Titanomessor Gustafsson & Bush, 2017
|
publication ID |
https://doi.org/10.11646/zootaxa.4313.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A5Fdfba5-F992-44A8-84C2-1756C943C19B |
|
DOI |
https://doi.org/10.5281/zenodo.5296943 |
|
persistent identifier |
https://treatment.plazi.org/id/832187E9-FF0E-FF46-FF74-634CFE12F9D7 |
|
treatment provided by |
Plazi |
|
scientific name |
Titanomessor Gustafsson & Bush |
| status |
gen. nov. |
Titanomessor Gustafsson & Bush , new genus
Type species. Titanomessor sexloba new species
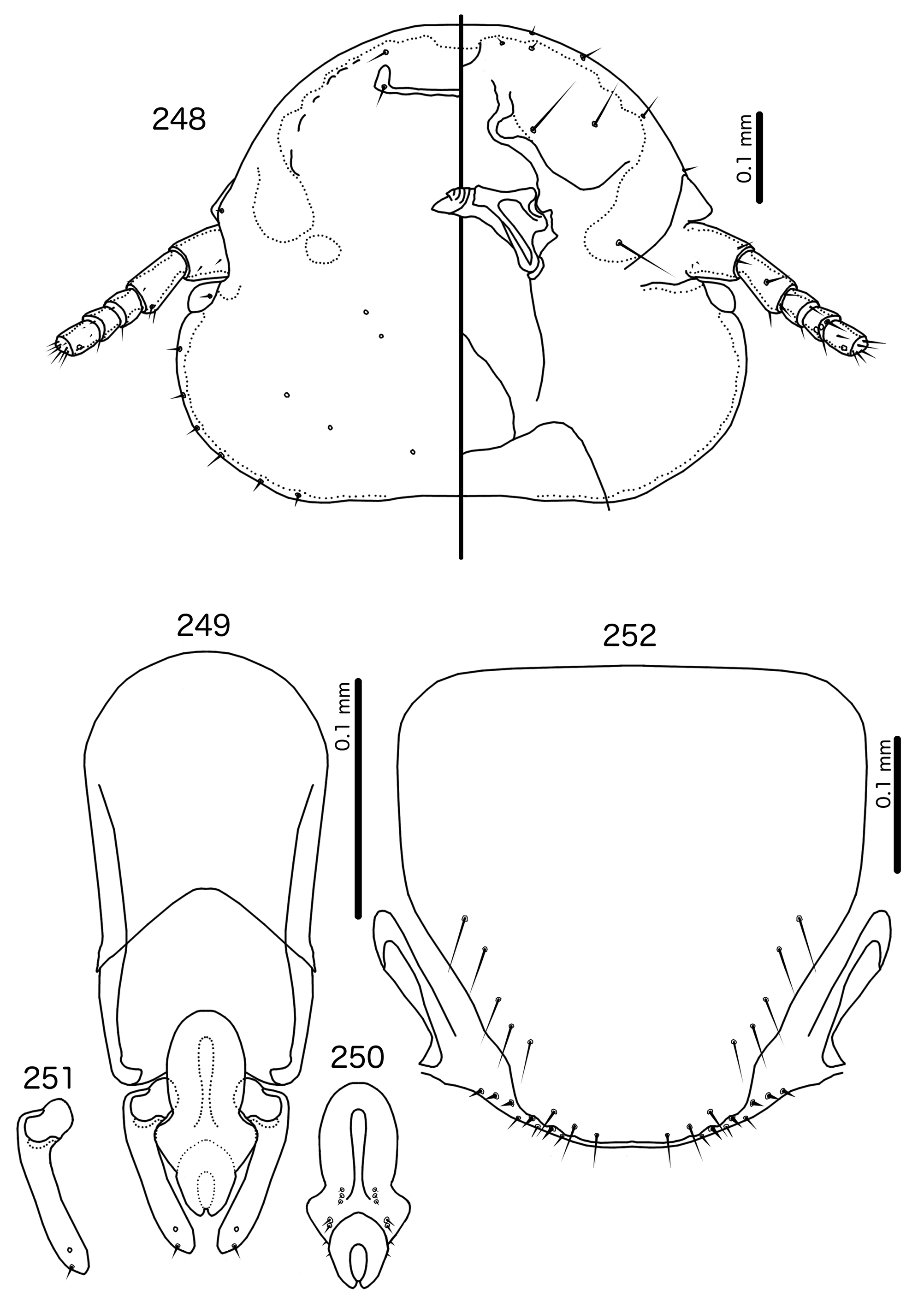
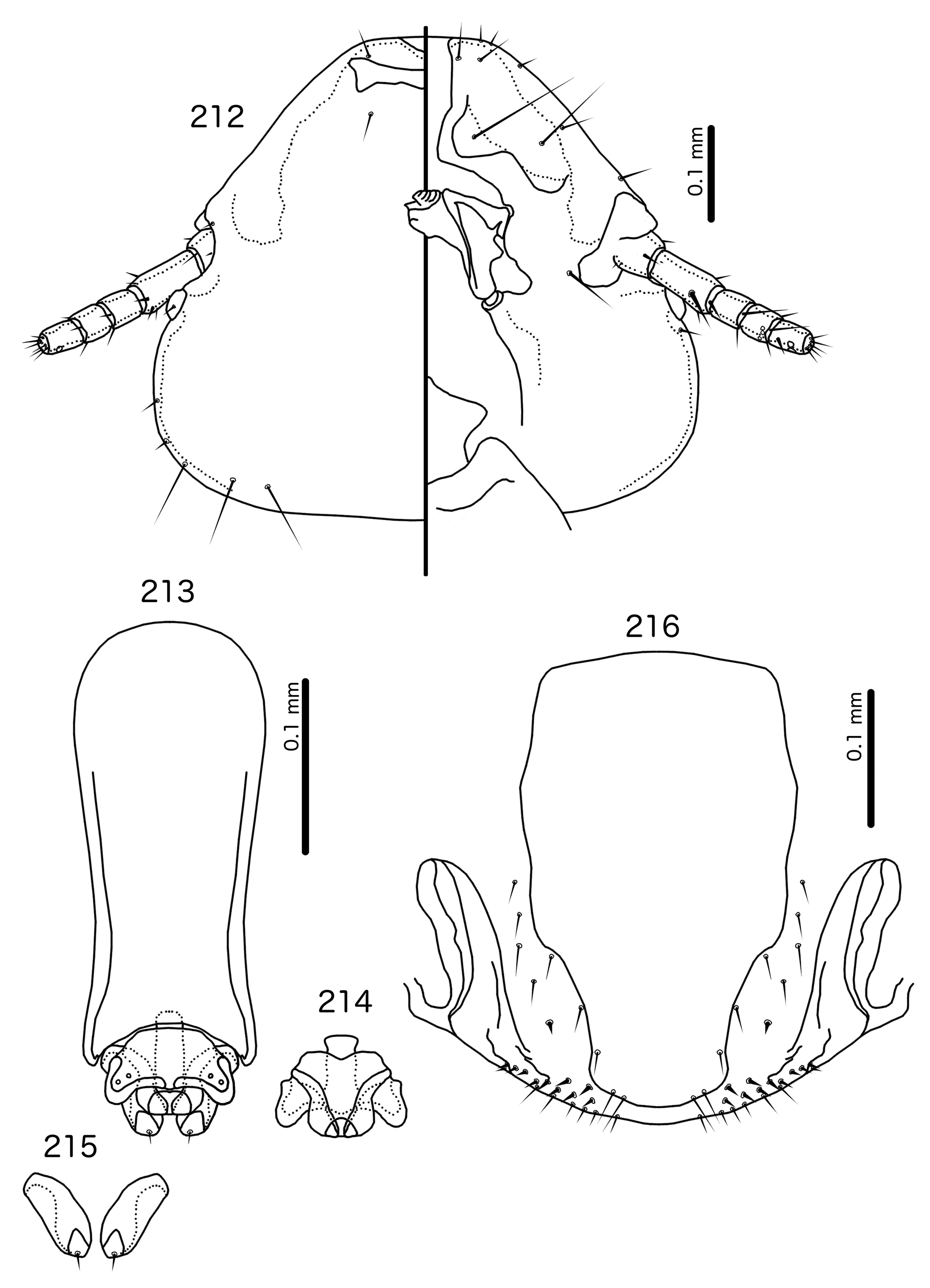
Diagnosis. Titanomessor n. gen. is separated from all other genera treated here by the unique male genitalia and the fact that mts 3–4 in both sexes, as well as mts 5 in males, are all of equal length, and much longer than mts 1–2. Within the Brueelia -complex, Harpactrox n. gen. ( Figs 248 View FIGURES 248 – 252 , 255, 260) is the only other genus that has a transversal dorsal preantennal suture, but these two genera are otherwise not similar in head chaetotaxy or structure, abdominal chaetotaxy, or the structure of the male genitalia.
