Meropoecus Eichler, 1940
|
publication ID |
https://doi.org/10.11646/zootaxa.4313.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A5Fdfba5-F992-44A8-84C2-1756C943C19B |
|
DOI |
https://doi.org/10.5281/zenodo.5297061 |
|
persistent identifier |
https://treatment.plazi.org/id/832187E9-FEAE-FEE5-FF74-673CFA8CFEB9 |
|
treatment provided by |
Plazi |
|
scientific name |
Meropoecus Eichler, 1940 |
| status |
|
Docophorus Nitzsch, 1818: 289 (in partim). Meropoecus Eichler, 1940: 102 .
Type species. Docophorus meropis Denny, 1842: 46 , by original designation.
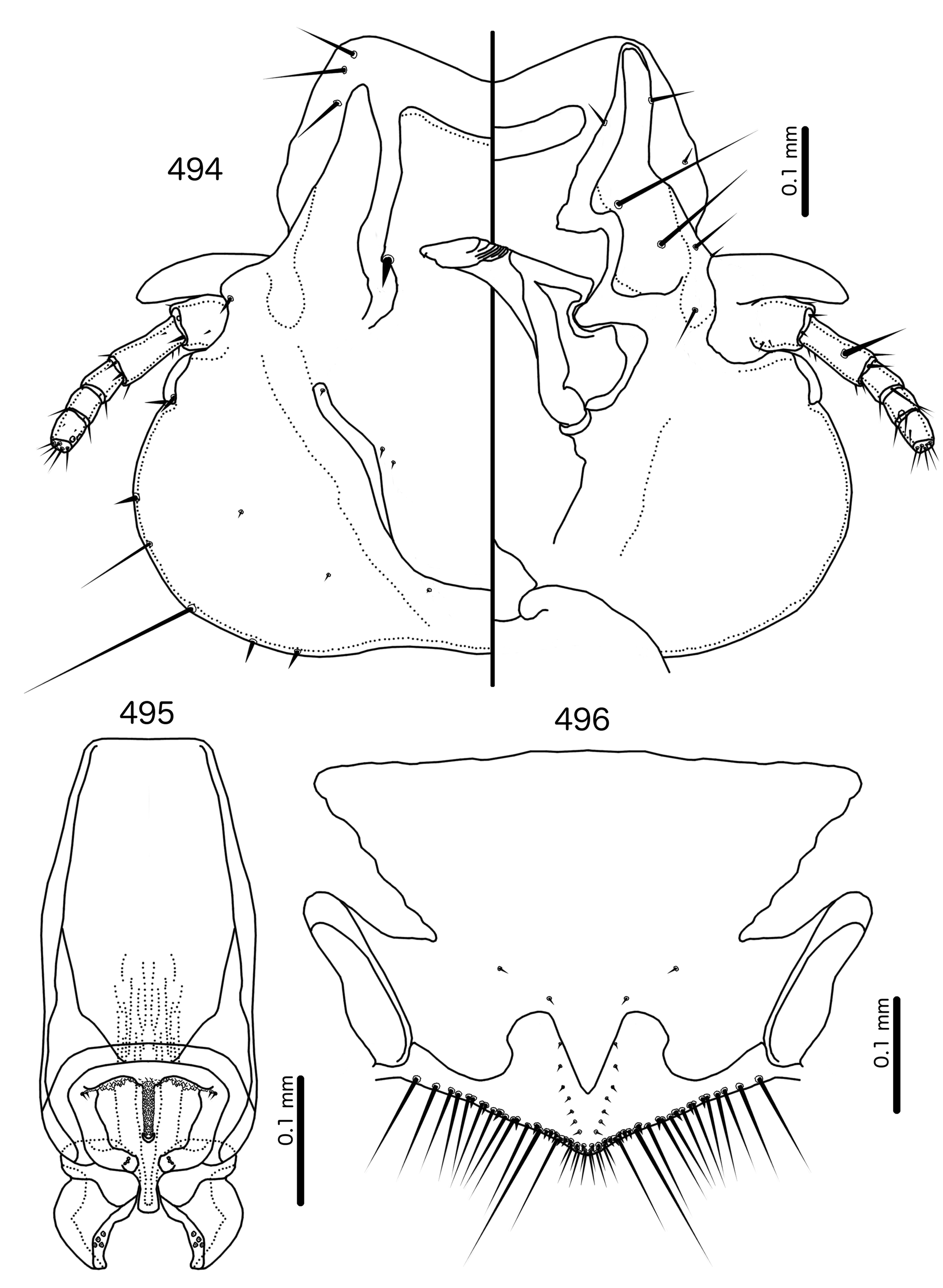
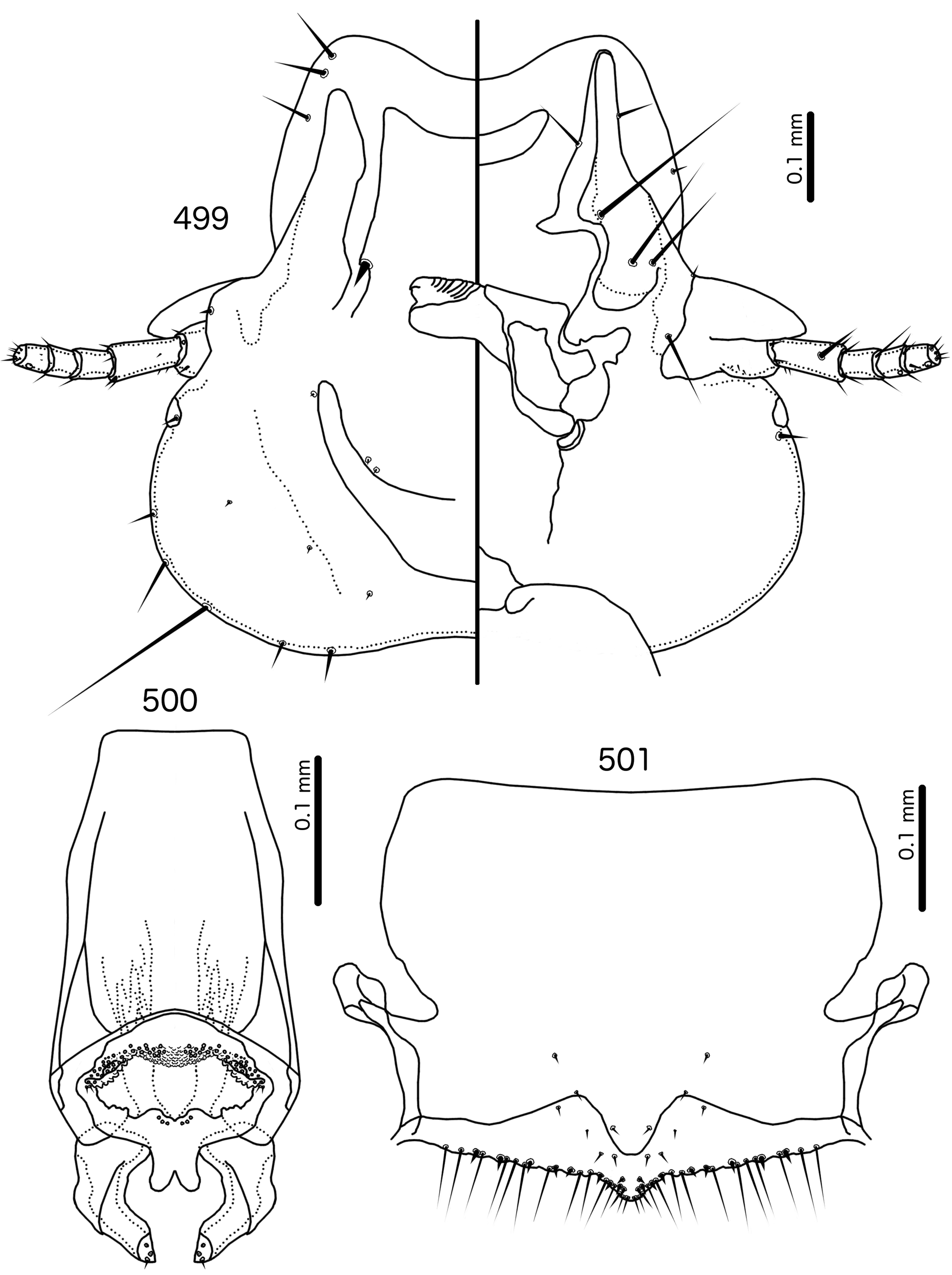
Diagnosis. Although Meropoecus is not very similar to other genera treated here, the female genitalia are similar to those of Meropsiella (see above). However, the male genitalia of Meropoecus ( Figs 495 View FIGURES 494 – 496 , 500 View FIGURES 499 – 501 ) are unlike any other genus in the Brueelia -complex. The presence of more than 2 pst is shared within the Brueelia -complex only with Aporisticeras n. gen. ( Fig. 529 View FIGURES 526 – 531 ), and while some other groups treated here have papillation on the anterior end of the mesosome (e.g. the Olivinirmus meinertzhageni group, Fig. 335 View FIGURES 334 – 337 ), this is not as extensive and elaborate as in Meropoecus .
Description. Both sexes. Head broad, bulb-shaped ( Figs 494 View FIGURES 494 – 496 , 499 View FIGURES 499 – 501 ). Marginal carina widely interrupted medianly, postmarginal carina reaching barely one third of preantennal head. Hyaline margin wide, anteriorly concave, continues laterally for most of preantennal head. Ventral carinae continue farther anterior than marginal carina, divergent anteriorly. Ventral anterior plate present, rounded crescent-shaped. Dorsal preantennal suture continuous with hyaline margin, reaching ads, but diffuse posterior to this. Dorsal anterior plate may be continuous with main head plate. Head setae as in Fig. 494 View FIGURES 494 – 496 , 499 View FIGURES 499 – 501 ; dsms and as1 on hyaline margin; as2–3 situated dorsally, on hyaline margin; ads very thick, thorn-like; pos ventral or lateral. Coni long, pointed. Antennae monomorphic.
Temples bulging. Temporal carinae visible, flanked medianly by a narrow dorsal postantennal suture; mts 3 only long setae (except in Meropoecus emersoni , see Tendeiro 1961), but mts 2 often longer than mts 1 and mts 4–5. Gular plate short and squat.
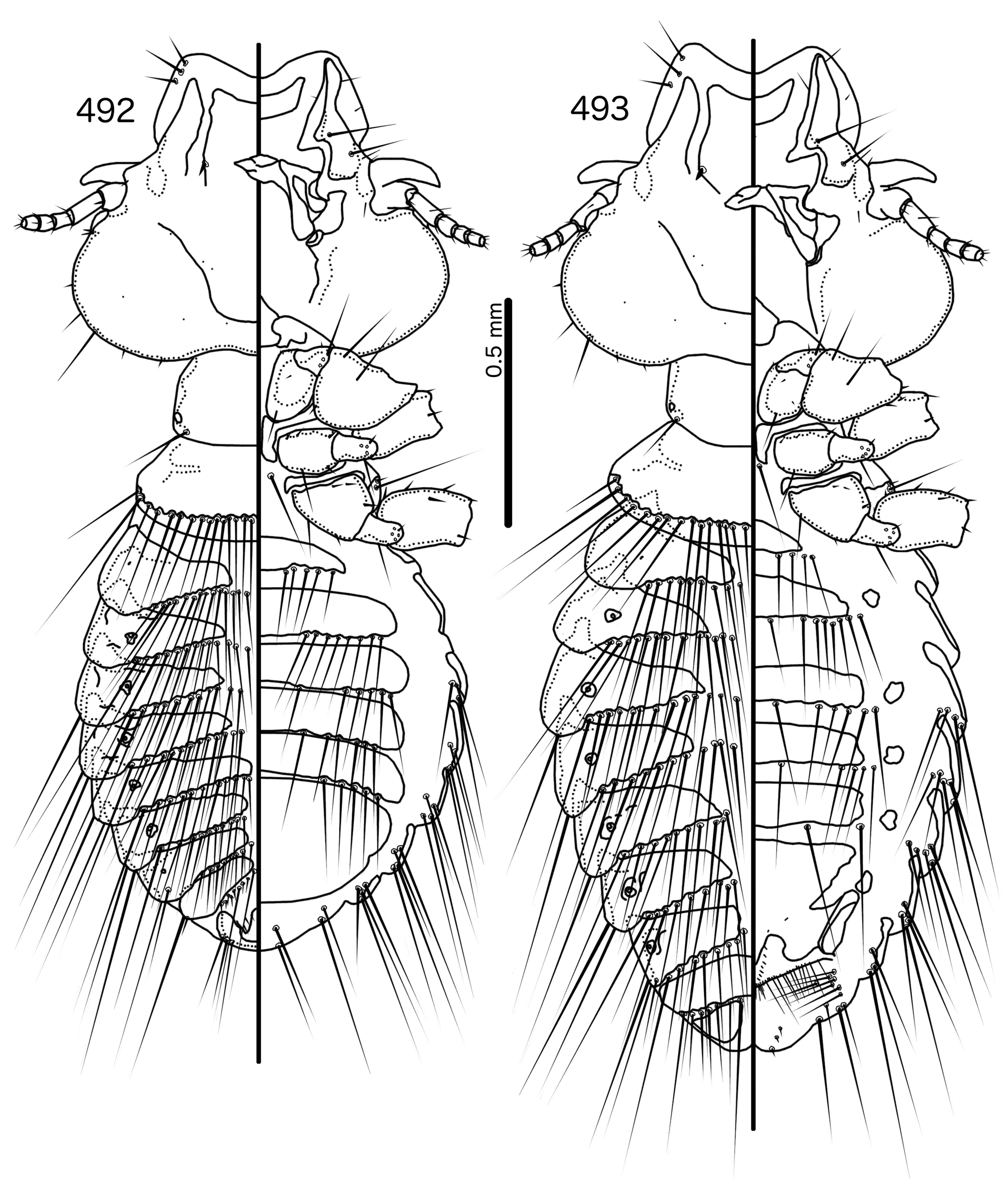
Prothorax rectangular ( Figs 492–493 View FIGURES 492 – 493 , 497–498 View FIGURES 497 – 498 ); ppss on postero-lateral corners. Proepimera long, hooked, reaching halfway around coxae II. Pterothorax rounded trapezoidal; lateral margins rounded; posterior margin flat or slightly rounded; mms form continuous row. Meso- and metasterna absent or present as pair of minute plates near median end of metepisterna; 1 seta on each side on ventral pterothorax median to coxae II. Metepisterna slender; median ends blunt. Leg chaetotaxy as in Fig. 25 View FIGURES 25 , except fI-v4, fI-v3, fI-p2, fII-v2, fIII-d2, fIII-v2 absent. Many leg setae long and spike-like.
Abdomen broadly oval in females and almost rounded in males ( Figs 492–493 View FIGURES 492 – 493 , 497–498 View FIGURES 497 – 498 ). Abdominal chaetotaxy as in Table 2. Tergopleurites triangular, more rectangular in posterior segments; tergopleurites II–IX+X in males and II–VIII in females moderately divided medianly. Sternal plates broad, may approach pleurites. Accessory sublateral plates may be present in females on segments III–VII. Pleural incrassations slender to moderate. Ventral section of tergopleurites typically slender. Re-entrant heads moderate. Male subgenital plate broad, rounded triangular, not reaching posterior margin of abdomen. Female subgenital plate broad, roughly rectangular or triangular, but does not reach vulval margin ( Figs 496 View FIGURES 494 – 496 , 501 View FIGURES 499 – 501 ); median section of plate with narrow, elongated bulge that may approach vulval margin. Lateral submarginal extensions present, wide. Vulval margin convergent to median point ( Figs 496 View FIGURES 494 – 496 , 501 View FIGURES 499 – 501 ) with numerous long, slender vms, numerous short, thorn-like vss; vss and vms not separated into distinct sets; vos in narrowly convergent rows, with distal vos approaching or median to vss.
Male genitalia ( Figs 495 View FIGURES 494 – 496 , 500 View FIGURES 499 – 501 ) dissimilar to those of other genera treated here. Basal apodeme roughly eggshaped, wider distally. Mesosome variable in shape, but in all species consists of rounded proximal part overlapping basal apodeme, and lesser distal part located median to parameres; 1 (e.g. Meropoecus smithi , not illustrated) or 2 (e.g. Mo. bartlowi n. sp., Fig. 500 View FIGURES 499 – 501 ) narrow and densely papillate lobes in anterior end of mesosome; 2 ames microsetae (in some species sensilla) near antero-lateral corners of mesosome on each side, just lateral to papillate lobes; 3 pmes microsetae or sensilla submedianly near distal margin of mesosome. Parameres stout, strongly curved, consisting of two parts: a more heavily sclerotized proximal-lateral end, and a translucent distal-median end. Up to 4 pst on median margin near distal end.
Host distribution. Limited to species of bee-eaters, family Meropidae . Preliminary studies indicate that most species are monoxenous, and that there are several undescribed species in Meropoecus .
Geographical range. Widely distributed across the Old World.
Remarks. Meropoecus was erected by Eichler (1940) for a single species that was characterized by its unique head structure ( Docophorus meropis Denny, 1842 ). Eichler compared this species to Cuculoecus Ewing, 1936 , and noted that Meropoecus has a widely bulging frontal head margin, more strongly arched portions of the marginal carina, a shorter clypeus, and a stouter and wider postantennal head. Later treatments of this genus include Emerson & Elbel’s (1956) key to the four species known at the time, and the checklist of Price et al. (2003), which listed seven valid species of Meropoecus , as well as one synonymized species ( Mo. bifrons = Mo. meropis ).
Two specimens of Meropoecus were included in the phylogeny of Bush et al. (2016). Their placement as a sister to Motmotnirmus , and as a sister clade to the larger part of the Brueelia -complex were both strongly supported. Adam (2004) has recently redescribed and reillustrated the type species, and we do not describe it here again.
Included species
* Meropoecus balisong new species
* Meropoecus bartlowi new species
* Meropoecus caprai Conci, 1941a: 102
* Meropoecus debeauxi Conci, 1941a: 102 Meropoecus eichleri Tendeiro, 1989: 101
* Meropoecus emersoni Tendeiro, 1961: 298
* Meropoecus meropis ( Denny, 1842: 46) [in Docophorus ] Docophorus bifrons Nitzsch, 1866: 116
Meropoecus mossambicensis Tendeiro, 1989: 100
* Meropoecus smithi Emerson & Elbel, 1956: 118 [1]
[1] Note that, in the original illustrations of this species, all head setae have mistakenly been placed on the dorsal side.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Meropoecus Eichler, 1940
| Bush, Sarah E. 2017 |
Meropoecus mossambicensis
| Tendeiro 1989: 100 |
Meropoecus emersoni
| Tendeiro 1961: 298 |
Meropoecus smithi
| Emerson 1956: 118 |
Meropoecus caprai
| Conci 1941: 102 |
Meropoecus debeauxi
| Tendeiro 1989: 101 |
| Conci 1941: 102 |
Meropoecus meropis ( Denny, 1842: 46 )
| Nitzsch 1866: 116 |
| Denny 1842: 46 |
Docophorus
| Eichler 1940: 102 |
| Nitzsch 1818: 289 |