Warimiri Tavares, de Mello & Mendes
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5057.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:6D2BE117-9EE7-47DB-BBC4-D4BC462B91AE |
|
DOI |
https://doi.org/10.5281/zenodo.5592614 |
|
persistent identifier |
https://treatment.plazi.org/id/846887E1-244E-5C4B-7BC9-F8C7FC3F2E98 |
|
treatment provided by |
Plazi |
|
scientific name |
Warimiri Tavares, de Mello & Mendes |
| status |
|
Warimiri Tavares, de Mello & Mendes View in CoL g en. nov.
urn:lsid:zoobank.org:act:DE1766C9-E04C-452C-9EA3-116D6DC9215C
Type species. Warimiri madiba View in CoL sp. nov. (described below).
Etymology. The name is an arbitrary combination of letters derived from two words of the Nheengatu: wariní = warrior + mirĩ = small. Nheengatu is a language created by the Jesuit priests to homogenize several native idioms of the Tupi branch, making it possible for them to communicate between the tribes. Nheengatu is considered the New Tupi language. The gender of the name is being established as neuter.
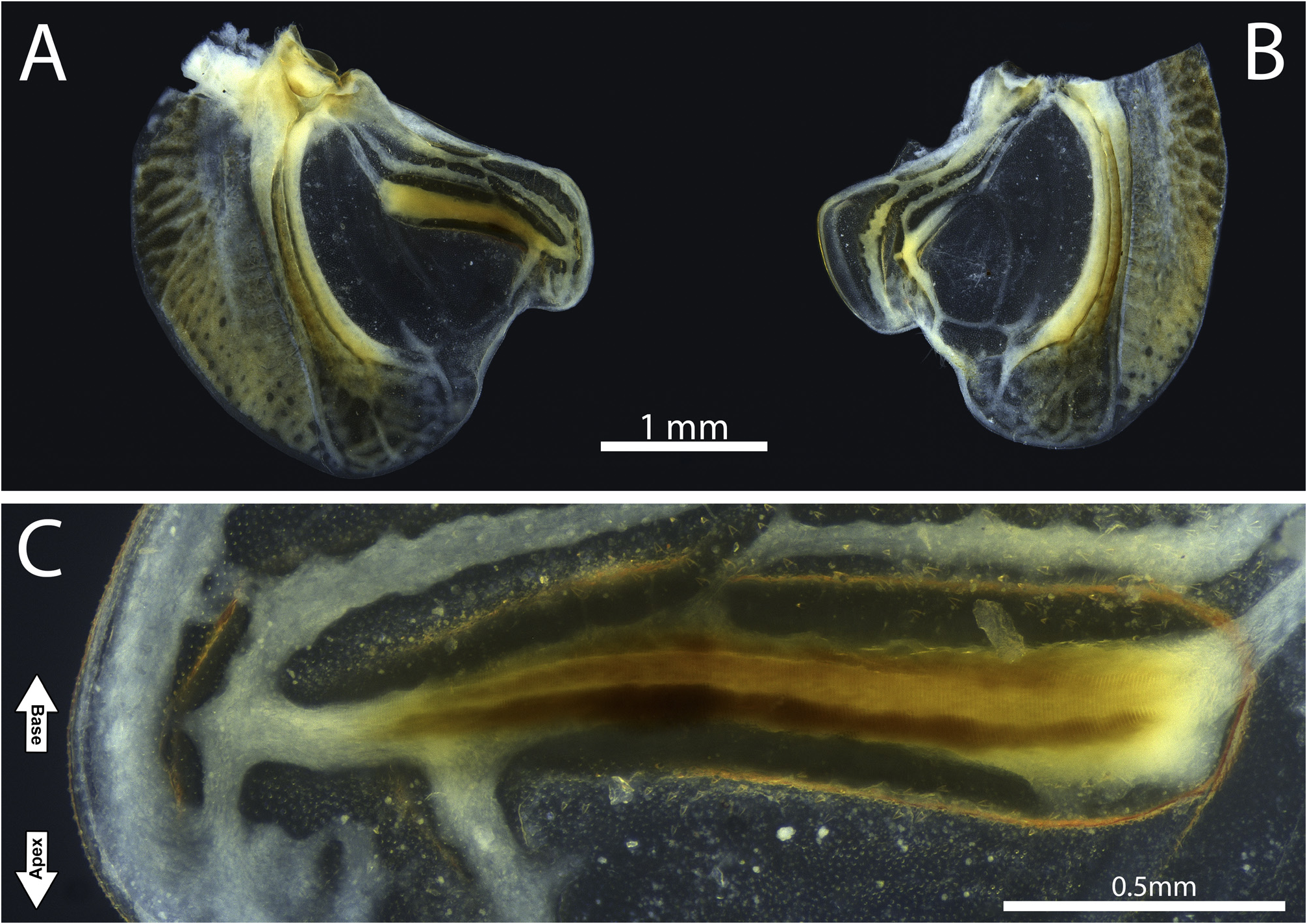
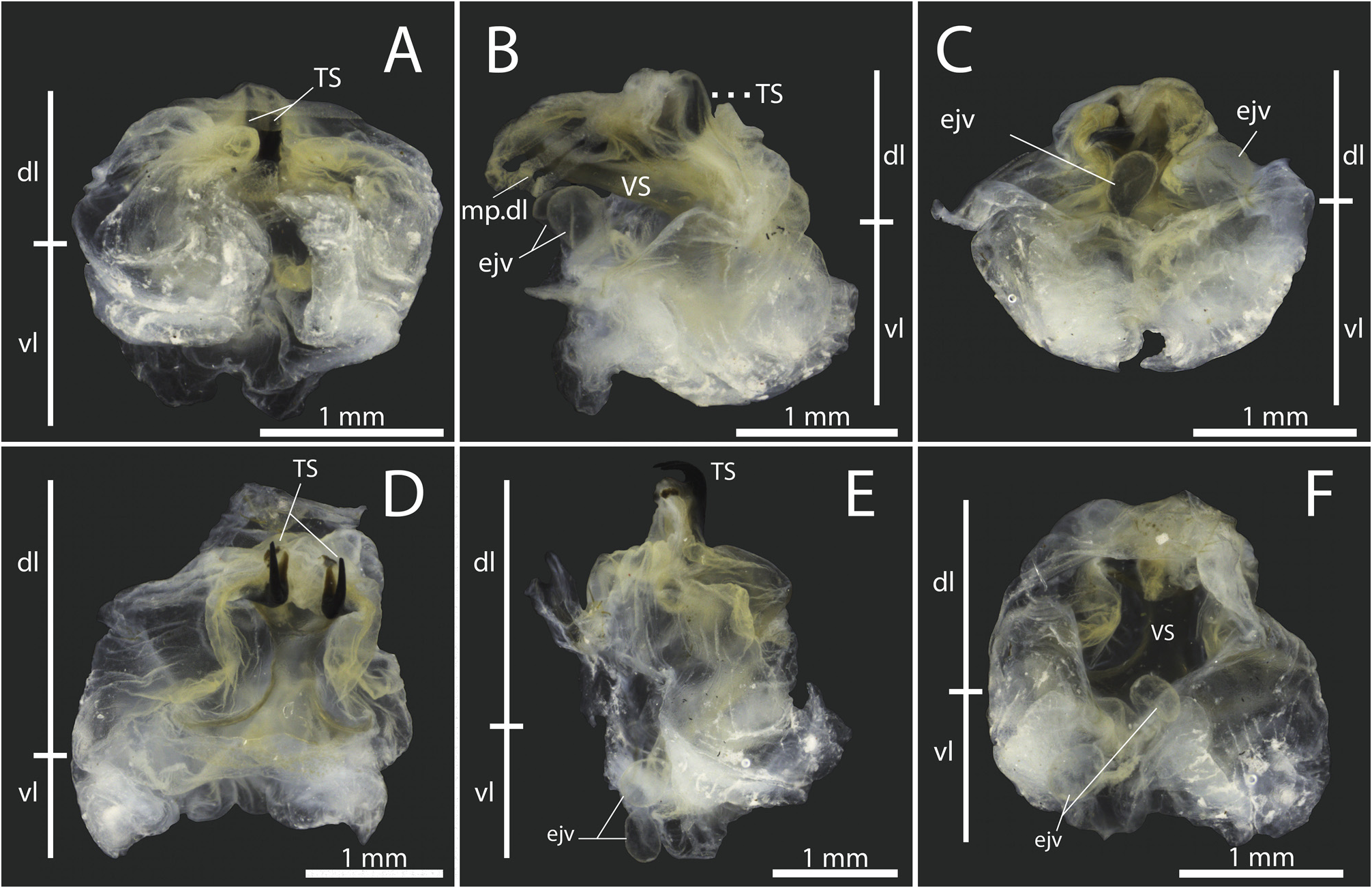
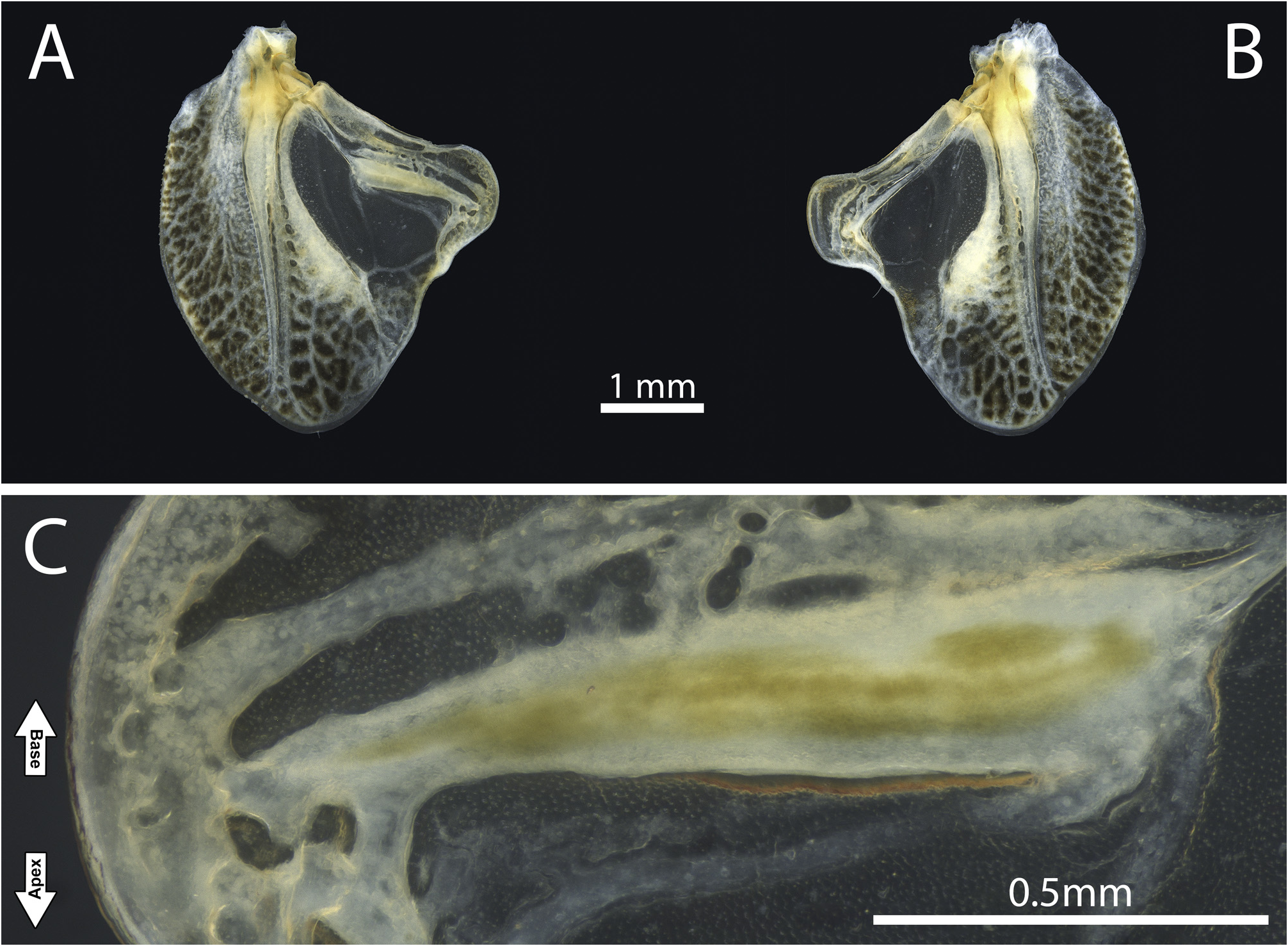
Diagnosis. Fastigium of vertex blunt, moderately protruding, wider than scapus, not dentate below, and contiguous with the fastigium of frons ( Figs. 2A–E, G–H View FIGURE 2 ; 6C–D View FIGURE 6 ; 7B–D View FIGURE 7 ; 11B–D View FIGURE 11 ; 14C–D, G–H View FIGURE 14 ; 19C–D View FIGURE 19 ). Dorsal surface of head, frons, and genae conspicuously rugose and punctuate ( Figs. 2C–E View FIGURE 2 ; 7B–C View FIGURE 7 ; 11B–C View FIGURE 11 ; 14C–E View FIGURE 14 ); antennal scape with an inward blunt tooth ( Figs. 2D View FIGURE 2 ; 11B View FIGURE 11 ; 14D View FIGURE 14 ), posterior portion of each antennomere darker than the remaining. Eyes subglobose, slightly broader than the fastigium of vertex ( Figs. 2A–E, G–H View FIGURE 2 ; 6C–D View FIGURE 6 ; 7B–D View FIGURE 7 ; 11B–D View FIGURE 11 ; 14C–D, G–H View FIGURE 14 ; 19C–D View FIGURE 19 ). Pronotum slightly convex in lateral view; pronotal disk distinctly lighter than the lateral lobes, with rounded lateral keels and an almost inconspicuous medial keel, more notable on the posterior half, resembling a roof ( Figs. 2B; H View FIGURE 2 ; 6B, D View FIGURE 6 ; 7C, E View FIGURE 7 ; 11C, E View FIGURE 11 ; 14B, H View FIGURE 14 ; 19B, D View FIGURE 19 ); anterior and posterior margins truncated, this last produced behind. Lateral lobes produced laterally, with cephalic margins straight and oblique, anteroventral angles obtuse, anteroventral margins sinuous and oblique, posteroventral angles obtuse, posterior margins sinuous, and humeral sinus inconspicuous ( Figs. 2A, G View FIGURE 2 ; 6A, C View FIGURE 6 ; 7A, D View FIGURE 7 ; 11A, D View FIGURE 11 ; 14A, G View FIGURE 14 ; 16A, C View FIGURE 16 ). Prosternum smooth, meso- and metatasternum wider than long, trapezoid ( Figs. 2F View FIGURE 2 ; 7F View FIGURE 7 ; 11F View FIGURE 11 ; 14F View FIGURE 14 ). Wings significantly reduced (micropterous), almost entirely covered by the pronotal disk posterior margin ( Figs. 2G–H View FIGURE 2 ; 4A–B View FIGURE 4 ; 6A–D View FIGURE 6 ; 7A, C–E View FIGURE 7 ; 11A, C–E View FIGURE 11 ; 14G–H View FIGURE 14 ; 16A–B View FIGURE 16 ; 19A–D View FIGURE 19 ). In males, both tegmina slightly surpassing the first tergite anterior margin ( Figs. 2G–H View FIGURE 2 ; 7A, C–E View FIGURE 7 ; 14G–H View FIGURE 14 ), and hind wings trifling ( Figs. 2A–B View FIGURE 2 ; 14A–B View FIGURE 14 ). Left tegmen anal margin conspicuously prominent, very similar to the right tegmen protruding plectrum ( Figs. 4A View FIGURE 4 ; 8A View FIGURE 8 ; 16A View FIGURE 16 ). Mirror of both tegmina membranous, hyaline, and subtriangular ( Figs. 4A–B View FIGURE 4 ; 8A–B View FIGURE 8 ; 16A–B View FIGURE 16 ). Right tegmen plectrum flexible, with a hyaline and more membranous cell formed between two anal veins ( Figs. 4B View FIGURE 4 ; 8B View FIGURE 8 ; 16B View FIGURE 16 ). In females, both pairs of wings even more reduced, trifling ( Figs. 6A–D View FIGURE 6 ; 7A, E View FIGURE 7 ; 19A–D View FIGURE 19 ). Legs short and stout; anterior tibiae’s dorsal surface elevated into two low lateral keels ( Figs. 3A–C View FIGURE 3 ; 7G View FIGURE 7 ; 11G View FIGURE 11 ; 15A–C View FIGURE 15 ); all femora dorsally unarmed ( Figs. 3A–B, D–E, G–H View FIGURE 3 ; 7A, G–H View FIGURE 7 ; 11A, G–H View FIGURE 11 ; 15A–B, D–E, G–H View FIGURE 15 ); anterior femora with minute ventral spines internally ( Figs. 3B View FIGURE 3 ; 7G View FIGURE 7 ; 11G View FIGURE 11 ; 15B View FIGURE 15 ) and externally generally smooth ( Figs. 3A View FIGURE 3 ; 15A View FIGURE 15 ); mid femora usually smooth internally ( Figs. 3E View FIGURE 3 ; 15E View FIGURE 15 ) and externally with minute ventral spines ( Figs. 3D View FIGURE 3 ; 7H View FIGURE 7 ; 11H View FIGURE 11 ; 15D View FIGURE 15 ). Male subgenital plate widely expanded laterally and dorsally, emarginated posteriorly, with two cylindrical styli ( Figs. 3J–L View FIGURE 3 ; 7I–K View FIGURE 7 ; 15J–L View FIGURE 15 ); cerci short, bearing inward spines distally or medio-proximally ( Figs. 3M–N View FIGURE 3 ; 7I View FIGURE 7 ; 15M–N View FIGURE 15 ). Titillatory process (ti) comprising a continuous sclerotized area that can be limited to the dorsal fold (df) ( Figs. 5A–B, E–F View FIGURE 5 ) or extends from the fold df to the dorsal cavity (dc) ( Figs.17A–B, D–F View FIGURE 17 ). Titillator’s sclerites (TS) paired and long, rod-shaped ( Figs. 1A–F View FIGURE 1 ; 5A–B, D–E View FIGURE 5 ; 9A, D–E View FIGURE 9 ; 17A–D View FIGURE 17 ); anterophallic membranous processes of the dorsal lobe (mp.dl) pared, attached to the apical most portion of sclerite VS ( Figs. 5B–C, F View FIGURE 5 ; 9B View FIGURE 9 ; 17A–C, F View FIGURE 17 ); sclerite of the ventral fold of the dorsal lobe (VS) large, inverted Yshaped. Female subgenital plate narrow (basally narrower than the proximal portion of ovipositor ventral valves), emarginated posteriorly, not produced dorsolaterally, and basally flanked by two membranous invaginations (better seen when the subgenital plate is lowered) ( Figs. 1H–I View FIGURE 1 ; 6G View FIGURE 6 ; 11K View FIGURE 11 ; 19G View FIGURE 19 ), located just before the anteriormost margins of the ventral valves ( Figs. 1H–I View FIGURE 1 , white arrows); ovipositor strongly upcurved ( Figs. 6E View FIGURE 6 ; 11I View FIGURE 11 ; 19E View FIGURE 19 ).
Warimiri gen. nov. is morphologically similar to Hyperomerus and Dectinomima and probably is closely related to them. However, Hyperomerus has two distinct sclerotized areas comprising the processes ti; processes mp.dl attached to the process es ti anterophallic surface; cerci usually elongated, bearing a compressed projection and a basal appendage or spine (only Hyperomerus almeirina Tavares, Sovano & Gutjahr has short cerci, with reduced compressed projection). On the other hand, Dectinomima has no processes ti; the sclerites TS are neither elongated nor rod-shaped, and the cerci bear an elongate mesointernal tapered projection anteriorly or posteriorly upcurved.
Comments. Montealegre-Z et al. (2011) recorded two undescribed species tentatively determined as “near Uchuca ” (i.e., Hyperomerus ) to the pacific coast of Colombia and Ecuador. Till the moment, it is not sure if these species belong to Hyperomerus or another undescribed genus. The available knowledge is that all species already described to Hyperomerus are limited to the East side of the Andes, extending to the Amazonian region ( Tavares & Nunes-Gutjahr 2021), and the two species within Dectinomima are recorded to Panama and the Pacific coast of Colombia, on the trans-Andean region ( Montealegre-Z & Morris 2003). Warimiri gen. nov. is the first genus of Agraeciini with blunt and large fastigium of vertex (larger than scapus) recorded in the Atlantic forest. Nowadays, this biome is isolated from the Amazon by two biomes of the ‘open formation diagonal’ (also known as ‘dry diagonal’): Cerrado and Caatinga ( Batalha-Filho et al. 2013; Werneck 2011).
The new species described here were collected in the States of Ceará, Alagoas (one species with disjoint distribution), and Bahia (two species). We believe that Warimiri gen. nov. is closer to Hyperomerus than Dectinomima due to the similarity of the phallic complex, but a phylogenetic approach is needed to confirm this hypothesis.
Comments on the phallic components. Males genital structures tend to diverge faster than other morphological structures, driven by different select pressures ( Simmons 2014). In katydids, the sclerites TS play an important role in female acceptance for copula by stimulating the internal membranes of the copulatory chamber (acting as a courtship device) ( Wulff et al. 2015, 2018), applying pressure that forces the subgenital plate to stay open ( Wulff et al. 2017) or mechanically supporting the mating position and the spermatophore transference ( Wulff & Lehmann 2020). The copula duration varies among Tettigoniidae subfamilies, but it is significantly longer in species with sclerites TS than in species without these structures, and more complex sclerites TS transfer spermatophorous quicker ( Vahed et al. 2011). In addition, many species of Tettigoniidae have processes ti, sclerotized areas that bear sclerotized microstructures, which probably also act as stimulation devices ( Chamorro-Rengifo & Lopes-Andrade 2014), increasing the stimulatory capability of the phallus. However, morphofunctional studies have not considered these last structures ( Vahed et al. 2011; Wulff et al. 2015, 2017, 2018; Wulff & Lehmann 2014, 2020). In fact, any other component of the phallic complex has not been considered, especially the internal ones.
In Warimiri gen. nov. and Hyperomerus , the posterior portion of the phallic complex bears very long and paired sclerites TS and paired or single processes ti. Internally (or anteriorly), a very large sclerite VS and a pair of processes mp.dl are also present in both genera. Despite being on different faces of the phallus, in these two genera, the sclerite VS supports the sclerite TS eversion, acting as an umbrella’s runner ( Figs. 1A–E View FIGURE 1 ). So when the phallus is everted (by the hydrostatic pressure of hemolymph), it is physically easier to push a unique structure (the runner) that, in turn, forces the two sclerites TS outward (acting as the umbrella’s stretchers) than directly forcing two independent mobile bars ( Fig. 1G View FIGURE 1 , black arrow). For us, this sizeable central sclerite also helps to maintain the phallus wholly everted.
On the other hand, it is also easier to retract the phallus by pulling a unique central structure. However, we believe that a second component facilitates the phallus retraction, the processes mp.dl. These processes are located on the dorsalmost portion of the anterophallic face in both genera and are connected directly to the sclerite VS apex (in Warimiri gen. nov.) or the processes ti (in Hyperomerus ). We believe these processes act like tendons, pulling back inside the sclerite VS ( Fig. 1G View FIGURE 1 , white arrow). Ventrally, a membranous projection of the phallus is connected to the subgenital plate and acts like a frenulum, helping to retract the phallus ( Fig. 1F View FIGURE 1 , white arrow).
Dectinomima has shorter sclerites TS and apparently no processes ti, but we do not have any information about the anterophallic components of this genus, so we will not discuss it.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |