Echiniscus granulatus ( Doyère, 1840 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5344.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:DCF48473-AC31-4CDB-808F-453F8F280002 |
|
DOI |
https://doi.org/10.5281/zenodo.8349260 |
|
persistent identifier |
https://treatment.plazi.org/id/8119D633-B951-FFEC-1CED-FB03BBEFFE7F |
|
treatment provided by |
Plazi |
|
scientific name |
Echiniscus granulatus ( Doyère, 1840 ) |
| status |
|
9. Echiniscus granulatus ( Doyère, 1840) View in CoL View at ENA
Figures 7–12 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12 , Tables 2–5 View TABLE 2 View TABLE 3 , Supplementary Material 3
Synonyms
Echiniscus abanti Maucci, 1973 View in CoL : Ramazzotti & Maucci (1983)
Echiniscus crassus Richters, 1904 View in CoL : Marcus (1936)
Echiniscus egnatiae Durante Pasa & Maucci, 1979 View in CoL syn. nov.
Echiniscus fortis Bartoš, 1935 : Ramazzotti & Maucci (1983)
Emydium granulatum View in CoL , Emydium granulosum Doyère, 1840
Echiniscus heterospinosus Maucci, 1954 View in CoL syn. nov.
Echiniscus granulatus inocelatus Mihelčič, 1938 syn. nov., Echiniscus inocellatus Mihelčič, 1964 View in CoL
Locus typicus: ca. 48°50’32”N, 2°21’23”E: France, Paris , Muséum national d’Histoire naturelle. Mosses from a skeleton of a sperm whale/cachalot Physeter macrocephalus Linnaeus, 1758 GoogleMaps .
New type locality: 48°48’35”N, 2°29’2”E, 46 m asl: France, Saint-Maur-des-Fossés (south-eastern suburbs of Paris). Moss from a tombstone in urban cemetery. Found together with E. testudo ( Doyère, 1840) and Dianea sattleri ( Richters, 1902) .
The neotype series consists of 101 specimens: 77 ♀♀, 5 ÔÔ, 13 juveniles and 6 larvae mounted on permanent slides FR.135.01–8 (neotype: FR.135.04). 12 specimens used for DNA extraction (four hologenophores preserved); 10 specimens on SEM stub 20.12.
Additional localities: See Table 1 View TABLE 1 for all sequenced populations. Comparative material embraced numerous populations of E. granulatus from France, Germany, Iceland, Italy, Kyrgyzstan, Poland, and Spain, which has already been recorded from all these countries ( McInnes 1994) but Kyrgyzstan:
• KG.039; 41°56'47"N, 77°39'4"E, 2715 m asl: Kyrgyzstan, south of Barskoon , mosses and lichens from rocks in Asian spruce ( Picea schrenkiana ) forest, Witold Morek & Bartłomiej Surmacz coll. on 16 th October 2018 (11 ♀♀) GoogleMaps .
• KG.047; 41°56'50"N, 77°38'55"E, 2795 m asl: Kyrgyzstan, south of Barskoon , mosses from rocks in Asian spruce forest, Witold Morek & Bartłomiej Surmacz coll. on 16 th October 2018 (2 ♀♀) GoogleMaps .
• KG.054; 41°56'50"N, 77°38'57"E, 2778 m asl: Kyrgyzstan, south of Barskoon , mosses from rocks in Asian spruce forest, Witold Morek & Bartłomiej Surmacz coll. on 16 th October 2018 (2 ♀♀) GoogleMaps .
Etymology: From Latin granulatus = grainy, which misleadingly refers to the dorsal sculpturing (or rather, how it was perceived with lower quality microscopes). In fact, there are no granular elements in the sculpturing (see below). An adjective in nominative singular.
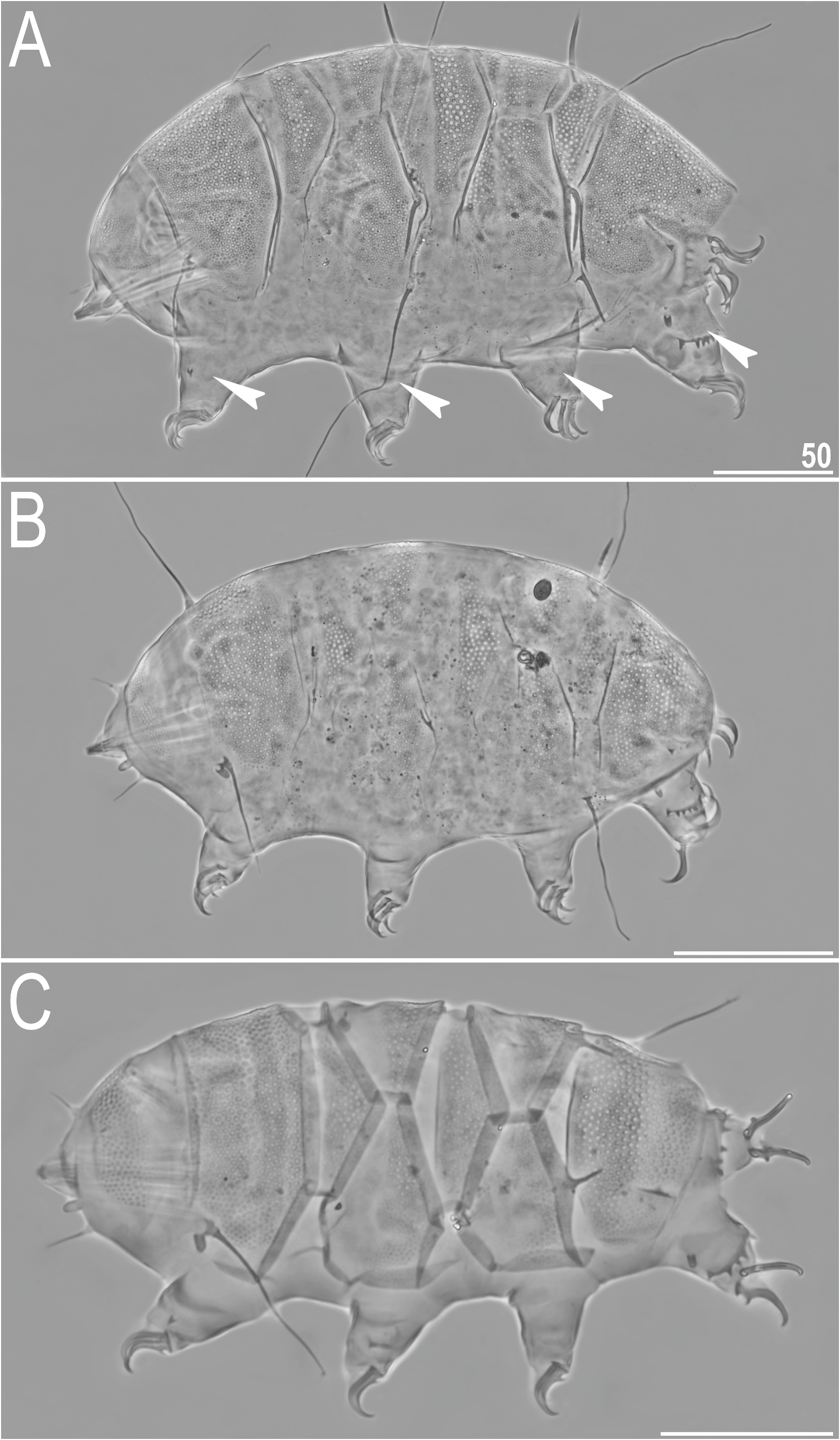
Redescription. Females (i.e. from the third instar onwards; measurements and statistics in Table 2 View TABLE 2 ). Medium-sized (ca. 210–280 µm in length) body ( Figs 7A View FIGURE 7 , 8–9A View FIGURE 8 View FIGURE 9 ), orange to red and with large red eyes, dissolving in Hoyer’s medium. Dactyloid cephalic papillae (secondary clavae) and (primary) clavae; cirri growing out from bulbous cirrophores ( Figs 7A View FIGURE 7 , 8A View FIGURE 8 ). Cirri A short (ca. 25% of the body length). Body appendage configuration A-(B)- C-Cd- D-Dd; cirrus B often absent, appendages Cd and Dd developed in a form of long spines, whereas lateral appendages C and D– long, filamentous cirri ( Figs 7A View FIGURE 7 , 8–9A View FIGURE 8 View FIGURE 9 ). Asymmetries in the development of trunk appendages frequent ( Fig. 8A View FIGURE 8 ). Minute spicule E present ( Figs 7A View FIGURE 7 , 8A View FIGURE 8 ).