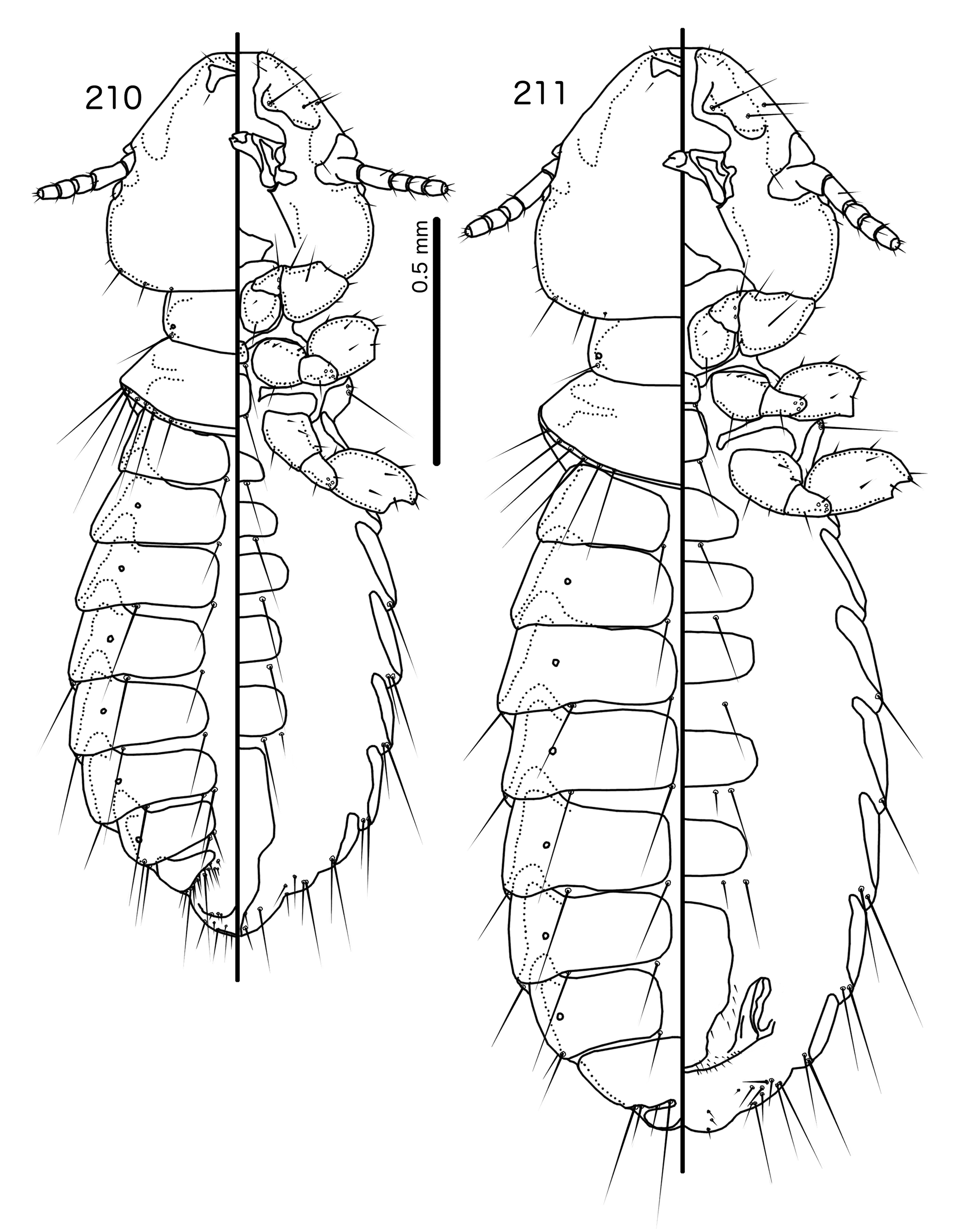
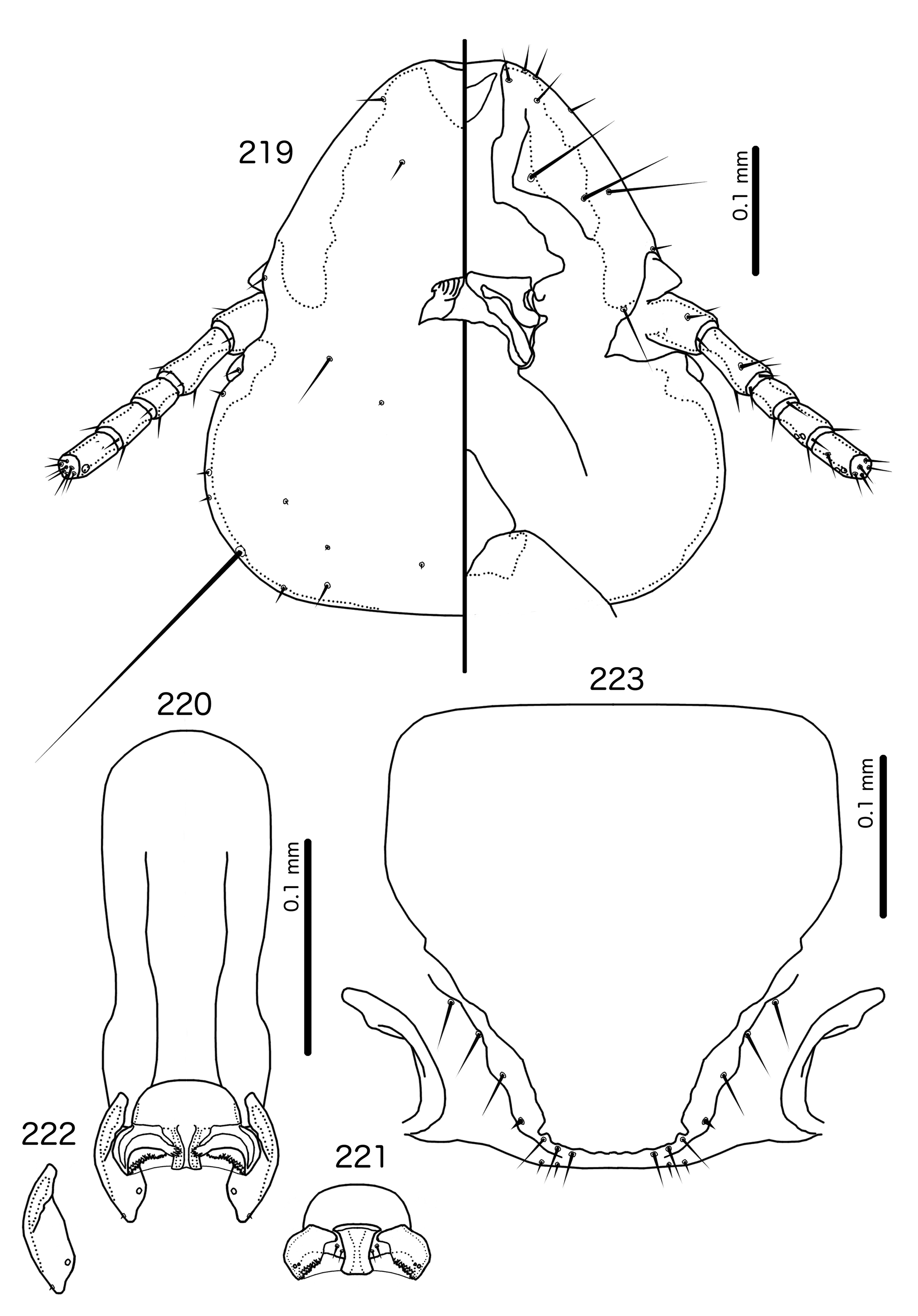
Overall, the genus most similar to Titanomessor is Indoceoplanetes n. gen., particularly In. ( Indoceoplanetes ) n. subgen. These two genera have similar abdominal chaetotaxy ( Table 2), except that tps are present in more anterior segments in males of In. ( Indoceoplanetes ) ( Fig. 217 View FIGURES 217 – 218 ) than in males of Titanomessor ( Fig. 210 View FIGURES 210 – 211 ). In both In. ( Indoceoplanetes ) ( Fig. 219 View FIGURES 219 – 223 ) and Titanomessor ( Fig. 212 View FIGURES 212 – 216 ), the marginal carina is uninterrupted but displaced at osculum, the female submarginal plate is broad distally, and approaches but does not reach the vulval margin ( Figs 216 View FIGURES 212 – 216 , 223 View FIGURES 219 – 223 ). In both genera, mts 5 and sometimes mts 4 are typically clearly dorsal, rather than marginal. However, there are large differences between the two groups. There is no dorsal preantennal suture in In. ( Indoceoplanetes ) ( Fig. 219 View FIGURES 219 – 223 ), but there is in Titanomessor ( Fig. 212 View FIGURES 212 – 216 ). In Titanomessor ( Figs 210–211 View FIGURES 210 – 211 ) mts 3–5 in male and mts 3–4 in female are mesosetae, whereas mts 3 is a macroseta and mts 4–5 in both sexes are microsetae in In. ( Indoceoplanetes ) ( Figs 217–218 View FIGURES 217 – 218 ). In females of In. ( Indoceoplanetes ) ( Fig. 218 View FIGURES 217 – 218 ) tergopleurite XI is not fused to tergopleurite IX+X, but these plates are fused in Titanomessor ( Fig. 211 View FIGURES 210 – 211 ). Finally, the male genitalia are very different between these two groups. The parameres in In. ( Indoceoplanetes ) ( Fig. 222 View FIGURES 219 – 223 ) are curved, wheras in Titanomessor ( Fig. 215 View FIGURES 212 – 216 ) the parameres are straight and convergent, and the structure of the mesosome of both genera are very different (cf. Fig. 214 View FIGURES 212 – 216 with Fig. 221 View FIGURES 219 – 223 ).
In Indoceoplanetes (Capnodella) n. gen. et n. subgen. female tergopleurite IX+X is fused to tergopleurite XI ( Figs 225 View FIGURES 224 – 225 , 232 View FIGURES 231 – 232 ) as in Titanomessor ( Fig. 211 View FIGURES 210 – 211 ), but unlike in Titanomessor ( Fig. 212 View FIGURES 212 – 216 ), the dorsal preantennal suture of In. (Capnodella) ( Figs 226 View FIGURES 226 – 230 , 233 View FIGURES 233 – 237 ) is longitudinal rather than latitudinal, and does interrupt the marginal carina submedianly. Male In. (Capnodella) also lack tps ( Figs 224 View FIGURES 224 – 225 , 231 View FIGURES 231 – 232 ), which are present in male Titanomessor ( Fig. 210 View FIGURES 210 – 211 ), and while both In. ( Indoceoplanetes ) ( Fig. 219 View FIGURES 219 – 223 ) and Titanomessor ( Fig. 212 View FIGURES 212 – 216 ) lack a ventral anterior plate, this is present in In. (Capnodella) ( Fig. 226 View FIGURES 226 – 230 ).
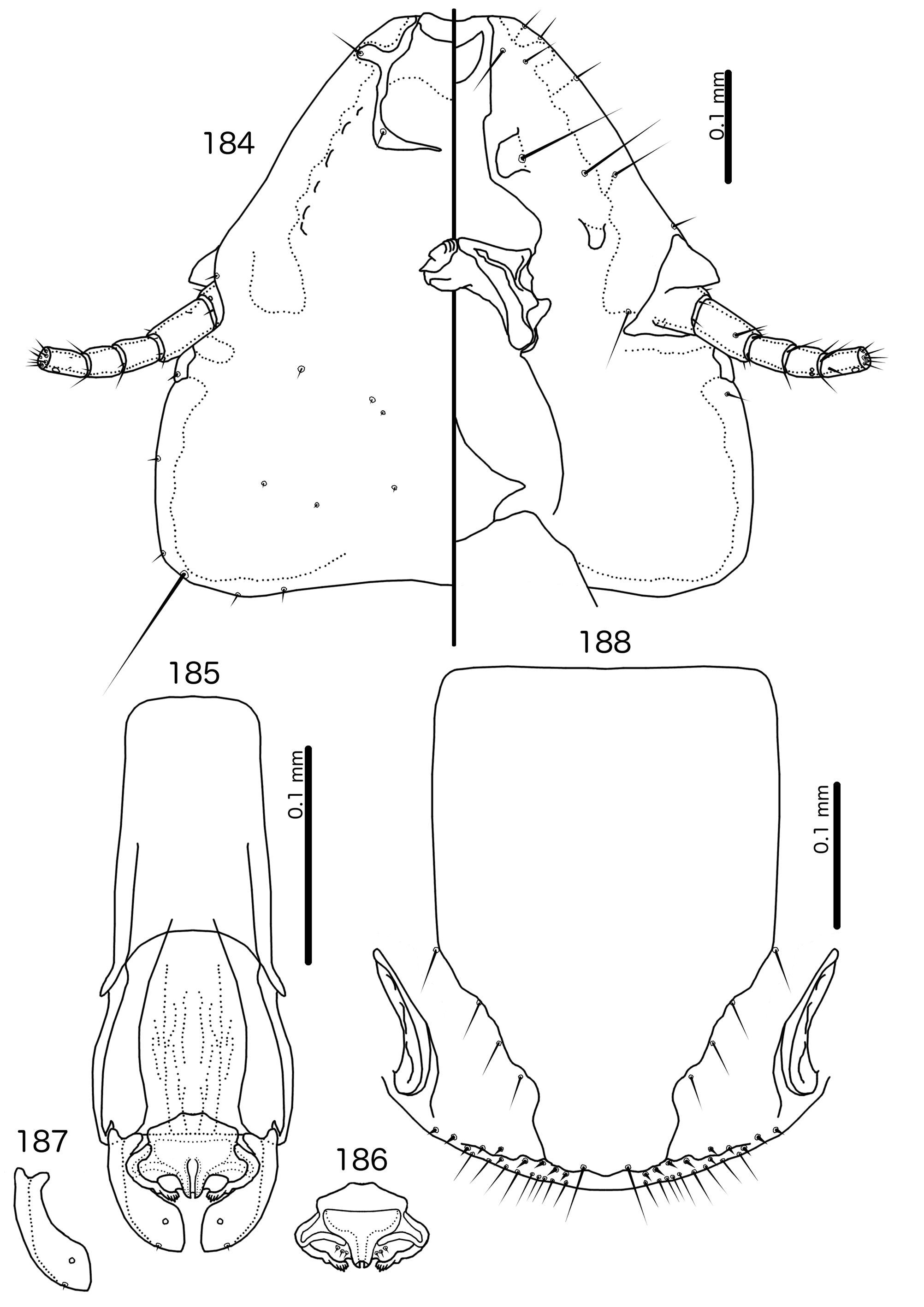
The large size (see Measurements below) and the shape of the mesomere ( Fig. 214 View FIGURES 212 – 216 ) suggests that Titanomessor could be closely related to Turdinirmus ( Fig. 186 View FIGURES 184 – 188 ). Abdominal chaetotaxy is identical between the two genera ( Table 2), except for the presence of tps on male tergopleurites VII–VIII in Titanomessor ( Fig. 210 View FIGURES 210 – 211 ), which are absent in all Turdinirmus . The marginal thickening of the mesosomal lobes of both genera do not deviate from the margin ( Figs 186 View FIGURES 184 – 188 , 214 View FIGURES 212 – 216 ), and in both genera the gonopore consists of a pair of convergent ventral thickenings. However, parameral shape differs between these two genera, and while pst1 is present in Turdinirmus ( Fig. 186 View FIGURES 184 – 188 ), it is absent in Titanomessor ( Fig. 214 View FIGURES 212 – 216 ). Preantennal structure also differs between the two, as the marginal carina is interrupted at least submedianly in Turdinirmus ( Fig. 184 View FIGURES 184 – 188 ), but not in Titanomessor ( Fig. 212 View FIGURES 212 – 216 ), and the dorsal preantennal suture is structured differently in the two genera.
For differences between Titanomessor and Maculinirmus , see the diagnosis of Maculinirmus above.
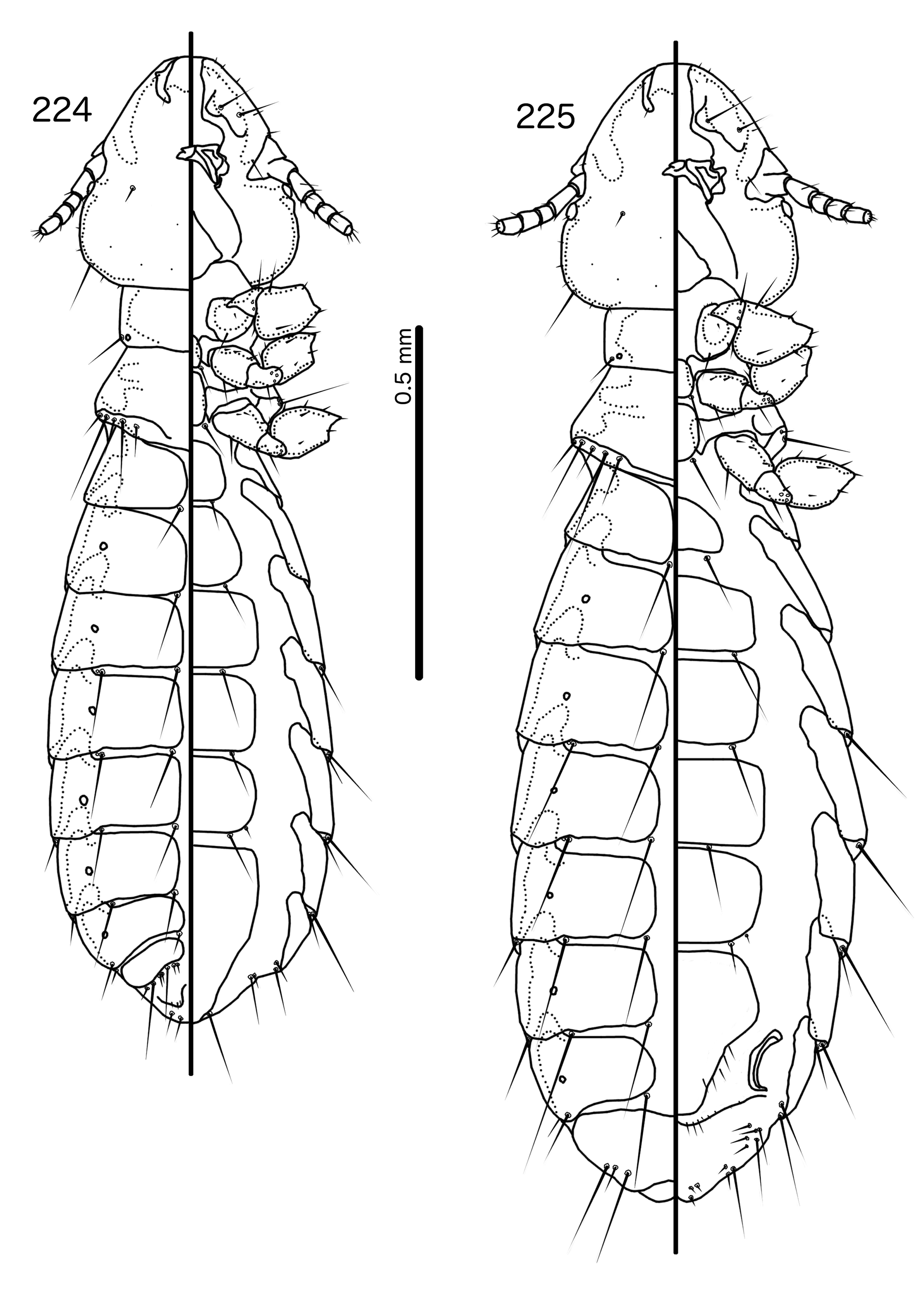
Description. Both sexes. Head rounded pentagonal, very broad ( Fig. 212 View FIGURES 212 – 216 ). Marginal carina broad, narrowing anteriorly, not interrupted, but displaced posteriorly and dorsally at osculum. Frons hyaline. Ventral carinae broad, diffuse anterior to pulvinus and not clearly continuous with marginal carina. Dorsal preantennal suture transversal, seemingly reaching dsms and maybe ads, but this cannot be established with certainty due to the state of examine dmaterial. Suture does not reach lateral margins of head. Dorsal anterior plate connected to main head plate laterally. Ventral anterior plate absent. Head chaetotaxy as in Fig. 212 View FIGURES 212 – 216 ; as3 present; pns, pts, and s1–4 not visible in the material examined; pos posterior to eye, ventral. Ventral anterior plate absent. Coni short, blunt. Antennae monomorphic. Marginal and occipital carinae not visible. Marginal temporal carina very narrow. Uniquely in complex, mts are sexually dimorphic: in males mts 3–5 long, of roughly equal length, in females mts 3–4 long, of roughly equal length, and mts 5 thorn-like; in both sexes mts 4–5 often clearly dorsal. Gular plate broad, diamondshaped.
Prothorax ( Figs 210–211 View FIGURES 210 – 211 ) rectangular; ppss on postero-lateral corner. Proepimera and metepisterna with blunt median ends. Pterothorax roughly trapezoidal; lateral margins divergent, posterior margin gently rounded; mms widely separated medianly. Meso- and metasterna not fused, 1 seta on postero-lateral corner on each side of each plate. Leg chaetotaxy as in Fig. 25 View FIGURES 25 , except fI-p2, fI-v4 absent; fII-a2, fIII-a2 dorsal.
Abdomen ( Figs 210–211 View FIGURES 210 – 211 ) oval. Distal end of abdomen bulging in male. Tergopleurites rectangular; tergopleurites II–IX+X in male and tergopleurites II–VIII in female narrowly separated medianly. Female tergopleurites IX+X and XI fused medianly. Sternal plates narrow, lateral margins rounded. Sternal plates II–IV translucent and illustrated approximately. Pleural incrassations moderate to large. Re-entrant heads large, blunt, translucent. Abdominal chaetotaxy as in Table 2. Male subgenital plate oblong, narrowing distally, and reaching distal part of abdomen. Female subgenital plate slender, roughly rectangular, narrowing only slightly posteriorly, approaching vulval margin. Vulval margin ( Fig. 216 View FIGURES 212 – 216 ) gently rounded, with slender vms, thorn-like vss; slender vos follow lateral margins of subgenital plate, vos typically in two parallel rows; distal vos median to vss.
Male genitalia ( Fig. 213–215 View FIGURES 212 – 216 ) unique within Brueelia -complex. Basal apodeme ( Fig. 213 View FIGURES 212 – 216 ) slender, roughly rectangular, anterior margin rounded. Proximal mesosome ( Fig. 214 View FIGURES 212 – 216 ) broadly flattened. Gonopore open distally and proximally, as broad convergent thickenings; protruding distally to lobes. Mesosomal lobes angular, in some specimens slightly rugose distally; 2 pmes sensilla visible on mesosome lateral to gonopore and near lateral margins. Parameral heads ( Fig. 215 View FIGURES 212 – 216 ) blunt. Parameral blades as fleshy lobes, converging distal to mesosome. Parameral blades rounded distally; pst1 not visible; pst2 as microseta on distal end of paramere.
Host distribution. Presently know only from the type host, Laniarius erythrogaster , belonging to the Malaconotidae . We have examined lice from the following malaconotid hosts: Dryoscopus cubla hamatus Hartlaub, 1863 , Dryoscopus gambensis gambensis (Lichtenstein, 1832) , Laniarius major major (Hartlaub, 1848) , Laniarius major mossambicus (Fischer and Reichenow, 1880) , Malaconotus blanchoti hypopyrrhus Hartlaub, 1844 , Malaconotus cruentus (Lesson, 1831) , Malaconotus blanchoti blanchoti Stephens, 1826 , Tchagra senegalus armenus (Oberholser, 1906) , Tchagra senegalus cucullatus (Temminck, 1840) , Telophorus nigrifrons sandgroundi (Bangs, 1931) , Telophorus sulfureopectus similis (Smith, A., 1936) . However, all this material belongs to Guimaraesiella rather than Titanomessor , making the occurrence of Ti. sexloba unique within the host family. The material we have examined is from two different collection events, suggesting that L. erythrogaster is the correct host.
Geographical range. Known only from Uganda, but the host genus Laniarius occurs across most of Sub- Saharan Africa ( Harris & Franklin, 2000).
Etymology. The genus name derived from Greek “ titan ” for the large size of these lice, combined with Latin “ messor ” for “harvester”. Gender: masculine.
Remarks. Titanomessor sexloba n. sp. was not included in the phylogeny of Bush et al. (2016), and its affinities within the Brueelia -complex are unknown. The great differences in the male genitalia between Titanimessor and all other genera treated here makes the genus hard to place, but abdominal chaetotaxy and overall pigmentation patterns are very similar to those of Maculinirmus , Turdinirmus , and Indoceoplanetes .
Included species
* Titanomessor sexloba n. sp.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |