Protospathidium lepidosomatum, Foissner & Wolf & Kumar & Quintela-Alonso, 2014
|
publication ID |
https://doi.org/ 10.4467/16890027AP.14.015.1596 |
|
persistent identifier |
https://treatment.plazi.org/id/7F52878B-FFC4-FFAA-FF54-8ACDFBEB0788 |
|
treatment provided by |
Tatiana |
|
scientific name |
Protospathidium lepidosomatum |
| status |
sp. nov. |
Protospathidium lepidosomatum nov. spec. ( Figs 4a–o View Figs 4 ,
5a–k View Figs 5 , 6a–g View Figs 6 ; Tables 2, 3)
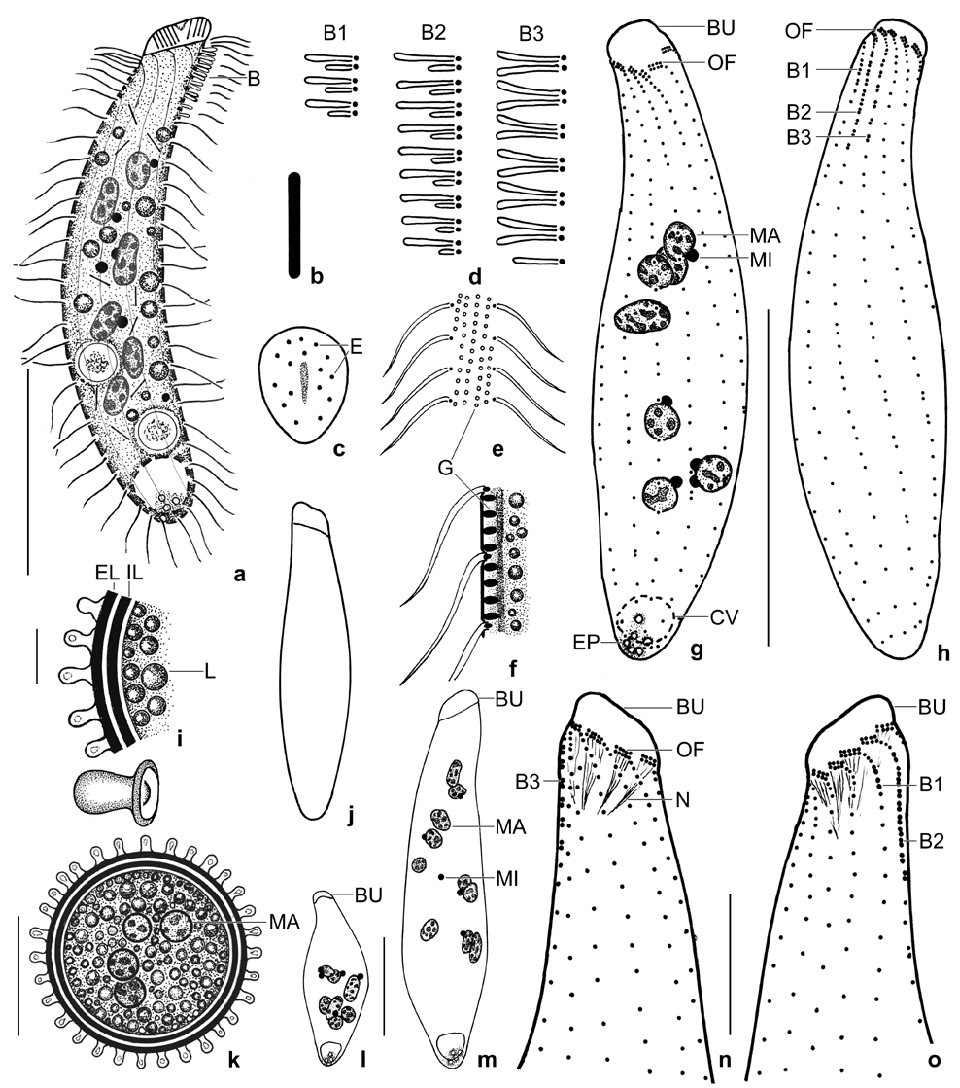
Diagnosis: Size about 70 × 15 µm in vivo; narrowly spatulate. On average 9 scattered, broadly ellipsoidal macronuclear nodules and 5 globular micronuclei. Contractile vacuole in rear body end. Extrusomes rod-shaped with rounded ends, in vivo about 3 × 0.3 µm in size. On average 11 ciliary rows. Dorsal brush distinctly heterostichad, row 1 shorter than the longest row 2 by about 58%, composed of an average of 3 dikinetids; rows 2 and 3 of similar length, composed of an average of 10 and 7 dikinetids, respectively. Oral bulge oblique, flat, obovate, on average 9 µm long in vivo. Resting cyst surface studded with 1–2 µm high, nipple-shaped lepidosomes.
Type locality: In tank bromeliads from the “Upper Cedar Valley”, southern slope of the Blue Mountains, Jamaica, 18°2′N 76°34′W GoogleMaps .
Type material: 1 holotype and 2 paratype slides with protargol-impregnated specimens have been deposited in the Biologiezentrum of the Oberösterreichische Landesmuseum in Linz (LI), Austria. Relevant specimens have been marked by black ink circles on the coverslip .
Etymology: Composite of Greek nouns lepidotos (scaled) and soma (body), referring to the lepidosomes on the cyst surface.
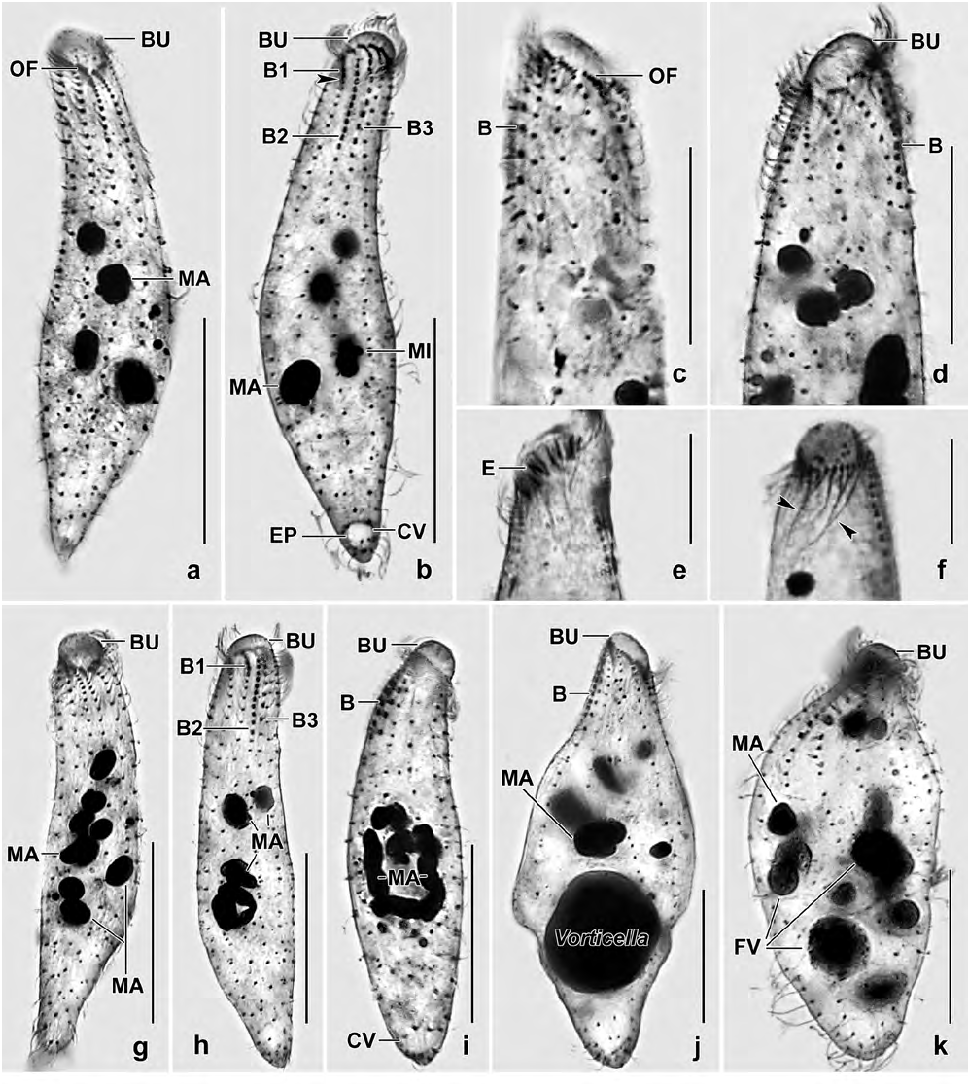
Description: Size and length:width ratio rather variable (CV ~ 15%) due to slender theronts and thick trophonts ( Table 2); in vivo 50–80 × 10–20 µm, on average 70 × 15 µm; in protargol preparations 63 × 16 µm and 72 × 18 µm when adding 15% for preparation shrinkage. Usually narrowly, rarely very narrowly spatulate and slightly curved dorsally or strongly inflated by food vacuoles ( Fig. 5k View Figs 5 ). Anterior end oblique, about 8 × 6 µm in protargol preparations, posterior end narrowly rounded to slightly acute ( Figs 4a, g, h, j, l, m View Figs 4 , 5a, b, g–i View Figs 5 ). Four to 14, on average nine globular to ellipsoidal macronuclear nodules, most in middle third of body; nucleoli 1–3 µm across ( Figs 4a, g, l, m View Figs 4 , 5a, d, g, k View Figs 5 ). Post-dividers with a nodulated macronuclear strand or a mixture of nodules and short strands ( Fig. 5h, i View Figs 5 ), as expected in multinucleate species ( Foissner et al. 2002); some post-conjugants with two macronuclear nodules. Two to eight, on average five micronuclei 1–2 µm across scattered among macronuclear nodules ( Figs 4a, g View Figs 4 , 5b View Figs 5 ). Contractile vacuole in rear body end, on average four excretory pores ( Figs 4a, g View Figs 4 , 5b View Figs 5 ). Extrusomes packed in oral bulge and scattered in cytoplasm, in vivo rod-shaped with rounded ends and about 2.5–3 × 0.3 µm in size; both oral bulge and cytoplasmic extrusomes sometimes impregnate with the protargol meth- od used ( Figs 4a–c View Figs 4 , 5e View Figs 5 ; Table 2). Cortex very flexible, gelatinous, about 0.8 µm thick, cortical granules rather strongly refractive and arranged in about four rows between each two kineties, about 0.5 × 0.25 µm in size ( Figs 4e, f View Figs 4 ). Cytoplasm with rather many lipid droplets 1–3 µm in diameter and food vacuoles with unidentifi- able contents up to 10 µm across; one specimen with a Vorticella sp. about 20 µm in size ( Figs 5j, k View Figs 5 ). Swims rather rapidly.
Cilia in vivo 8 µm long, ordinarily spaced. On average 11 meridional, ordinarily spaced ciliary rows more densely ciliated anteriorly than posteriorly, arranged in typical Protospathidium pattern ( Foissner and Xu 2007). Dorsal brush distinctly heterostichad and isomorphic, row 1 with 1.5 µm long slightly inflated bristles, composed of 2–4 dikinetids, shorter than row 2 by about 60%. Rows 2 and 3 with 2 µm long slightly inflated bristles, composed of 9–12 and 4–8 dikinetids, respectively, of almost the same length with the longest row 2 occupying only 16% of body length on average. Row 3 with widely spaced dikinetids and a short posterior tail composed of 2–3 monokinetidal bristles; all rows with a short anterior tail of ciliated monokinetids ( Figs 4a, d, g, h, n, o View Figs 4 , 5a–d, g–i View Figs 5 ; Table 2).
Oral bulge about half as long as widest trunk region, oblique by about 25–45°, surface flat to slightly convex, obovate in ventral view, in protargol preparations on average 8 × 6 × 3 µm in size; studded with extrusomes ( Figs 4a, c, j, n View Figs 4 , 5e, j View Figs 5 ; Table 2). Circumoral kinety broadly elliptical, composed of dikinetidal kinetofragments attached obliquely to the respective ciliary rows and separated from each other by gaps 1–3 dikinetids wide; kinetofragments composed of 3–6 dikinetids each associated with an 8 µm long cilium and a short nematodesma. Oral basket composed of cuneate, about 10–15 µm long nematodesma bundles originating from oral kinetofragments ( Figs 4g, h, n, o View Figs 4 , 5a–d, f, g View Figs 5 ).
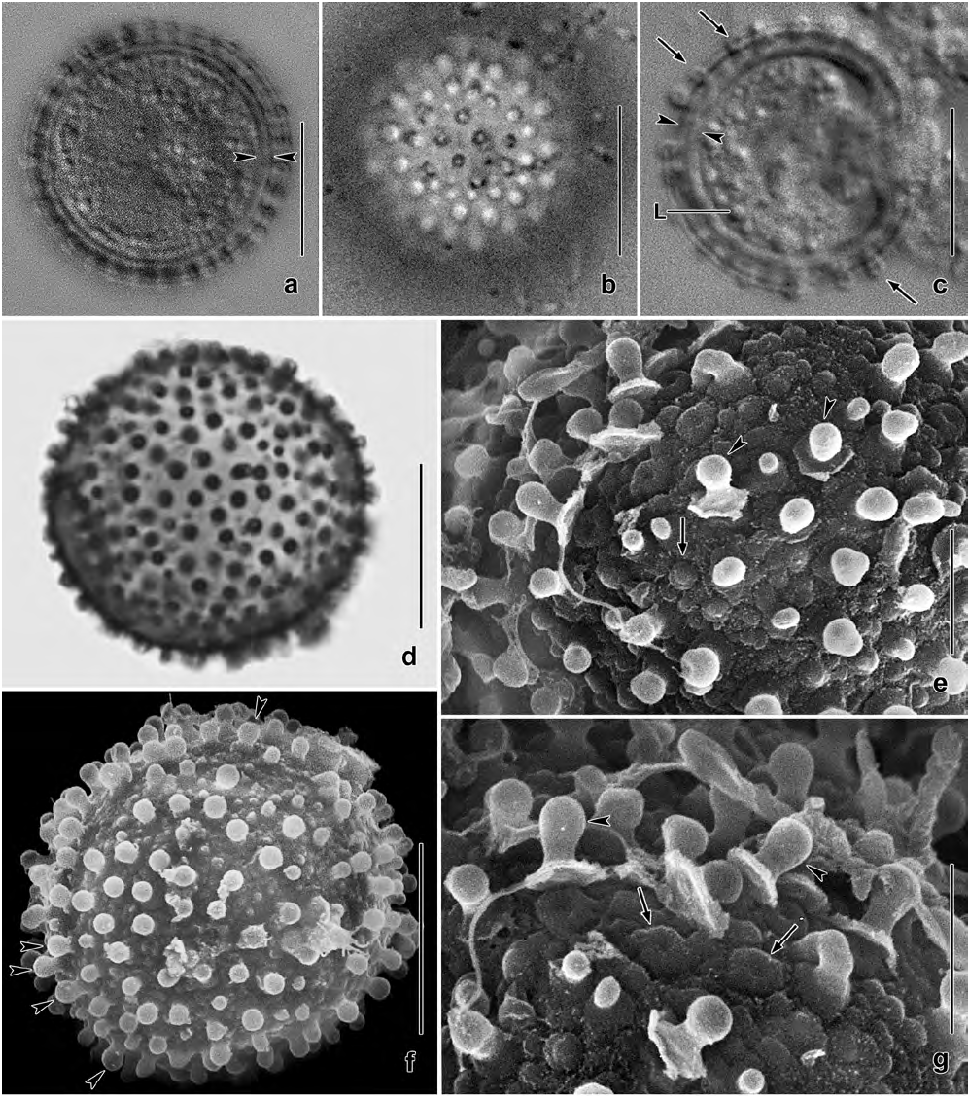
Resting cysts very refractive due to the lepidosomes and the thick wall, in vivo 15–25 µm across, on average 20 µm with lepidosomes included ( Table 2). Cyst wall yellowish, about 2–2.5 µm thick including lepidosomes, composed of an external and an internal layer both structureless in the light microscope. Cyst surface studded with 1–2 µm high, usually nipple-shaped lepidosomes attached to minute wall convexities and having a less refractive centre of varying size; impregnate with protargol. Cyst contents close to wall, composed of lipid droplets 1–3 µm across and globular macronuclear nodules faintly impregnated with protargol ( Figs 4i, k View Figs 4 , 6a–g View Figs 6 ).
Occurrence and ecology: Discovered in tank bromeliads from Jamaica, became moderately abundant in raw cultures. Surprisingly, later we found this species with its highly characteristic lepidosomes in a non-flooded Petri dish culture with leaf litter and surface soil from a park at the margin of the town of Salzburg, Austria.
Remarks: Protospathidium lepidosomatum is very similar to P. muscicola (for a review, see Foissner and Xu 2007), from which it differs by the nipple-shaped (vs. conical) lepidosomes covering the cyst wall. All other important features are near or within the variability of P. muscicola ( Table 3).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.