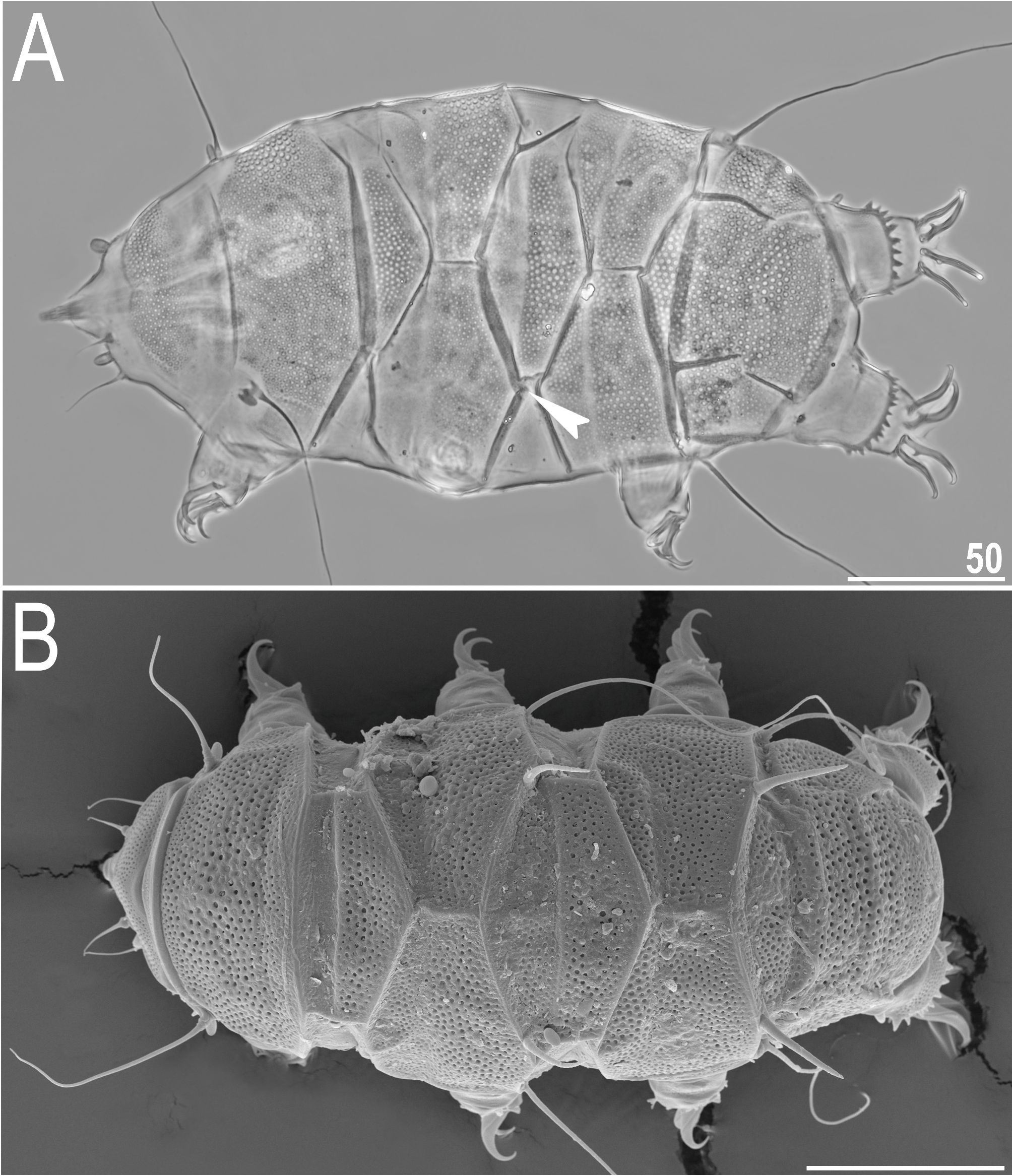
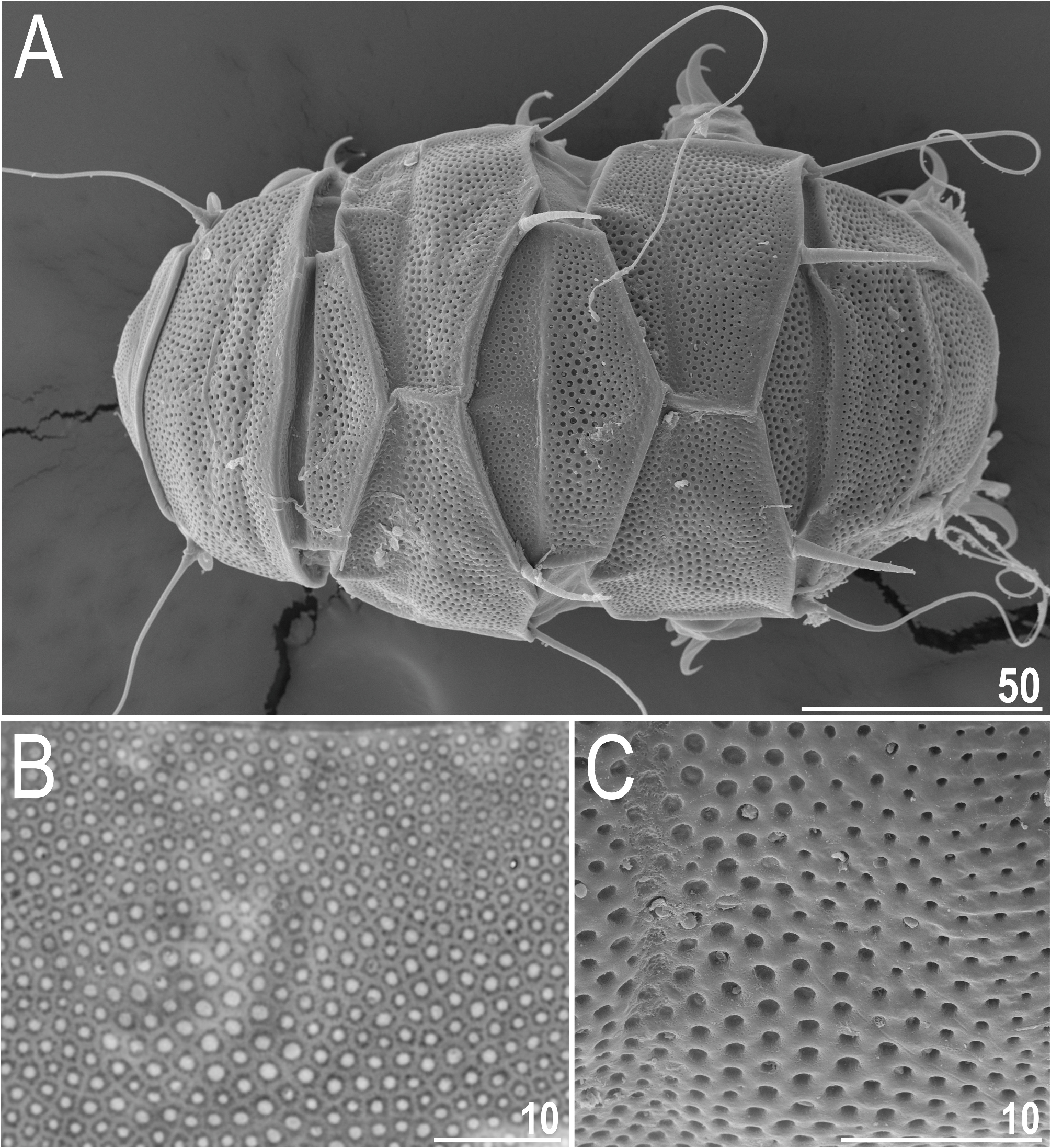
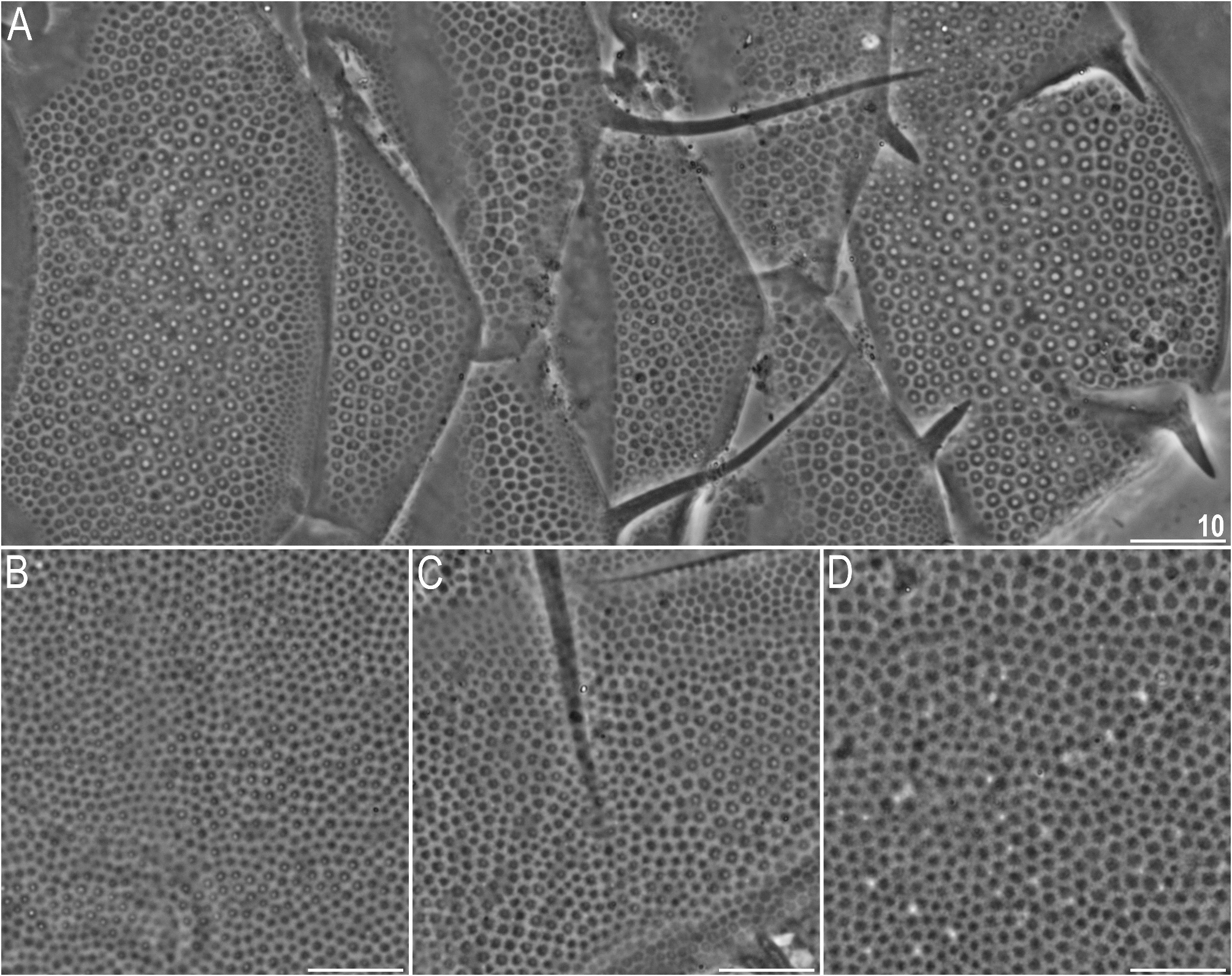
Dorsal plates with the sculpturing close to that in the blumi-canadensis complex or in Testechiniscus , i.e. large cuticular pores surrounded by polygonal thickenings regularly and tightly distributed in all plates, and occasionally a reticulum in anterior plate portions of pI–II and m2, indistinguishable from pores in PCM ( Figs 7A View FIGURE 7 , 8–9 View FIGURE 8 View FIGURE 9 ). The largest pores are present in the central portions of all plates, with the tendency to reduction towards the lateralmost portions. Cephalic plate with an anterior chalice-like incision ( Fig. 8A View FIGURE 8 ); cervical plate unsculptured and narrow, tightly inserted between cephalic and scapular plates ( Fig. 8 View FIGURE 8 ). Scapular plate large, without any median sutures and with faintly marked lateral sutures separating lateralmost rectangular portions ( Fig. 8A View FIGURE 8 ). Median plates 1 and 3 unipartite, and m2 divided into the anterior and posterior part, the latter with a more pronounced sculpturing ( Figs 7A View FIGURE 7 , 8A View FIGURE 8 ). Paired segmental plates I–II with poorly delineated anterior and posterior parts by faint transverse belts ( Figs 7A View FIGURE 7 , 8–9A View FIGURE 8 View FIGURE 9 ). Caudal plate with two short incisions ( Figs 7A View FIGURE 7 , 8A View FIGURE 8 ); epicuticular ridge connecting incisions visible only in SEM ( Figs 8B View FIGURE 8 , 9A View FIGURE 9 ).
