIGURE 3 D). Third and fourth pereiopods similar in shape and length, both with ischium armed with spiniform seta on ventrolateral surface; merus longer than propodus, about four times as long as wide, distoventral margin unarmed; carpus unarmed, about half merus length and slightly shorter than propodus length ( Fig. 3 View FIGURE 3 E, G, H); propodus with about 12 strong spiniform setae along ventral margin, often arranged in pairs, plus one distal pair of spiniform setae near dactylus; dactylus around 0.4 propodus length, simple, conical, slightly curved, acute distally ( Fig. 3 View FIGURE 3 F, I). Fifth pereiopod with ischium and merus unarmed; merus slender, about six times as long as wide; carpus about 3/4 merus length ( Fig. 3 View FIGURE 3 J); propodus slightly longer than carpus, with nine spiniform setae along ventral margin plus one distal pair of spiniform setae near dactylus; distolateral surface with cleaning brush consisting of about 12 transverse rows of short setae; dactylus similar in shape to that of third and four pereiopods, proportionally slightly longer, corresponding to almost 1/2 propodus length ( Fig. 3 View FIGURE 3 K).
Uropods with bifid protopods, each lobe ending in acute tooth ( Fig. 1 View FIGURE 1 H); exopod slightly longer than endopod; distolateral spiniform setae slender, distinctly shorter than posterior margin of exopods, not pigmented; exopodal diaeresis with two slightly lobes separated by median notch; distolateral tooth acute, approximately 1/2 length of distolateral spiniform seta ( Fig. 1 View FIGURE 1 H, J).
Gill formula typical for genus.
Morphological variation: The V-shaped notch on the cutting edge of the pollex is in general obvious in larger individuals and inconspicuous or absent in smaller individuals. The shape of the notch varies from obtuse to acute in larger individuals (see Fig. 2 View FIGURE 2 D, H). However, this notch may also be inconspicuous in large individuals, as in the material from Venezuela (OUMNH.ZC. 2011-06.003, 2011-06.009, 8.7–9.5 mm CL).
Another important variation lies in the sculpturing of the palm of the male minor chelae, which resembles that of the major chelae in fully adult male specimens (see Fig. 2 View FIGURE 2 A, B, E, F). In larger individuals, palm sculpturing (depth of the grooves on the dorsal and ventral margins and depressions on the mesial and lateral surfaces) is well developed, whereas the sculpturing in small individuals is, in general, little developed. Medium-sized individuals vary in sculpturing, with some degree of overlapping. Similar variation was noted for A. lobidens (see Banner & Banner 1981).
The presence of a tooth on the distal portion of the ventromesial margin of the meri of the major and minor chelipeds is a typical trait of A. buckupi spec. nov. (see Fig. 2 View FIGURE 2 A, E). In general, this tooth is strong and acute, but it may be less developed and sometimes is obsolescent or missing, especially on the minor cheliped. This type of variation was also noted in A. lobidens (see Banner & Banner 1981).
The eastern Atlantic material consists of two small specimens from São Tomé and Príncipe (male, CL = 6.1 mm; female, CL = 5 mm) that agree well with the western Atlantic material. The sculpturing on the male minor chela is well developed, with deep dorsal and ventral grooves on the palm extending to the mesial and lateral margins. On the other hand, the V-notch on the cutting edge of the pollex of the major chela is absent in the male and inconspicuous in the female. The ischium of pereiopod 5 is unarmed in the male and armed in the female. Although rare, the condition observed in this female is also present in some eastern Atlantic specimens.
Color pattern (based on analyses of color photographs of fresh specimens): Carapace, abdomen, and uropods dark green in larger animals (paired females frequently darker than males) ( Fig. 4 View FIGURE 4 D); palm major and minor chelae usually pale greenish, fingers darker than palm; walking legs semitransparent ( Fig. 4 View FIGURE 4 B), sometimes with reddish to brownish chromatophores ( Fig. 4 View FIGURE 4 D). Body of smaller individuals usually greenish, semitransparent, frequently with reddish to brownish chromatophores on chelipeds, carapace, abdomen, and uropods ( Fig. 4 View FIGURE 4 C). Ovaries and eggs light green ( Fig. 4 View FIGURE 4 E).
Etymology: It is our great pleasure to name this new species in honor of the Brazilian carcinologist, Prof. Dr. Ludwig Buckup (Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil) in recognition of his dedication and contributions to the knowledge of crustaceans.
Type locality: Northeastern Brazil, state of Pernambuco, Paulista, Timbó River (07º51’38.3”S, 34º50’21.4”W).
Distribution: Western Atlantic: Venezuela (Orinoco Delta) and Brazil (Ceará, based on color photographs provided by Arthur Anker; Rio Grande do Norte, Pernambuco, Alagoas, Bahia, and São Paulo). Eastern Atlantic: São Tomé and Príncipe.
Ecology: Free-living species, occurring mainly in estuaries, but also in bays and protected beaches, living in male-female pairs under rocks and in cavities of an unidentified estuarine sponge, on mud, mud-sand, and finesand bottoms, salinity range 21–38, depth range 0–0.3 m. At the type locality, two other alpheids were observed occurring syntopically, A. carlae and S. carvachoi . They were obtained together with unidentified gobiid fish, with small hand-net under the same rocks and stones where the type material was collected.
Discussion
In the Atlantic basin, A. buckupi spec. nov. has some morphological similarities with other species of the A. edwardsii group, such as A. heterochaelis Say, 1818 (western Atlantic), A. pontederiae de Rochebrune, 1883 (amphi-Atlantic), A. chacei Carvacho, 1979 (western Atlantic), and A. estuariensis Christoffersen, 1984 (western Atlantic), and also with species of the A. armillatus H. Milne Edwards, 1837 complex (western Atlantic). Most of those taxa also occur in the same habitats where the new species is found.
Similarly to A. buckupi spec. nov., A. heterochaelis and A. pontederiae have well-developed balaeniceps setae on the male minor chelae, differing from A. chacei and A. estuariensis , in which the male minor chelae lack these setae (see Christoffersen 1984). Alpheus buckupi spec. nov. differs from A. heterochaelis by the presence of a tooth on the distal portion of the ventromesial margin of the meri of the major and minor chelipeds (absent in A. heterochaelis ), by the stronger sculpturing of the male minor chelae, and by the presence of spiniform setae on the ischium of pereiopods 3 and 4 [vs. on pereiopods 3–5 in A. heterochaelis , see McClure (1995)]. The presence of a ventromesial tooth on the distal portion of the meri of the chelipeds also distinguishes A. buckupi spec. nov. from A. pontederiae , A. euphrosyne De Man, 1897 , and A. microrhynchus De Man, 1897 , two Indo-Pacific species close to A. pontederiae . These three species also differ from A. buckupi spec. nov. by the presence of an oblique groove flanked by two ridges on the mesial surface of the pollex of the major chela (absent in A. buckupi spec. nov.) and by the distolateral tooth of the scaphocerite usually reaching the distal margin of the adjoining blade or sometimes shorter (vs. distinctly overreaching the distal margin of the blade in A. buckupi spec. nov.).
Members of the A. armillatus species complex also have a tooth on the distal portion of the ventromesial margin of the meri of the major and minor chelipeds (Anker 2012). Based on morphology alone, males of A. buckupi spec. nov. can easily be differentiated from species of the A. armillatus complex by the presence of rows of balaeniceps setae on the mesial and lateral margins of the minor cheliped (absent in species of the A. armillatus complex; see Anker 2012). Females of the two species are similar, especially young females. Adult females of A. buckupi spec. nov. can be differentiated from those of the A. armillatus complex by the shallower adrostral furrows that are not abruptly delimited posteriorly, and the rostral carina that is not flattened posteriorly (vs. deeper adrostral furrows that are markedly delimited posteriorly and the carina flattened posteriorly, forming a more-orless V- or U-shaped post-rostral plate; see Anker 2012).
Alpheus buckupi spec. nov. is morphologically very similar to members of the A. lobidens species complex, including A. inopinatus Holthuis & Gottlieb, 1958 and possibly various undescribed taxa (Anker 2001; Yang & Anker 2003; A. Anker pers. com.), especially regarding on major and minor chelae configuration. For this reason, material from Australia (NMV J5860 and J21615 View Materials ) that we previously identified as A. cf. lobidens was analyzed and compared to our material.
The most conspicuous difference between A. buckupi spec. nov. and A. inopinatus is the presence of a tooth on the distal portion of the ventromesial margin of the meri of the major and minor chelipeds in the former species. Based on illustrations and descriptions provided by Holthuis & Gottlieb (1958), A. buckupi spec. nov. also differs by the narrower stylocerite (broadly oval in A. inopinatus ) and by the weaker distolateral tooth of the scaphocerite (narrower than the adjoining blade in A. buckupi spec. nov. vs. as broad as or broader than the adjoining blade in A. inopinatus ).
Similarly to A. inopinatus , the specimens of A. cf. lobidens have no tooth on the distal portion of the ventromesial margin of the meri of the major and minor chelipeds. In contrast, this tooth is typically well developed in A. buckupi spec. nov. However, other authors did mention the existence of this tooth in A. lobidens (Banner & Banner 1982; Yang & Anker 2003), as well as variation in the degree of its development (Banner & Banner 1981). Therefore, this is apparently not a reliable character to separate A. buckupi spec. nov. from A. cf. lobidens and other members of the A. lobidens species complex. The V-shaped notch on the cutting edge of the pollex anterior to the fossa is frequently obvious in larger individuals of A. buckupi spec. nov. and inconspicuous or absent in smaller individuals. In the three specimens of A. cf. lobidens from Australia, this character was not observed. Available descriptions and illustrations of A. lobidens (e.g., Banner & Banner 1981, 1982; Yang & Anker 2003) are too limited and insufficiently detailed and do not mention the existence of a V-shaped notch on the cutting edge of the pollex. Revision of the A. lobidens complex may indicate if this is a useful morphological character in distinguishing A. buckupi spec. nov. from the species of the A. lobidens complex.
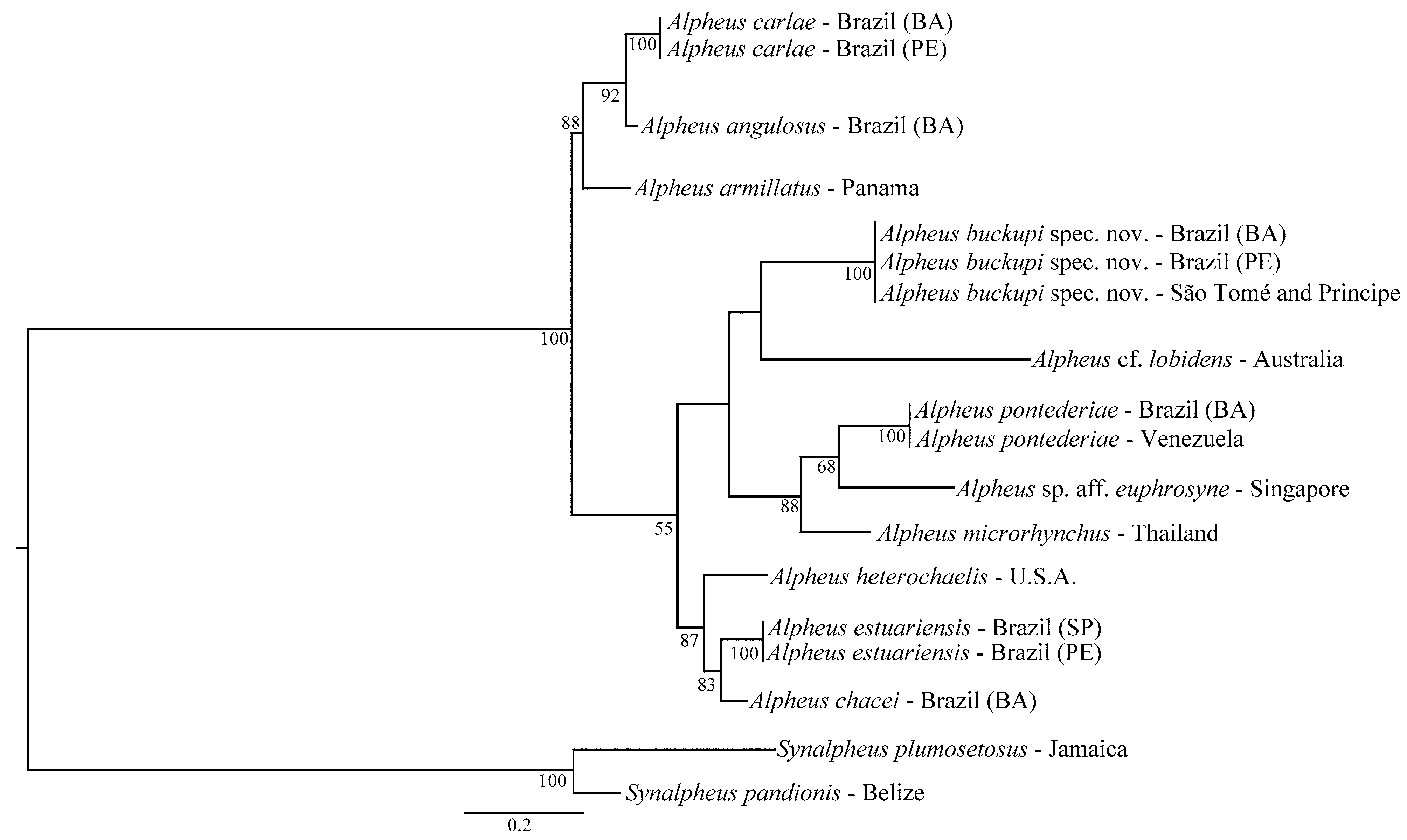
However, and importantly, our molecular data indicated that the genetic divergence between A. buckupi spec. nov. and A. cf. lobidens was much higher than the level of intraspecific variation in this analysis (0.170, see below). The new species was clearly contextualized in terms of DNA sequencing using the ribosomal mt16S gene, which provided enough data to support our findings in establishing the classification of the new species. The multiple sequence alignment of the 16S gene obtained had 541 positions. Low values of genetic distance (K2P) occurred with morphologically well-defined species ( Table 2 View TABLE 2 ): two specimens of A. carlae ( Brazil: Pernambuco and Bahia; 0.008), two specimens of A. estuariensis ( Brazil: Pernambuco and São Paulo; 0) and two specimens of A. pontederiae ( Brazil and Venezuela; 0). Therefore, in this analysis the intraspecific variation among specimens of the genus Alpheus ranged from 0 to 0.008.
In the current analysis, the interspecific variation among species of the genus Alpheus ranged from 0.058 to 0.232 ( Table 2 View TABLE 2 ). Thus, the gap between intraspecific and interspecific variations was from 0.008 to 0.058. These interspecific values agree with another study based on the 16S gene, in which the genetic distances (K2P) between the Pacific species A. tenuis Kim & Abele, 1988 and the Caribbean A. armillatus complex ranged from 0.070 to 0.100 (Mathews 2006).
The genetic distance estimated among the three specimens of A. buckupi spec. nov. was 0 ( Table 2 View TABLE 2 ); this value is lower than the maximum intraspecific value found (0.008). The genetic distances estimated among the specimens of A. buckupi spec. nov. and other species of Alpheus ranged from 0.136 to 0.197 ( Table 2 View TABLE 2 ). These values are much higher than the maximum intraspecific value (0.008), and they are within the range of interspecific variation (0.058 to 0.232).
The phylogenetic tree generated by ML analyses showed a clear separation of the specimens of A. buckupi spec. nov. from the other species analyzed ( Fig. 5 View FIGURE 5 ). This analysis included other species of the A. edwardsi group, including some morphologically similar species such as A. cf. lobidens , A. heterochaelis , A. pontederiae , A. sp. aff. euphrosyne , and A. microrhynchus .
Therefore, the genetic analysis (K2P distances and ML) corroborated the morphological results and confirmed the validity of A. buckupi spec. nov. Additionally, based on the small distance (0) between the western and eastern Atlantic specimens, the amphi-Atlantic distribution of the new species was confirmed. Finally, the close morphological resemblance between A. buckupi spec. nov. and A. lobidens , together with the considerable genetic differences, indicate that they are cryptic taxa.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |