Hecatrishula Gustafsson & Bush, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4313.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A5Fdfba5-F992-44A8-84C2-1756C943C19B |
|
DOI |
https://doi.org/10.5281/zenodo.5296901 |
|
persistent identifier |
https://treatment.plazi.org/id/832187E9-FFDC-FF9B-FF74-6298FBD4FBDC |
|
treatment provided by |
Plazi |
|
scientific name |
Hecatrishula Gustafsson & Bush |
| status |
gen. nov. |
Hecatrishula Gustafsson & Bush , new genus
Nirmus Nitzsch, 1818: 291 (in partim).
Degeeriella Neumann, 1906: 60 (in partim). Brueelia Kéler, 1936a: 257 (in partim).
Corvonirmus Eichler, 1944 (in partim).
Type species. Brueelia atherae Ansari, 1957a: 161
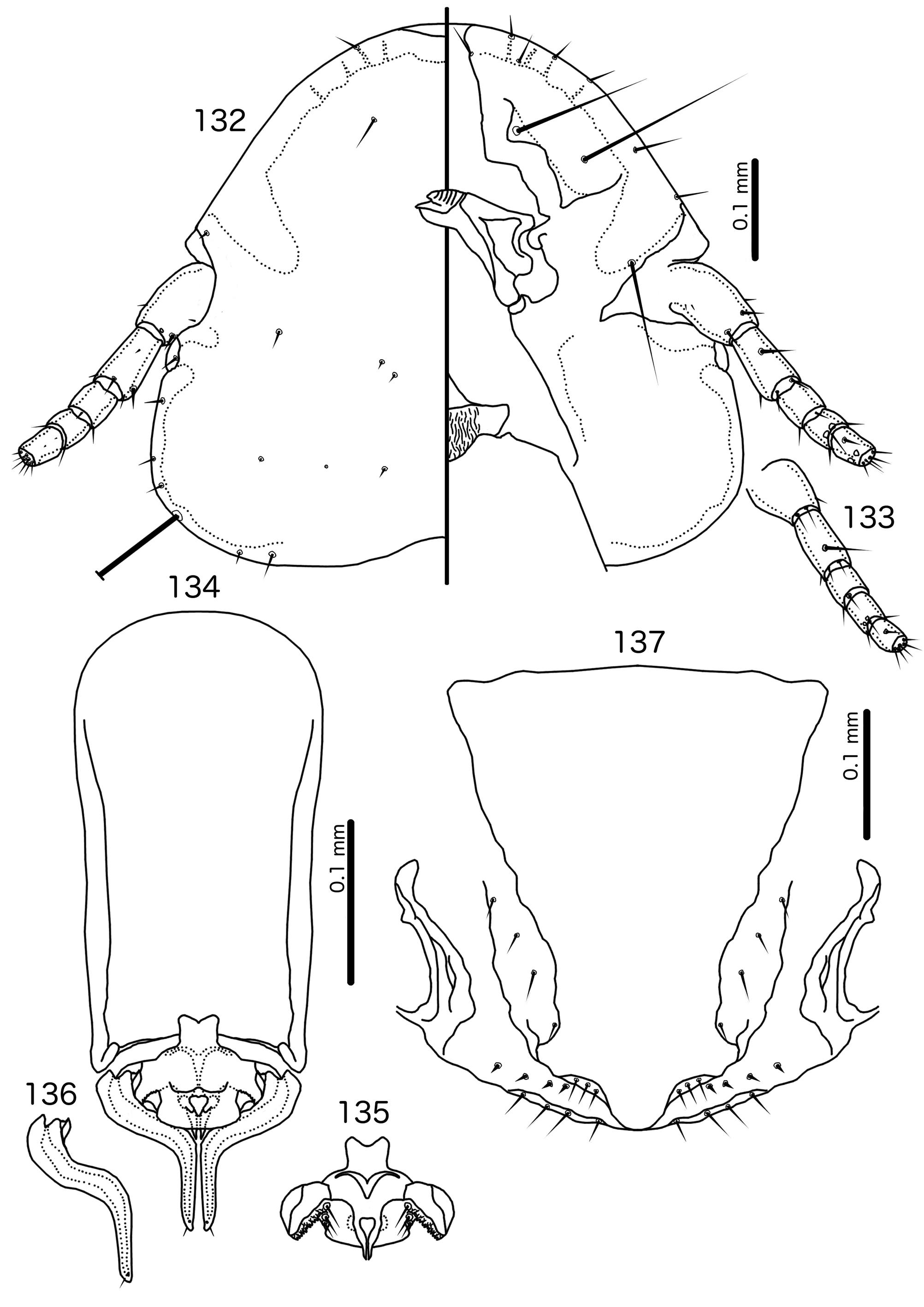
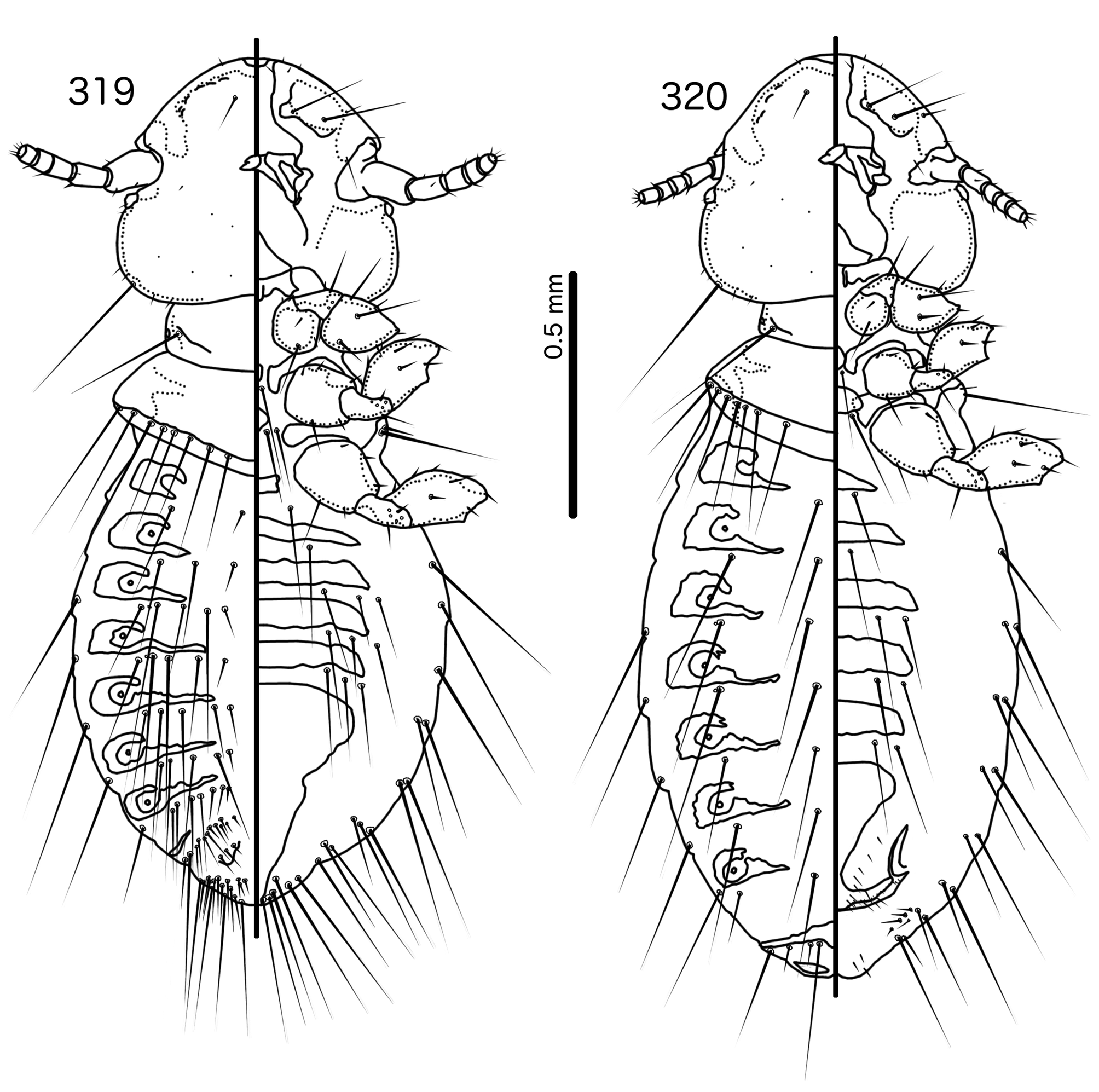
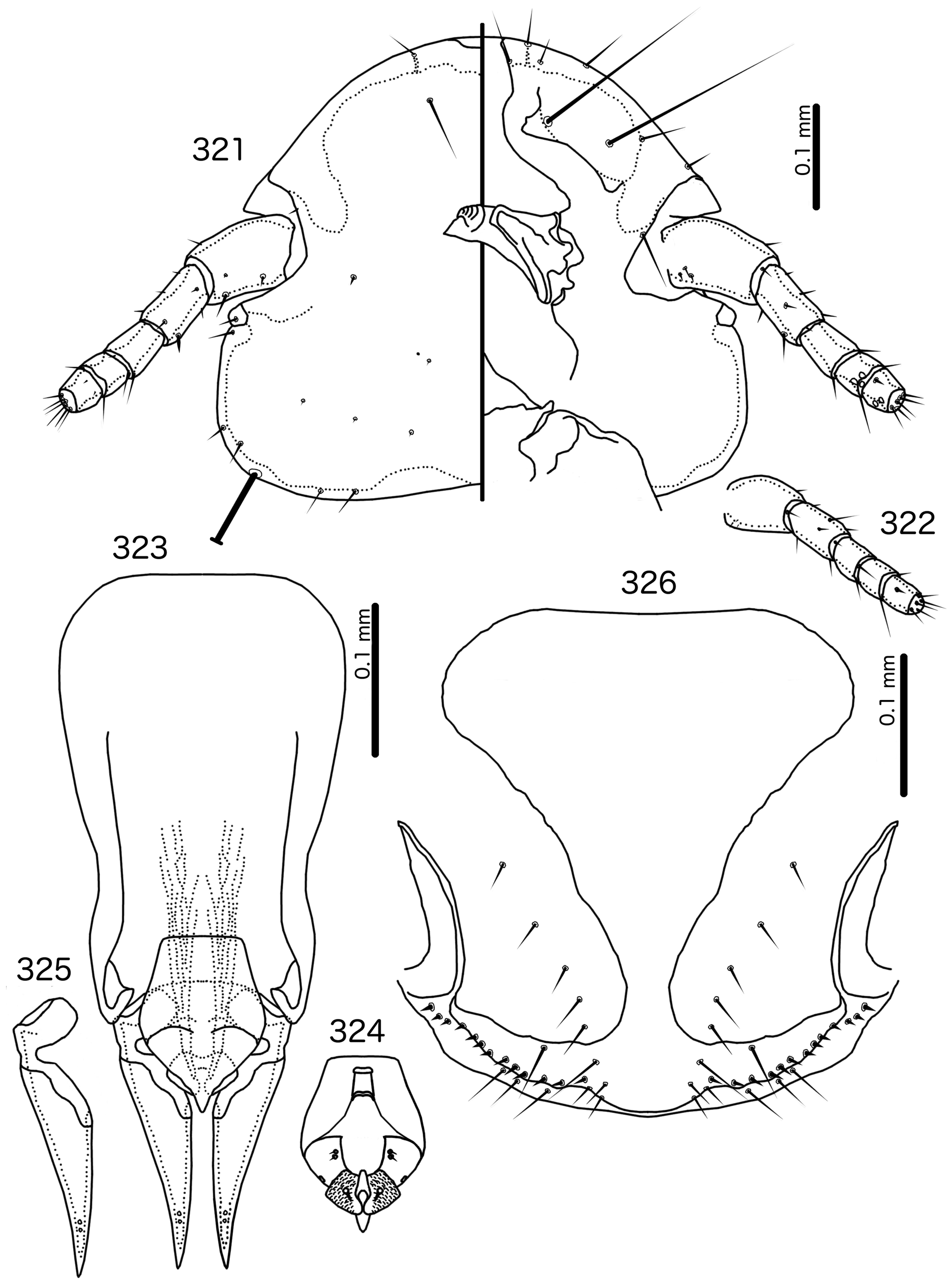
Diagnosis. Hecatrishula atherae ( Figs 130–137 View FIGURES 130 – 131 View FIGURES 132 – 137 ) species group are most similar to Corvonirmus ( Figs 319–326 View FIGURES 319 – 320 View FIGURES 321 – 326 ), and both occur on the same host species. Both groups typically have reduced tergopleurites, limited dark-brown to black pigmentation, wide, rounded head without dorsal preantennal sutures, and setose abdomens that include accessory ventral setae on at least some segments. However, the two groups are easily separated by the following characters: as3 absent in Corvonirmus ( Fig. 321 View FIGURES 321 – 326 ) but present in Hecatrishula n. gen. ( Fig. 132 View FIGURES 132 – 137 ); aps present in male Corvonirmus ( Fig. 319 View FIGURES 319 – 320 ) but absent in male Hecatrishula ( Fig. 130 View FIGURES 130 – 131 ); female subgenital plate flares into cross-piece in Corvonirmus ( Fig. 326 View FIGURES 321 – 326 ) but only lateral submarginal bulges present in Hecatrishula ( Fig. 137 View FIGURES 132 – 137 ); parameres are gently tapering and elongated, with blunt median extensions of the heads in Corvonirmus ( Fig. 325 View FIGURES 321 – 326 ), but strongly curved, and with 2–4 fingers on heads in Hecatrishula ( Fig. 136 View FIGURES 132 – 137 ); pst2 is sensillus located centrally near pst 1 in Corvonirmus ( Fig. 325 View FIGURES 321 – 326 ), but microseta located laterally in Hecatrishula ( Fig. 136 View FIGURES 132 – 137 ); mesosomal lobes not extended laterally in Corvonirmus ( Fig. 324 View FIGURES 321 – 326 ), whereas in Hecatrishula mesosomal lobes are much extended laterally ( Fig. 135 View FIGURES 132 – 137 ).
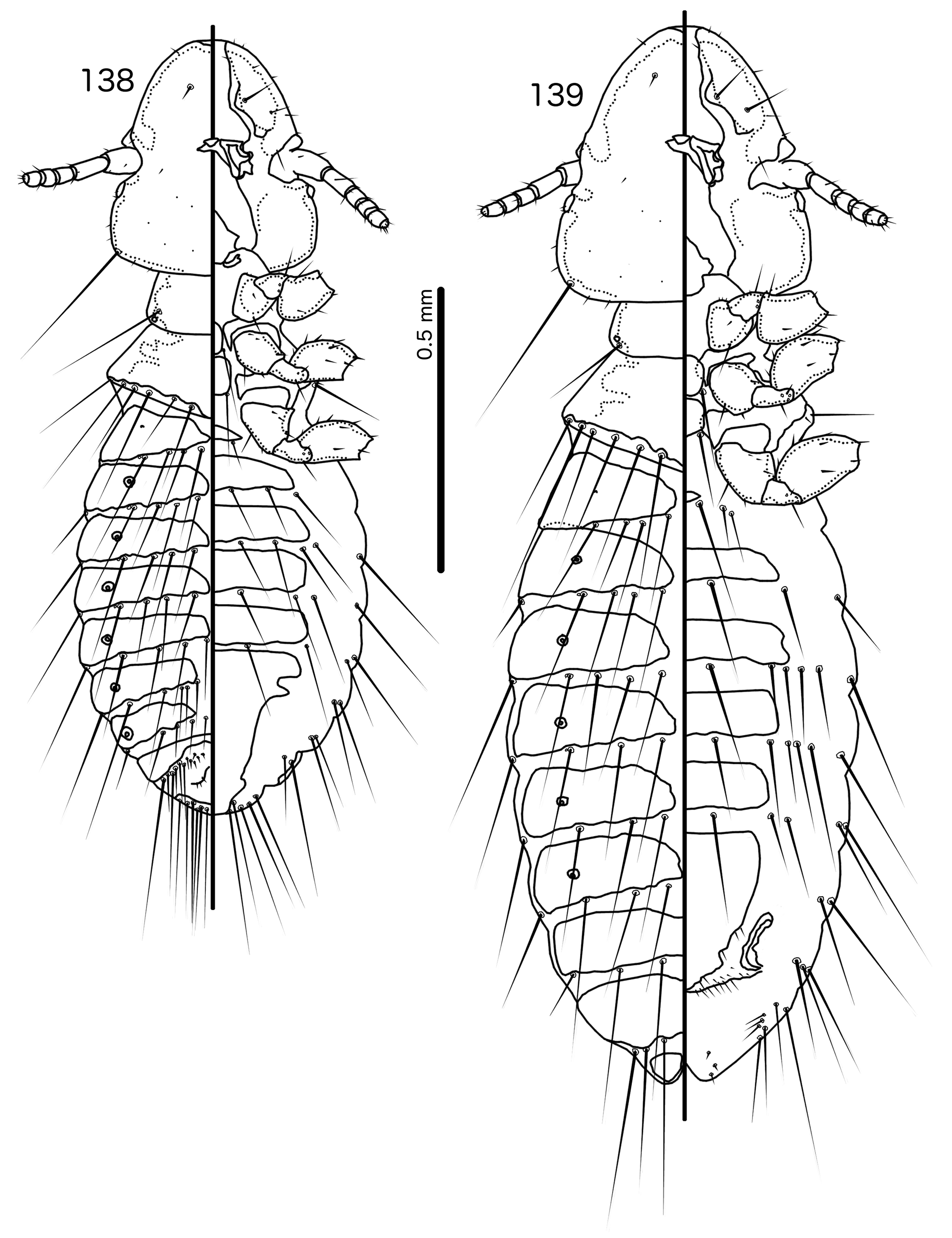
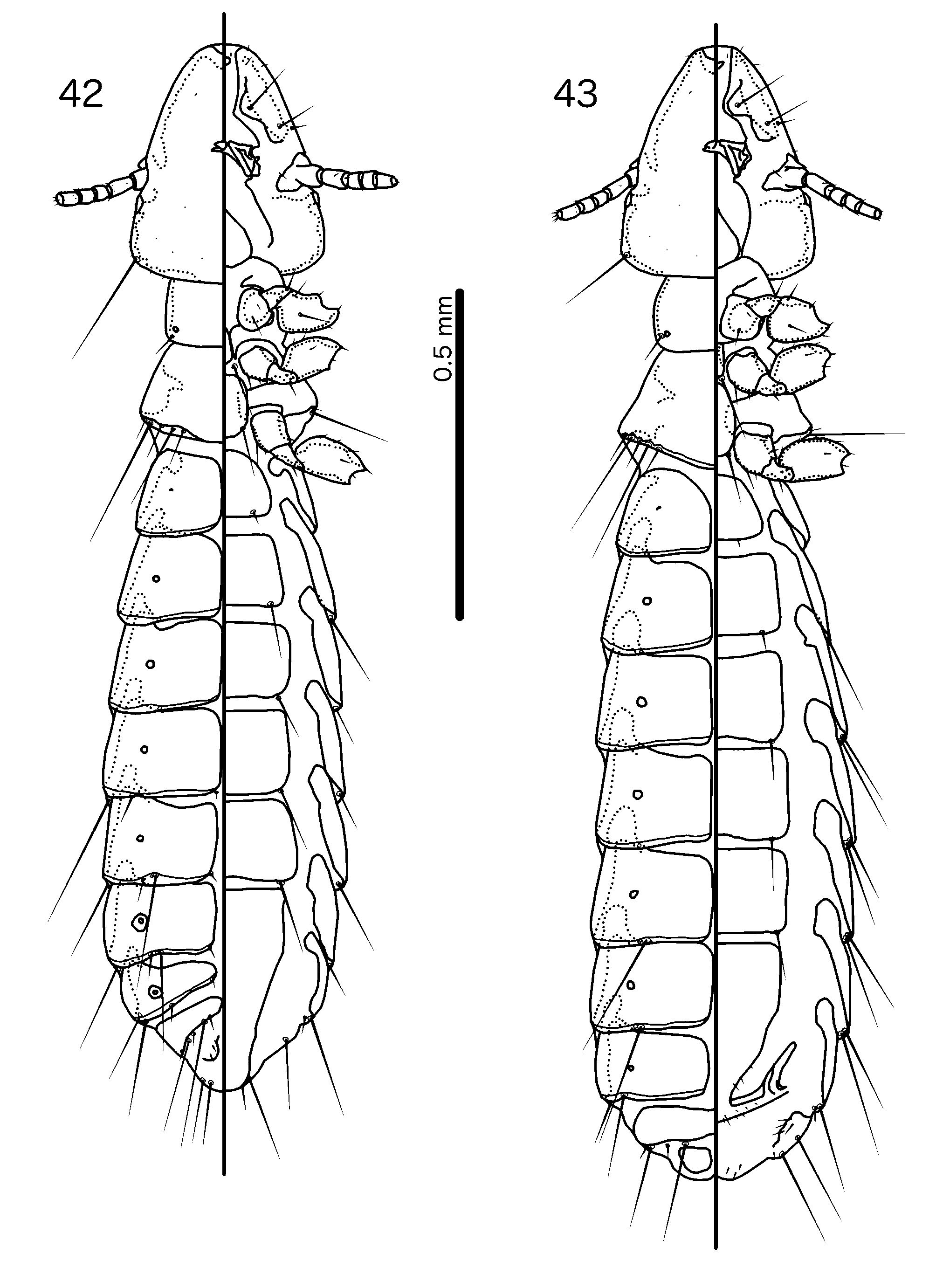
Members of the Hecatrishula biguttata species group ( Figs 138–145 View FIGURES 138 – 139 View FIGURES 140 – 145 ) are most similar to Brueelia s. str. ( Figs 42–48 View FIGURES 42 – 43 View FIGURES 44 – 48 ) in overall habitus, and like many members of Brueelia s. str., members of the He. biguttata species group typically have an even, brown pigmentation. These groups are distinguished by the following characters: as3 is absent in Brueelia s. str. ( Fig. 44 View FIGURES 44 – 48 ), but present in Hecatrishula ( Fig. 140 View FIGURES 140 – 145 ), and the abdomen is much more setose in Hecatrishula ( Figs 138–139 View FIGURES 138 – 139 ) than in Brueelia s. str. ( Figs 42–43 View FIGURES 42 – 43 ). No Brueelia s. str. have accessory sternal setae, but these are present in all Hecatrishula . psps, tps , and ss are absent in anterior abdominal segments of both sexes in Brueelia s. str., but present in Hecatrishula (see Table 2). The male genitalia of these two genera are also quite distinct: parameral heads blunt or cup-shaped in Brueelia s. str. ( Fig. 47 View FIGURES 44 – 48 ) but polyfid in Hecatrishula ( Fig. 144 View FIGURES 140 – 145 ); mesosomal lobes do not extend into lateral wings in Brueelia s. str. ( Fig. 46 View FIGURES 44 – 48 ), but do in Hecatrishula ( Fig. 143 View FIGURES 140 – 145 ); gonopore is terminal in Brueelia s. str. ( Fig. 46 View FIGURES 44 – 48 ) but ventral in Hecatrishula ( Fig. 143 View FIGURES 140 – 145 ). The female subgenital plate flares into cross-piece in all Brueelia s. str. ( Fig. 48 View FIGURES 44 – 48 ), but form only lateral submarginal bulges in ( Fig. 145 View FIGURES 140 – 145 ).
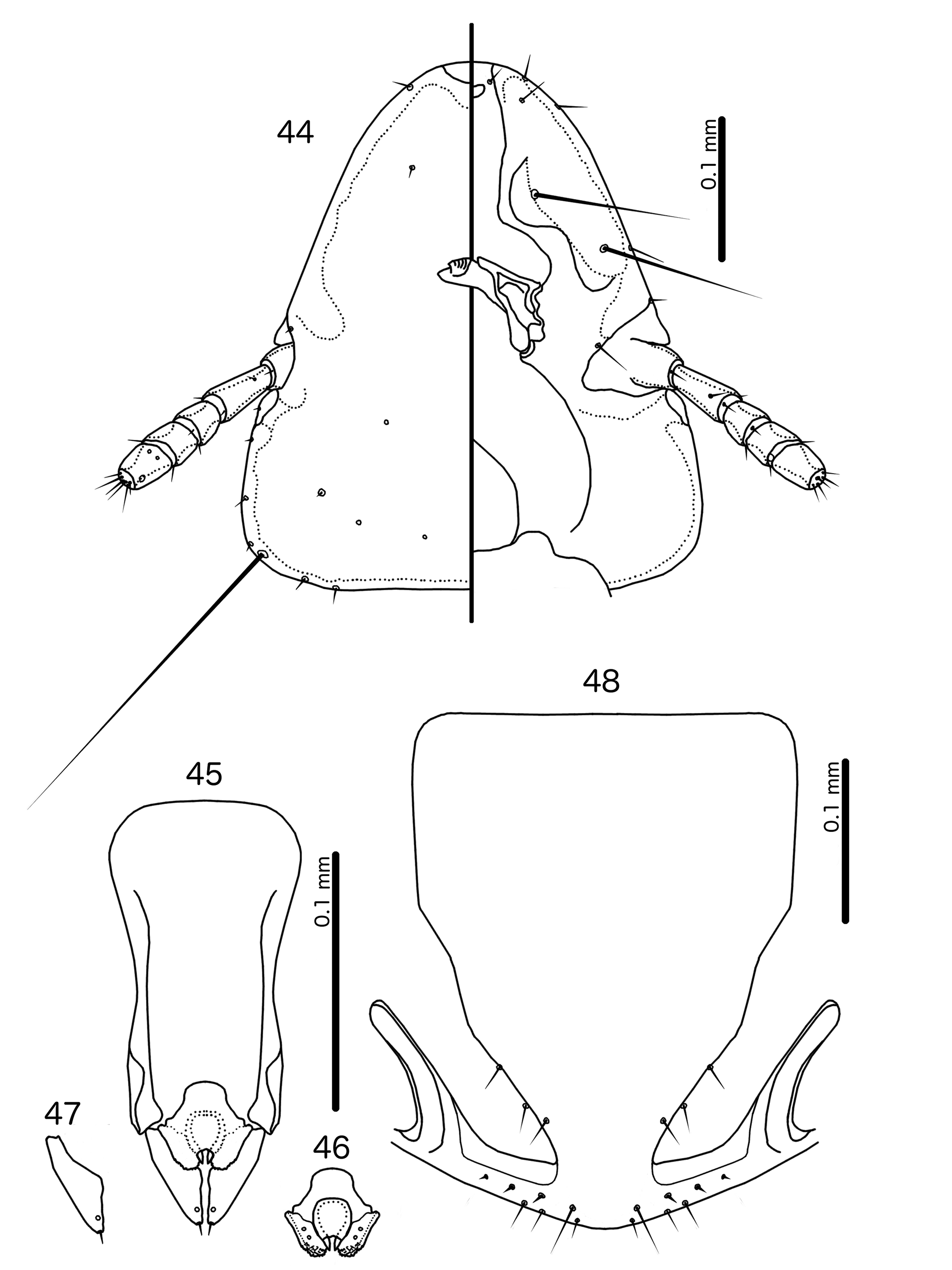
Description. Both sexes. Head convex- to indented-dome shaped ( Figs 132 View FIGURES 132 – 137 , 140 View FIGURES 140 – 145 ). Marginal carina uninterrupted, displaced dorsally and posteriorly at osculum. Dorsal preantennal suture, dorsal anterior plate, and ventral anterior plate absent. Ventral carinae diffuse anteriorly, not clearly continuous with marginal carina. Head setae as in Figs 132 View FIGURES 132 – 137 , 140 View FIGURES 140 – 145 ; pts and s1–4 all with visible microsetae. Preantennal nodi with “hollow” interior in Hecatrishula atherae species-group. Coni small, blunt. Antennae sexually dimorphic, male scapes larger than female scapes; pedicel and flagellomeres largely the same between sexes. Temporal carinae not visible; mts 3 only macrosetae. Gular plate broad, triangular. Pigmentation patterns of head variable among species.
Prothorax rectangular ( Figs 130–131 View FIGURES 130 – 131 , 138–139 View FIGURES 138 – 139 ); ppss on postero-lateral corner in Hecatrishula biguttata species-group, more median in He. atherae species-group. Proepimera slender, median ends hook-shaped. Pterothorax short, pentagonal ( Figs 130–131 View FIGURES 130 – 131 , 138–139 View FIGURES 138 – 139 ); lateral margins widely divergent; posterior margin convergent to median point. Meso- and metasterna not fused; 1 seta on postero-lateral corner on each side of each plate. Metepisterna slender to broad, median ends widened, blunt. mms not or only narrowly separated medianly. Leg chaetotaxy as in Fig, 25, except fI-p2–4, fI-v4 absent.
Abdomen oval ( Figs 130–131 View FIGURES 130 – 131 , 138–139 View FIGURES 138 – 139 ). Abdominal chaetotaxy as in Table 2. Both sexes with at last some accessory sternal setae situated between central sternal plate and abdominal margin in all or most segments. Tergopleurites bluntly triangular; tergopleurites II–IX+X in male, and tergopleurites II–VIII in female separated medianly; in Hecatrishula docilis (not illustrated) tergopleurites V–VII in male and tergopleurites VI–VII in female medianly continuous, connected by narrow postero-median bridge; in He. biguttata ( Figs 138–139 View FIGURES 138 – 139 ) male tergopleurites IX+X and female tergopleurites VIII medianly continuous; 1–2 fenestrae on tergopleurites II–VIII in both sexes of He. atherae species-group, none in He. biguttata species-group. Tergopleurites not or only barely reaching lateral margins of abdomen. Pleural incrassations and re-entrant heads absent. Sternal plates rectangular, transversally continuous, not approaching lateral margins of abdomen. Male subgenital plate triangular, with narrow point reaching posterior margin of abdomen. Female subgenital plate triangular, reaching or approaching vulval margin, with lateral submarginal bulges but no cross-piece. Vulval margin ( Figs 137 View FIGURES 132 – 137 , 145 View FIGURES 140 – 145 ) with slender vms, thorn-like vss; vos follows lateral margins of subgenital plate; distal vos median to vss.
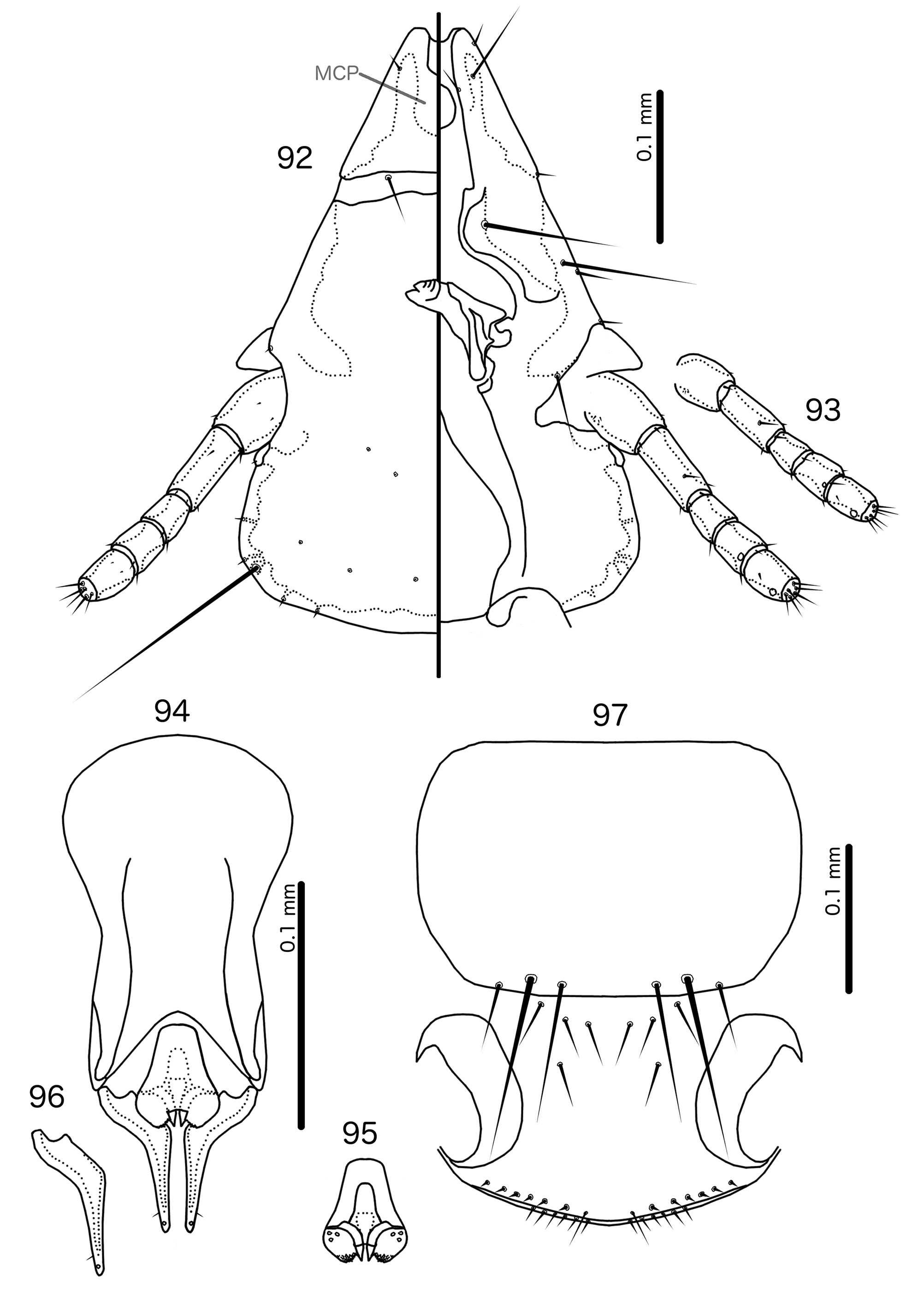
Male genitalia distinct ( Figs 134–136 View FIGURES 132 – 137 , 142–144 View FIGURES 140 – 145 ). Basal apodeme roughly rectangular. Proximal mesosome small, with prominent U or V-shaped ventral thickening. Gonopore ( Figs 135 View FIGURES 132 – 137 , 143 View FIGURES 140 – 145 ) subterminal and ventral, narrowly open distally, often projecting far distal to mesosomal lobes. Mesosomal lobes large, extending laterally to overlap with parameral heads. Lobes may be fused dorsal to mesosomal opening ( Fig. 135 View FIGURES 132 – 137 ). Distal margin of lobes papillate or fringed on ventral side ( Figs 95 View FIGURES 92 – 97 , 143 View FIGURES 140 – 145 ); 2–3 robust pmes on each side submedianly on distal part of mesosome, often hard to see due to papillation of mesosome. Small rugose nodi may be present near gonopore. Parameral heads with 2–4 fingers (Fig, 136, 144). Dorsal finger often appears to be separate sclerite, but connected to paramere through largely translucent neck. Parameral blades stout, heavily curved around the mesosome ( Figs 136 View FIGURES 132 – 137 , 144 View FIGURES 140 – 145 ), and not much elongated distally; pst1 sensillus, near distal tip, often very hard to see in He. atherae species group; pst2 microseta, lateral near distal tip.
Species groups. Based on preantennal and abdominal characters, we recognise two species groups, as follows: Hecatrishula atherae species-group ( Figs 130–137 View FIGURES 130 – 131 View FIGURES 132 – 137 ). Head indented-dome shaped. Ventral carinae broad, diffusely continuous with marginal carina. ppss on posterior margin of pronotum. Tergopleurites II–VIII of both sexes typically each with 1–2 fenestrae. Tergopleurites IX+X in male and tergopleurites VIII in female not connected medianly. Ex Pica spp., Nucifraga multipunctata , and Corvus spp.
Hecatrishula biguttata species-group ( Figs 138–145 View FIGURES 138 – 139 View FIGURES 140 – 145 ). Head convex-dome shaped. Ventral carinae slender, not visible anterior to pulvinus, thus not clearly continuous with marginal carina. ppss on postero-lateral corners of pronotum. Tergopleurites II–VIII of both sexes usually without fenestrae, or with only one small fenestra around spiracle openings. Tergopleurites IX+X in male and tergopleurites VIII in female medianly continuous; more anterior tergopleurites may be connected by narrow bridge. Ex Pyrrhocorax spp. and Podoces spp.
Host distribution. Known only from the Corvidae , where they are patchily distributed on Pica , Pyrrhocorax , Podoces , Nucifraga , and large species of Corvus . Hecatrishula hamatofasciata is known only from a member of the Bucerotidae , but this is likely to be a straggler, a contaminant, or a host mix-up.
Geographical range. Widely distributed across Africa, Eurasia, and North America. There appears to be a concentration of species in the mountains and highlands of Central Asia, where most of the hosts of Hecatrishula occur. The lack of any known Hecatrishula on other species of Nucifraga and on most Corvus spp. may indicate that the genus is rare or only patchily distributed across the potential host range.
Etymology. The genus name is derived from the Greek goddess Hekátē , who is often depicted holding a pair of torches, and the Hindu trident trishula. Both these words refer to the shape of the mesosome, specifically the shape of the mesosomal lobes. Gender: feminine.
Remarks. No member of Hecatrishula was included in the phylogeny of Bush et al. (2016) and, because of the unique structure of the male genitalia, this genus is difficult to place within the Brueelia -complex. Some species of Hecatrishula were included in Corvonirmus by Złotorzycka (1964a, 1997), likely due to similarities in pigmentation patterns rather than structural characters. The illustrations of the male genitalia of Co. perforatus and Co. varius by Złotorzycka (1964a: fig. 1d,e) are overly simplified, with the genitalia of Co. perforatus drawn from everted and folded genitalia, which makes comparisons difficult.
Included species
He. atherae group
* Hecatrishula atherae ( Ansari, 1957a: 161) n. comb. [in Brueelia ]
* Hecatrishula biocellata ( Piaget, 1880: 666) n. comb. [in Nirmus ] [1] Nirmus nigropictus ( Carriker, 1902: 219) [in Nirmus ] [1, 2]
* Hecatrishula bipunctata ( Rudow, 1870: 467) n. comb. [in Nirmus ] [3]
* Hecatrishula cryptoleuca ( Ansari, 1957a: 164) n. comb. [in Brueelia ] [1, 4]
* Hecatrishula multipunctata ( Clay, 1936: 906) n. comb. [in Degeeriella ]
* Hecatrishula nawabi ( Ansari, 1957a: 166) n. comb. [in Brueelia ]
* Hecatrishula varia ( Burmeister, 1838: 430) n. comb. [in Nirmus ] [5] Hecatrishula perforata ( Złotorzycka, 1964a: 244)
He. biguttata group
* Hecatrishula biguttata ( Kellogg & Paine, 1914: 234) n. comb. [in Nirmus ] * Hecatrishula docilis ( Ansari, 1956b: 393) n. comb. [in Brueelia ] [6] * Hecatrishula koslovae ( Clay, 1936: 908) n. comb. [in Degeeriella ]
[1] Williams (1986) examined material from Pica hudsonia , Corvus cryptoleucus (though her material was from Illinois, i.e. well outside the known range of this species) and Corvus corax [sinuatus], but she could not find any differences among the samples from those three host species. Therefore, Williams (1986) regarded both Brueelia cryptoleuca and Br. nigropicta as junior synonyms of Br. biocellata . We have examined material from those three hosts, as well as from Pica pica , P. nuttallii , and Old World subspecies of Corvus corax . While we agree with Williams (1986) in regarding Br. nigropicta as a junior synonym of Br. biocellata (now He. biocellata ), we consider that lice from both Corvus cryptoleucus and North American C. corax are separate from those from Old World subspecies of C. corax , and are here regarded as conspecific with He. cryptoleuca .
[2] This species may ultimately be separable from He. biocellata , but based on the few specimens we have seen from Pica spp. are morphologically very similar. Thus, we prefer to keep these samples together under He. biocellata until more morphological or molecular data are available.
[3] Ansari (1957a: 151, 171) mentions an unpublished account by Hopkins & Clay that states that Nirmus bipunctatus is not a nomen novum for Nirmus quadrangularis Rudow, 1869 (= Corvonirmus quadrangularis ), as the head shapes are different. In Ansari's illustrations of Br. bipunctata (ibid., p. 157, fig. 23), all three as can clearly be seen, thus Br. bipunctatus is a Hecatrishula , while Br. quadrangularis is a Corvonirmus . We therefore resurrect Br. bipunctatus from synonymy with Br. quadrangularis .
[4] The type material of He. cryptoleuca that we examined includes paratypes ostensibly collected from Corvus cryptoleucus from far outside the range of this species (Illinois). The material was collected by Richard Meinertzhagen, whose host identification and collection circumstances cannot always be trusted. It is likely this material was collected from C. corax ssp., which does occur at the collection locality. This North American material differs from He. atherae from C. corax ssp. from the Old World in the male genitalia, pigmentation patterns, and preantennal head shape, but is similar to He. cryptoleuca from the type host. It thus appears like North American C. corax ssp. are parasitised by a different species of Hecatrishula than Old World subspecies of the same host.
[5] Louse samples from Corvus frugilegus and C. monedula are morphologically very similar, and appear to differ only in size. Therefore, we tentatively consider He. varia and He. perforata as synonyms.
[6] The type series of this species is from Morocco, making the type host Pyrrhocorax pyrrhocorax barbarous and not Pyrrhocorax pyrrhocorax docilis , which is distributed from the Balkans to Pakistan.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Hecatrishula Gustafsson & Bush
| Bush, Sarah E. 2017 |
Hecatrishula atherae ( Ansari, 1957a: 161 )
| Ansari 1957: 161 |
Hecatrishula cryptoleuca ( Ansari, 1957a: 164 )
| Ansari 1957: 164 |
Hecatrishula nawabi ( Ansari, 1957a: 166 )
| Ansari 1957: 166 |
Hecatrishula multipunctata ( Clay, 1936: 906 )
| Clay 1936: 906 |
Hecatrishula biguttata ( Kellogg & Paine, 1914: 234 )
| Ansari 1956: 393 |
| Clay 1936: 908 |
| Kellogg 1914: 234 |
Degeeriella
| Keler 1936: 257 |
| Neumann 1906: 60 |
Hecatrishula biocellata ( Piaget, 1880: 666 )
| Carriker 1902: 219 |
| Piaget 1880: 666 |
Hecatrishula bipunctata ( Rudow, 1870: 467 )
| Rudow 1870: 467 |
Hecatrishula varia ( Burmeister, 1838: 430 )
| Zlotorzycka 1964: 244 |
| Burmeister 1838: 430 |
Nirmus
| Nitzsch 1818: 291 |