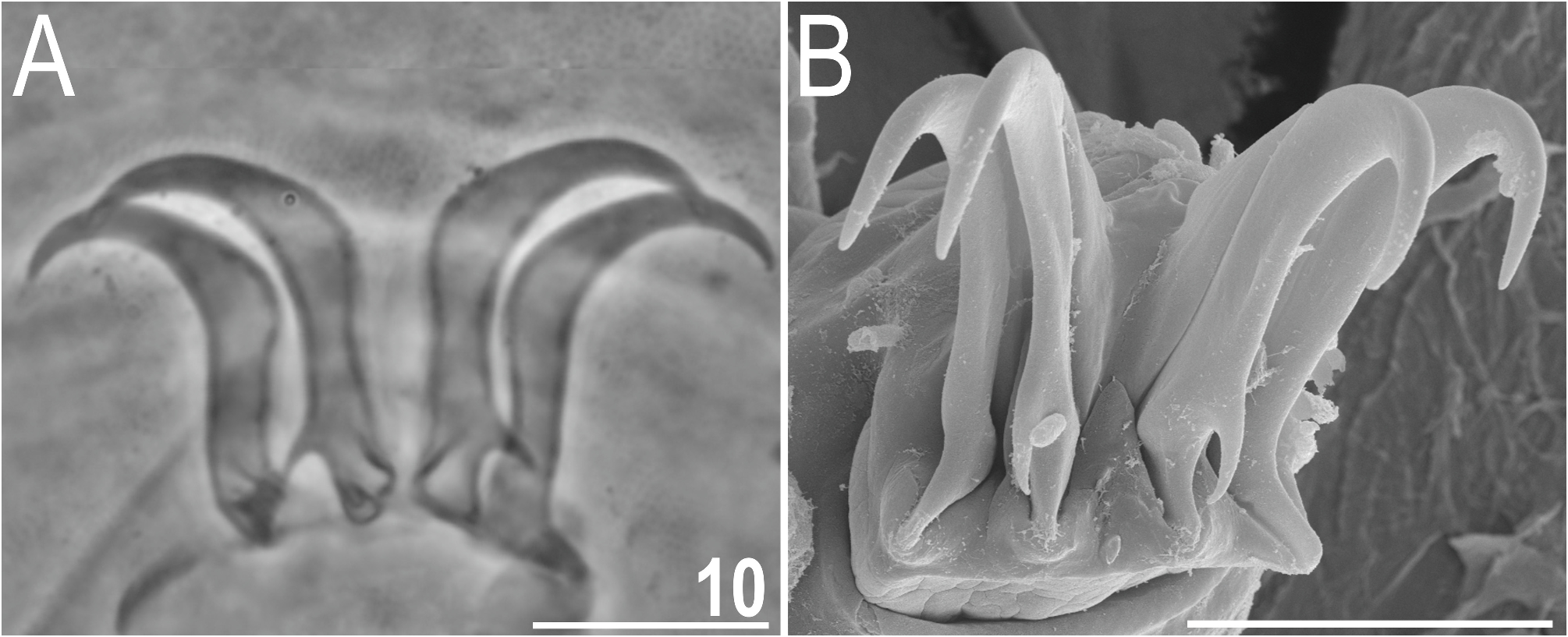
Venter uniformly granulated; subcephalic plates absent. Hexapartite gonopore placed between genital plates, and trilobed anus between legs IV. Legs short, with pedal plates barely visible in PCM as dark areas in their central portions ( Fig. 7A View FIGURE 7 ); the sculpturing of pedal plates comprises faint endocuticular pillars. Pulvini also poorly developed. Spine on leg I and papilla on leg IV present ( Fig. 7A View FIGURE 7 ). Claws elongated and heteronych ( Fig. 10 View FIGURE 10 ): claws I–III of equal heights, but claws IV considerably higher. External claws on all legs smooth; internal claws with primary spurs of a typical size and positioned at ca. 20% of the claw height, highly divergent from the branch.
Males (i.e. from the third instar onwards; measurements and statistics in Table 3 View TABLE 3 ). Quantitatively and qualitatively like females, males are probably smaller than females on average, but we refrain from a definite statement given how few males were found. Circular gonopore.
Juveniles (i.e. the second instar; measurements and statistics in Table 4). Smaller than sexually mature individuals ( Fig. 7B View FIGURE 7 ). Body appendage configuration: A-Cd -D - Dd, with Cd frequently reduced.A possibility of allometric growth visible in cirrus A length, which reaches ca. 1/3 of the body length. Gonopore absent.
Larvae (i.e. the first instar; measurements and statistics in Table 5). There is a slight overlap in body length ranges of larvae and juveniles, yet otherwise the dorsal plate sculpturing developed as in older life stages ( Fig. 7C View FIGURE 7 ). Body appendage formula: A-D-Dd. No gonopore and anus.
Eggs. Up to six orange eggs per exuvia.
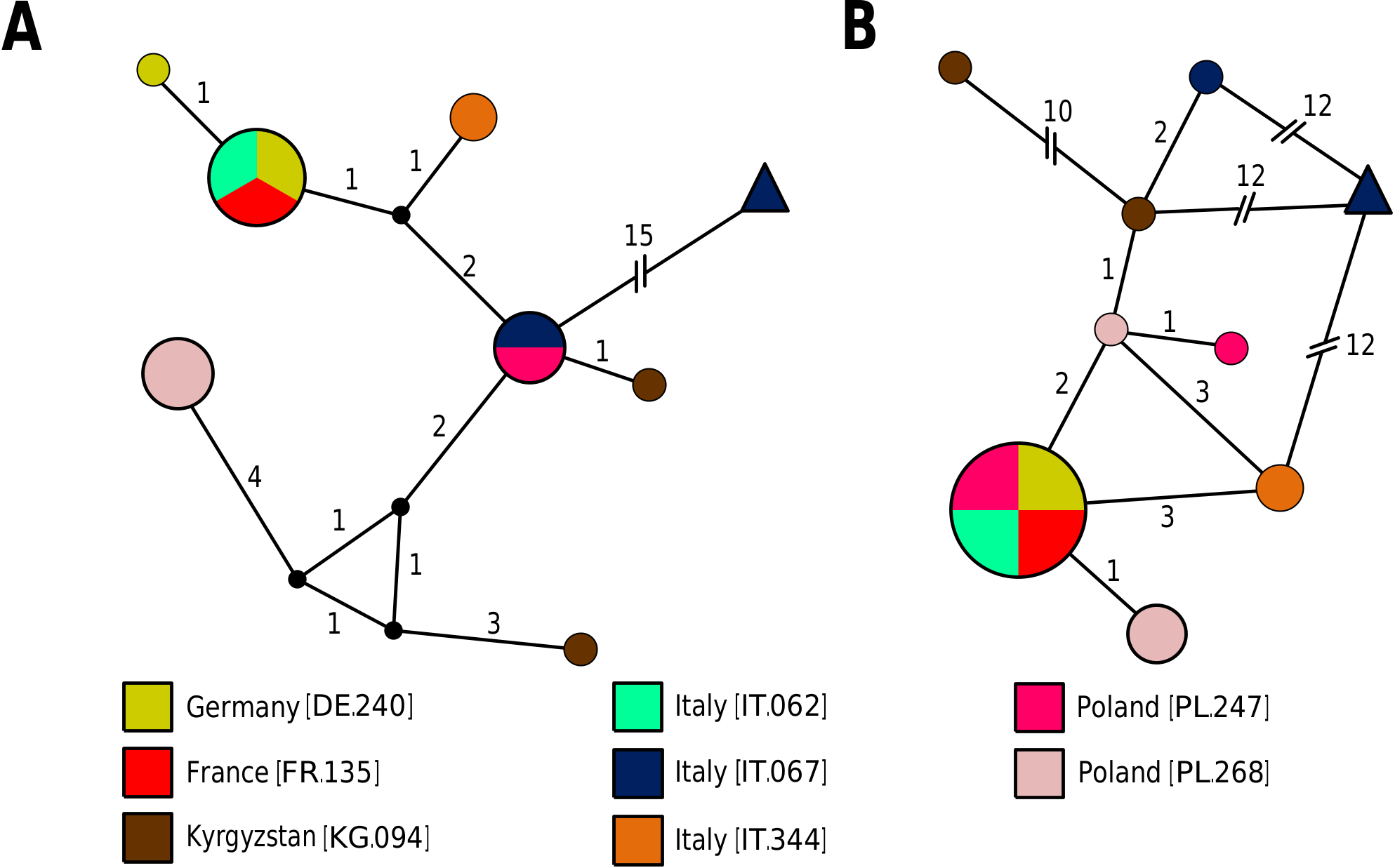
Phylogenetic position: Echiniscus granulatus is most closely related to E. militaris Murray, 1911 , and both species constitute a sister group of E. quadrispinosus (Figs 1–2). The genetic variability between Eurasian populations of E. granulatus is low ( Fig. 12 View FIGURE 12 ), and the ITS-2 haplotype network supports a probable incomplete lineage sorting between Echiniscus granulatus and E. militaris ( Fig. 12B View FIGURE 12 ), evidenced in the phylogenetic reconstructions (Figs 1–2).
Differential diagnosis: Echiniscus granulatus is similar to members of the blumi-canadensis complex with respect to the dorsal sculpturing, but it differs from them by higher and less massive claws, the lack of secondary/ tertiary spurs, and overall smaller body size. Other species with the similar polygonal pores can be distinguished from E. granulatus on the basis of:
• E. elaeinae Pilato et al., 2005 , a likely New Zealand endemic: the presence of long spine E (only a triangular spicule in E. granulatus ), similarity in lengths between lateral and dorsal appendages (lateral cirri typically much longer than dorsal spines in E. granulatus ), and weakly developed, minute spine on leg I (a typically developed spine in E. granulatus );
• E. jamesi Claxton, 1996 , a likely Australian endemic: different chaetotaxy (A-C-D-E in E. jamesi vs A-(B)- C-Cd- D-Dd in E. granulatus ), much lower claws (claws I 10.3–14.1 μm, claws IV 10.8–15.7 μm in E. jamesi vs claws I 15.7–19.0 μm, claws IV 19.2–23.0 μm in E. granulatus ), and more pronounced secondary sexual dimorphism (longer clavae and higher claws in males compared with females in E. jamesi vs no morphometric disparities between sexes in E. granulatus );
• E. militaris , a likely West Palaearctic endemic (see below): the presence of cirrus Bd (absent in E. granulatus ), the increment in thickness of dorsal appendages from Bd to Dd (Cd and Dd of equal thickness in E. granulatus ), and elongated, acute teeth in the dentate fringe IV (shorter and less prominent teeth in E. granulatus ).
Remarks: Males are recorded for the first time in this species, suggesting that parthenogenesis is facultative. Echiniscus granulatus is commonly found in the Mediterranean, and less frequent in other parts of Europe; it always inhabits carbonate bedrock ( Dastych 1980, 1988). The geographic range extends eastwards to as far as Mongolia and China ( McInnes 1994; Beasley & Miller 2007), but the species probably does not reach easternmost regions of the Palaearctic, like the Korean Peninsula or Japan ( Suzuki 2017). The presence of this species in the Nearctic region remains a matter of debate ( Maucci 1987; Kaczmarek et al. 2016), and can be now verified against the redescription performed herein. In our opinion, it is likely that E. granulatus occurs in the Canadian High Arctic as reported before, and we found it in the samples from Disko Island (western coast of Greenland); more southwards records are not well-documented and should not be treated as trustworthy. Historically, the identification of E. granulatus gained less attention of taxonomists than the problems associated with the blumi-canadensis complex or the distinction between E. merokensis and E. quadrispinosus , which is unfortunate given the high degree of variability in the development of chaetotaxy in this species. This evinces in three new synonymies established herein. Echiniscus granulatus inocelatus Mihelčič, 1938 ( E. inocelatus ) syn. nov., with an entangled taxonomic history (see Degma & Guidetti 2007), completely falls within the scope of intraspecific variability of E. granulatus . Echiniscus heterospinosus Maucci, 1954 syn. nov. could be distinguished from E. granulatus using two characters: (1) spines Dd significantly shorter than spines Cd, and (2) the presence of robust spines E ( Fig. 11A View FIGURE 11 ). However, given the highly unstable configuration of appendages and frequent asymmetries in E. granulatus , these two chaetotaxy traits cannot be taken as decisive. We aimed at collecting E. heterospinosus syn. nov. in the type locality in the Carnic Alps ( Maucci 1954) and in the vicinity of Trieste, where it was reported as relatively common (Maucci 1973–4), but in both cases we found only E. granulatus . Therefore, the former should be treated as a junior synonym unless re-sampled and the genetic data indicate otherwise. Finally, E. egnatiae Durante Pasa & Maucci, 1979 syn. nov. from Greece has an identical dorsal plate sculpturing as E. granulatus ( Figs 11B–D View FIGURE 11 ) and similar claw morphology (elongated claw branches with primary spurs formed exactly as in E. granulatus ; the original description says that secondary spurs are present on claws IV and sometimes on claws I–III, but those are frequently missing in type specimens and thus are an unstable character that cannot be a firm diagnostic criterion in this case); the only presumed differences with E. granulatus are shortened lateral appendages C and D, and, as in the case of E. heterospinosus syn. nov., spines Dd significantly shorter than spines Cd. We believe that in the case of such a variable species as E. granulatus , these minor dissimilarities do not warrant keeping E. egnatiae syn. nov. separate from E. granulatus . Admittedly, the shortening of spines Cd with respect to spines Dd is rare in E. granulatus , but not unknown. Echiniscus punctus McInnes, 1995 does not appear to be well-delimited from E. granulatus either, as all traits used in the original description fall within the intraspecific variability of the latter, but given its type locality is in the Southern Hemisphere (South Orkney Islands; McInnes 1995), we designate it as nomen inquirendum, requiring further analyses to unravel its status.
TABLE 2. Measurements [in µm] of selected morphological structures of adult females of Echiniscus granulatus mounted in Hoyer’s medium. Abbreviations: sp—the proportion between the length of a given structure and the length of the scapular plate.
| CHARACTER | N | RANGE | MEAN | SD | Neotype | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µm | sp | µm | sp | µm | sp | µm | sp | ||||||
| Body length | 15 | 211 | – | 279 | 447 | – | 576 | 254 | 514 | 17 | 39 | 262 | 534 |
| Scapular plate length | 15 | 42.7 | – | 54.6 | – | 49.6 | – | 3.5 | – | 49.1 | – | ||
| Head appendage lengths | |||||||||||||
| Cirrus internus | 14 | 10.9 | – | 13.5 | 20.9 | – | 31.6 | 12.3 | 24.9 | 0.9 | 3.0 | 11.1 | 22.6 |
| Cephalic papilla | 15 | 5.5 | – | 7.4 | 10.7 | – | 16.2 | 6.8 | 13.7 | 0.5 | 1.5 | 6.7 | 13.6 |
| Cirrus externus | 15 | 16.2 | – | 21.5 | 33.4 | – | 44.5 | 18.7 | 37.8 | 1.6 | 3.1 | 17.8 | 36.3 |
| Clava | 15 | 5.8 | – | 7.2 | 11.5 | – | 15.0 | 6.6 | 13.3 | 0.4 | 0.9 | 5.8 | 11.8 |
| Cirrus A | 15 | 35.9 | – | 85.0 | 76.2 | – | 191.0 | 66.8 | 135.2 | 11.8 | 26.5 | 68.0 | 138.5 |
| Cirrus A /Body length ratio | 15 | 17% | – | 35% | – | 26% | – | 4% | – | 26% | – | ||
| Body appendage lengths | |||||||||||||
| Cirrus C | 14 | 88.4 | – | 153.8 | 187.7 | – | 301.6 | 117.1 | 234.1 | 19.2 | 33.2 | 97.3 | 198.2 |
| Spine Cd | 15 | 19.6 | – | 38.5 | 42.5 | – | 70.5 | 27.8 | 55.9 | 5.5 | 9.5 | 24.6 | 50.1 |
| Cirrus D | 14 | 85.7 | – | 160.7 | 184.4 | – | 315.1 | 120.9 | 244.8 | 23.6 | 40.5 | 104.6 | 213.0 |
| Spine Dd | 15 | 20.7 | – | 36.1 | 38.3 | – | 70.5 | 28.8 | 58.2 | 4.4 | 8.7 | 30.4 | 61.9 |
| Spine on leg I length | 14 | 3.6 | – | 4.8 | 6.6 | – | 10.2 | 4.0 | 8.2 | 0.3 | 0.9 | 3.6 | 7.3 |
| Papilla on leg IV length | 15 | 4.5 | – | 6.0 | 8.8 | – | 11.8 | 5.2 | 10.5 | 0.5 | 1.0 | 5.7 | 11.6 |
| Number of teeth on the collar | 13 | 6 | – | 14 | – | 11.7 | – | 2.1 | – | 12 | – | ||
| Claw I heights | |||||||||||||
| Branch | 15 | 16.0 | – | 19.0 | 33.7 | – | 39.9 | 17.9 | 36.2 | 0.9 | 1.7 | 17.9 | 36.5 |
| Spur | 15 | 2.5 | – | 4.2 | 4.7 | – | 8.3 | 3.2 | 6.4 | 0.5 | 1.0 | 2.8 | 5.7 |
| Spur/branch height ratio | 15 | 13% | – | 23% | – | 18% | – | 3% | – | 16% | – | ||
| Claw II heights | |||||||||||||
| Branch | 15 | 15.3 | – | 18.3 | 31.3 | – | 36.7 | 16.9 | 34.2 | 1.0 | 1.7 | 16.9 | 34.4 |
| Spur | 15 | 2.5 | – | 3.6 | 5.0 | – | 7.7 | 3.1 | 6.2 | 0.4 | 0.9 | 2.7 | 5.5 |
| Spur/branch height ratio | 15 | 14% | – | 22% | – | 18% | – | 3% | – | 16% | – | ||
| Claw III heights | |||||||||||||
| Branch | 15 | 14.9 | – | 18.3 | 28.6 | – | 37.3 | 16.7 | 33.7 | 1.1 | 2.1 | 17.4 | 35.4 |
| Spur | 15 | 2.4 | – | 3.4 | 4.6 | – | 7.2 | 2.9 | 6.0 | 0.3 | 0.7 | 2.4 | 4.9 |
| Spur/branch height ratio | 15 | 14% | – | 22% | – | 18% | – | 2% | – | 14% | – | ||
| Claw IV heights | |||||||||||||
| Branch | 15 | 19.2 | – | 23.0 | 37.6 | – | 48.2 | 21.2 | 42.9 | 1.2 | 2.7 | 20.8 | 42.4 |
| Spur | 15 | 3.1 | – | 4.6 | 5.8 | – | 8.8 | 3.6 | 7.4 | 0.5 | 0.9 | 3.7 | 7.5 |
| Spur/branch height ratio | 15 | 13% | – | 23% | – | 17% | – | 3% | – | 18% | – | ||
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Echiniscus granulatus ( Doyère, 1840 )
| Gąsiorek, Piotr & Vončina, Katarzyna 2023 |
Echiniscus egnatiae
| Durante Pasa & Maucci 1979 |
Echiniscus abanti
| Maucci 1973 |
Echiniscus inocellatus Mihelčič, 1964
| Mihelcic 1964 |
Echiniscus heterospinosus
| Maucci 1954 |
Echiniscus granulatus inocelatus Mihelčič, 1938
| Mihelcic 1938 |
Echiniscus fortis Bartoš, 1935
| Bartos 1935 |
Echiniscus crassus
| Richters 1904 |
Emydium granulatum
| Doyere 1840 |
Emydium granulosum Doyère, 1840
| Doyere 1840 |