Gigiella platnicki Rix & Harvey, 2010
|
publication ID |
https://doi.org/ 10.3897/zookeys.36.306 |
|
publication LSID |
lsid:zoobank.org:pub:ADCACC88-6C78-4386-8E33-3F98234ECE92 |
|
DOI |
https://doi.org/10.5281/zenodo.3789440 |
|
persistent identifier |
https://treatment.plazi.org/id/AF683CEF-BF54-4140-9B26-C2AB85F9DEE1 |
|
taxon LSID |
lsid:zoobank.org:act:AF683CEF-BF54-4140-9B26-C2AB85F9DEE1 |
|
treatment provided by |
Plazi |
|
scientific name |
Gigiella platnicki Rix & Harvey |
| status |
sp. nov. |
Gigiella platnicki Rix & Harvey , sp. n.
urn:lsid:zoobank.org:act:AF683CEF-BF54-4140-9B26-C2AB85F9DEE1
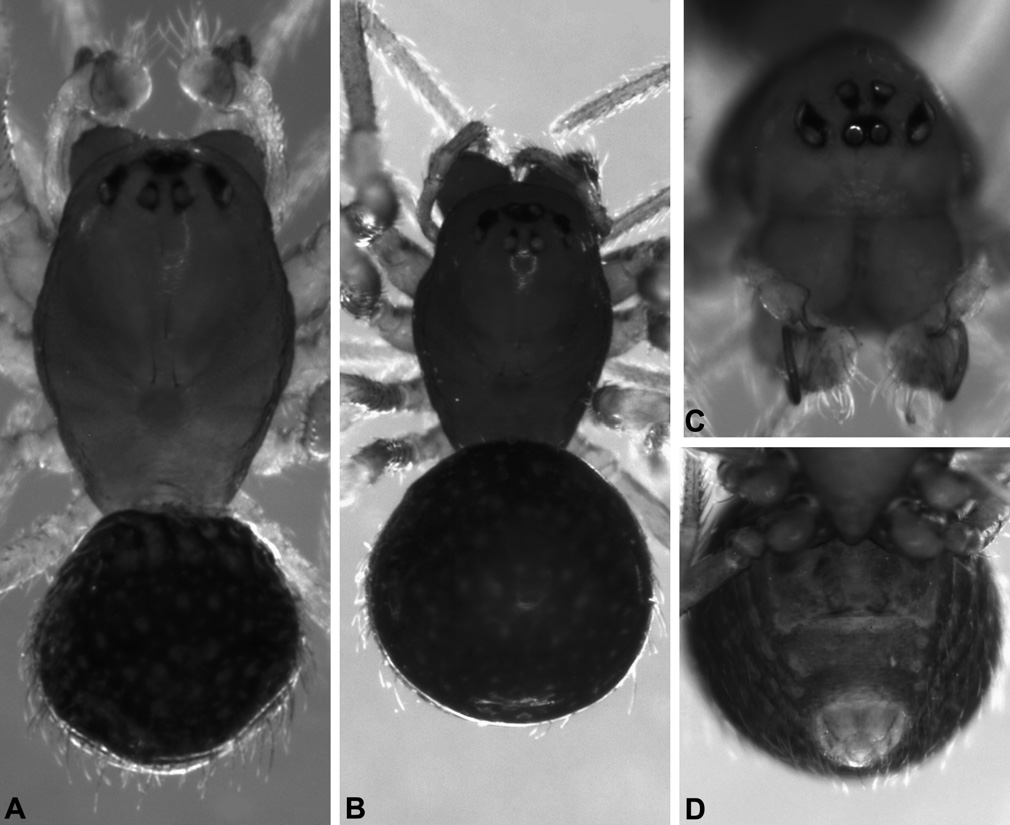
Figs 7R View Figure 7 , 193A–B, 193D, 193F, 195B, 197C–D, 203–209, 211
Type material. Holotype male: 102 km S. of Puerto Puyuguapi , Aisén province, Región Ibáñez del Campo, Chile, 220 m, wet forest, 19.I.1986, N. Platnick, P. Goloboff, T. Schuh ( AMNH).
Paratypes: Allotype female, same data as holotype ( AMNH); 1 male and 7 females, same data as holotype (AMNHSEM♁ ♀) .
Other material examined. CHILE: Región XI (Ibáñez del Campo): Aisén: Parque Nacional Queulat, near Puerto Cisnes , 500 m, wet forest, 6.II.1985, N. Platnick, O. Francke, 1♁ ( AMNH). Región X (Los Lagos): Chiloé : Chiloé Island , 5 km N. of Quellon , 105 m, Berlese , modified forest, floor litter and moss, 1.XII.1981, N. Platnick, T. Schuh, 1♀ ( AMNH). Palena: 70 km S. of Chaitén, 500 m, wet streambank, moss Berlese, 16.I.1986, N. Platnick, P. Goloboff, T. Schuh, 1♀ ( AMNH) ; vicinity of Chaitén , 0–100 m, moss in forest, 7.XII.1981, N. Platnick, T. Schuh, 2♁ ( AMNH) ; 25–27 km N. of Chaitén, 40 m, wet virgin forest, moss Berlese, 17.I.1986, N. Platnick, P. Goloboff, T. Schuh, 1♁ ( AMNH). Valdivia : 34 km WNW. of La Unión , 700 m, mixed evergreen forest, 17.XII.1984 – 7.II.1985, S. & J. Peck, 1♀ ( AMNH) .
Etymology. The specific epithet is a patronym in honour Norman I. Platnick, of the American Museum of Natural History (New York), for his enormous contribution to the study of micropholcommatid spiders, and for collecting many specimens of this species, including the type series.
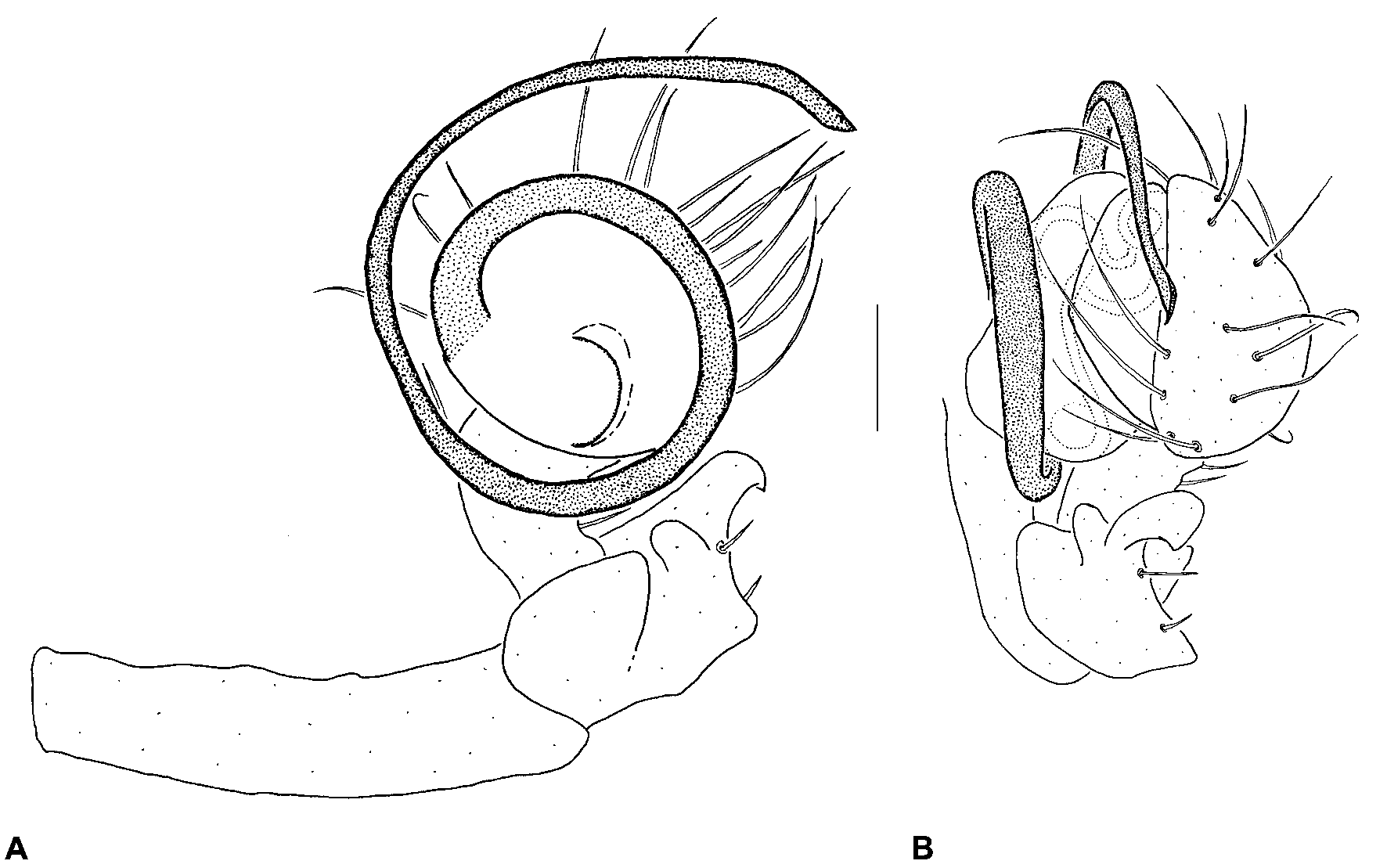
Diagnosis. Males of Gigiella platnicki can be distinguished from G. milledgei by the longer, coiled embolus ( Fig. 204 View Figure 204 ). Females can be distinguished from G. milledgei by the coiled insemination ducts (Figs 197C–D). Both sexes can also be recognised by the Chilean distribution (Fig. 211).
Description. Holotype male: Total length 1.41. Carapace 0.86 long, 0.59 wide. Abdomen 0.73 long, 0.59 wide. Leg I femur 0.88. Cephalothorax dark tan-yellow; legs tan-yellow; abdomen dark grey with paler sclerotic spots. Carapace raised anteriorly, fused to sternum via pleural sclerites; dorsal surface of pars cephalica slightly convex in lateral view. Eight subequal eyes present on anterior margin of pars cephalica; PME separated by slightly less than their own diameter. Chelicerae without bulging anterior projections; promargin without peg teeth. Legs relatively short (leg I femur-carapace ratio 1.02); macrosetae absent. Abdomen oval-globose, covered with hair-like setae, each seta projecting from small sclerotic spot; dorsal scute and lateral sclerotic strips absent. Pedipalpal patella with distally-directed, forked lRPA; tegulum smooth, with curved ETR; embolus long (length> 5× width), coiled 1.5x around margin of bulb ( Fig. 204 View Figure 204 ).
Allotype female: Total length 1.47. Carapace 0.76 long, 0.54 wide. Abdomen 0.94 long, 0.76 wide. Leg I femur 0.71. Cephalothorax brown; legs dark tan-yellow; abdomen dark grey with paler sclerotic spots. Carapace raised anteriorly, fused to sternum via pleural sclerites; dorsal surface of pars cephalica slightly convex in lateral view. Eight subequal eyes present on anterior margin of pars cephalica; PME separated by slightly less than their own diameter. Chelicerae without bulging anterior projections; promargin without peg teeth. Legs relatively short (leg I femur-carapace ratio 0.93); macrosetae absent. Abdomen oval-globose, covered with hair-like setae, each seta projecting from small sclerotic spot; dorsal scute and lateral sclerotic strips absent. Pedipalp entire, five-segmented. Epigyne with distinctive external morphology ( Fig. 203D View Figure 203 ); re- ceptacula globular, twisted; insemination ducts coiling around receptacula; fertilisation ducts looped (Figs 197C–D).
Distribution. Known only from southern Chile, in Región de los Lagos (Chiloé, Palena and Valdivia provinces) and Región Ibáñez del Campo (Aisén province) (Fig. 211).
Remarks. Gigiella platnicki is a relatively large species from the cool-temperate rainforests of southern Chile, in the region between Valdivia and Aisén (Fig. 211). It has mostly been collected from moss in wet, virgin forest, but nothing else is known of its biology.
Biogeography
As first suggested by Forster and Platnick (1981: 264), the taxonomy of the Micropholcommatidae “would be of greater value to biogeographic analysis if a more fully resolved classification were available”. Such a classification is now available, and with a newly proposed phylogenetic hypothesis for the family, the distribution of the Micropholcommatidae warrants biogeographic analysis under a cladistic framework. As discussed by Forster and Platnick (1981), and as highlighted by the results of the current study, temperate Chilean-Australian patterns are evident in several micropholcommatid genera, raising the question of whether Gondwanan vicariance could be responsible for these biogeographic patterns.
The southern-temperate micropholcommatid fauna. The Micropholcommatidae are a distinctively southern-temperate family (see Platnick 1991), with a south-eastern Australian centre of diversity, and over 80% of all known genera occurring within just 10 degrees of latitude, between 35°S and 45°S (Figs 210, 217). In the cool-temperate rainforests of the Otway Ranges, Yarra Ranges and Tasmania (Fig. 218), micropholcommatid spiders can be extremely abundant, and the Southern Beech ( Nothofagus cunninghamii ) forests of south-eastern Australia (Fig. 218D) are the only places in the world where 10 or 11 genera of Gigiellinae , Micropholcommatinae and Taphiassinae can be found living in close sympatry. Similarly, in extreme south-western Western Australia, the temperate Walpole region is the only area where all five Western Australian genera occur in sympatry, and two of these genera ( Austropholcomma and Normplatnicka ) are locally endemic (Fig. 214). Even in tropical New Guinea, New Caledonia and north-eastern Queensland, micropholcommatid species are largely restricted to montane habitats, which are cooler in climate, and home to a diversity of otherwise temperate taxa (Platnick 1991). In South America, micropholcommatid species have been collected only in the cooltemperate rainforests of southern Chile, and this fauna has a clear biogeographic connection to south-eastern Australia (see below). A very few micropholcommatid species have adapted to more xeric inland or tropical lowland habitats, however these species are the exception, and all such taxa have otherwise southern-temperate congeneric relatives.
The evidence for Gondwanan vicariance. Models of Gondwanan vicariance are a central tenet of ‘vicariance biogeography’ (see Brundin 1966; Platnick 1976; Nelson and Platnick 1981; Nelson and Ladiges 2001; Sanmartin and Ronquist 2004), generally invoked to explain the current distributions of Southern Hemisphere taxa by recourse to the continental rifting of Gondwana (see Li and Powell 2001; McLoughlin 2001). To address vicariant biogeographic hypotheses under a cladistic framework, ‘area cladograms’ can be constructed to reconcile the geographic distributions of taxa with their possible phylogenetic history ( Nelson and Platnick 1981), under a testable framework which assumes cladogenic events are congruent with the temporal order of vicariance ( Platnick 1976; Nelson and Platnick 1981). Vicariance biogeography is powerful in that concordant or ‘repetitious’ patterns can be compared across taxa ( Platnick 1976; Sanmartin and Ronquist 2004), and biogeographic hypotheses can be developed accordingly. Many studies have tested Gondwanan vicariant patterns using an area cladogram approach, in both plants (e.g. Swenson et al. 2001; Ladiges et al. 2003; Cook and Crisp 2005; Meudt and Simpson 2006) and animals (e.g. Griswold and Ledford 2001; Daniels et al. 2004; Sparks and Smith 2004; Kuntner 2006).
As suggested by Forster and Platnick (1981), the southern-temperate distribution of the Micropholcommatidae (Fig. 210) is amenable for exploring vicariant biogeographic hypotheses under the testable assumption that this distribution is the result of Gondwanan vicariance. Figure 217 summarises the phylogeny and biogeography of the 18 micropholcommatid genera with an area cladogram, illustrating those taxa found on different continental landmasses, and highlighting those clades with potentially vicariant Gondwanan patterns. Multiple micropholcommatid taxa in multiple subfamilies and tribes exhibit sister-group relationships on separate Gondwanan landmasses, with reciprocally-distributed Chilean-Australian (e.g. Gigiella ), Australian-New Zealand (e.g. Rayforstia ), Australian-New Caledonian (e.g. Taphiassa ) and Australian-New Guinean (e.g. Guiniella - Epigastrina / Eterosonycha ) clades, as highlighted (*) in Figure 217. Four genera in three separate lineages exhibit a remarkable Chilean-Australian distribution, and the Micropholcommatidae are one of only a few arachnid groups known to exhibit such a strong trans-Pacific connection between congeneric taxa (but see also certain Pseudoscorpiones, Hydracarina and Oribatida ) ( Harvey 1996, 1998a, b; Colloff 2009).
The case of the Textricellini is a further, compelling example of how phylogenetic patterns in the Micropholcommatidae are congruent with patterns of Gondwanan vicariance. The basal textricellin taxa in the genera Tinytrella , Eperiella and Algidiella are all restricted to New Zealand (the first of the eastern Gondwanan landmasses to separate; Li and Powell 2001; Sanmartin and Ronquist 2004), with more derived taxa in the genera Eperiella and Normplatnicka shared between Australia and South America, and terminal taxa in the ‘ Eterosonycha clade’ found only in Australia and montane Papua New Guinea (Fig. 217). This cladogenic pattern is largely congruent with the geological area cladogram of Sanmartin and Ronquist (2004, fig.1), and strong evidence for the Gondwanan ancestry of the textricellin clade. No other major micropholcommatid lineage exhibits such a characteristically Gondwanan phylogeny, and the Textricellini may yet prove to be one of the more striking examples of Gondwanan vicariance in the Arachnida. Interestingly, with the exception of an undescribed species of Raveniella from Western Australia (M. Rix, unpubl. data), species of Textricellini are entirely restricted to mesic, temperate habitats throughout their range, an observation which is consistent with a relictual, vicariant model of distribution for this group of spiders.
Other biogeographic models. In the case of the Micropholcommatidae , the evidence for Gondwanan vicariance is compelling (Fig. 217), and strong evidence to the contrary is required to invoke an alternative explanation for the distribution patterns observed. Models of ‘dispersal biogeography’ are often cited as alternatives to vicariance biogeography, under the assumption that widespread taxa may have dispersed in the past from former centres of origin ( Platnick 1976; Sanmartin and Ronquist 2004). One of the problems with dispersal biogeography is that such hypotheses are rarely falsifiable ( Sanmartin and Ronquist 2004) – taxa could of course, potentially, have travelled anywhere at any time – although with the advent of DNA sequencing, the molecular dating of clades is one way of approaching the dispersal-vicariance debate (e.g. see Buckley et al. 2009). For the Micropholcommatidae , no molecular dating data are currently available, and preliminary observations on live micropholcommatid specimens suggest that these spiders are ecologically restricted and highly prone to desiccation (see Natural History, below), rendering long-distance dispersal across the Pacific Ocean unlikely. Similarly, few convincing observations can be made regarding transoceanic distributions that might be explained by dispersal (e.g. see Vidal and Hedges 2009), with the exception that species of Rayforstia on Lord Howe Island and New Zealand may have had an Australian ancestor, if a vicariant Gondwanan biogeography is accepted for the tribe Textricellini (see Fig. 217). Similarly, the ancestor/s of species of Taphiassa on New Caledonia may also have dispersed there relatively recently, if a ‘Darwinian Island’ hypothesis is accepted for New Caledonia (see Grandcolas et al. 2008). Several other micropholcommatid taxa have been recorded from offshore islands (e.g. species of Patelliella , Taphiassa , Algidiella and Rayforstia on Lord Howe Island, Campbell Island and Auckland Island) (Fig. 210), although the way in which these taxa arrived is unknown, and in the case of all three islands an older vicariant or at least partially-vicariant (e.g. island ‘stepping-stone’) explanation is feasible (e.g. see Forster 1964; Buckley et al. 2009). As for most animals and plants, dispersal can never be completely ruled out for the micropholcommatid taxa, but a further discussion on this hypothesis requires calibrated molecular data and a more rigorous understanding of micropholcommatid ecology.
One other biogeographic model is also worthy of mention with respect to the Micropholcommatidae – that of Northern Hemisphere extinction, and a possible older, Pangaean origin for the group. This model is often overlooked with respect to southern-temperate taxa, but must be considered here given the well-documented past or present ‘bipolar’ distributions of certain Arachnida (e.g. pseudoscorpions of the families Syarinidae , Pseudogarypidae and Garypidae , and spiders of the family Archaeidae ; see Forster and Platnick 1984; Harvey 1998b; Penney 2003; Selden et al. 2008; Harvey and Št’áhlavský, in press), and given the recent description of a fossil micropholcommatid taxon from the Northern Hemisphere. Cenotextricella simoni was described by Penney et al. (2007) from the Eocene amber of the Paris Basin, France, and tentatively placed in the Micropholcommatidae . This placement was based on several characters typical of certain textricellin genera (see Taxonomy, above), although the morphology of Cenotextricella was described as being unlike that of any extant species ( Penney et al. 2007). Unfortunately, the affinities of Cenotextricella remain unknown, as several critical micropholcommatid characters cannot be determined using the X-Ray Computed Tomography method utilised by Penney et al. (2007), and no additional fossil specimens are known. As a result, the previous occurrence of micropholcommatid species in the Northern Hemisphere cannot be rejected nor confirmed, and a Pangaean hypothesis remains un-falsifiable in the absence of further taxa.
Natural history
The natural history of most micropholcommatid spiders remains poorly known, and there is much scope for future studies in this area. Hickman (1944, 1945) and Forster (1959) provided some important contributions regarding the webs and egg sacs of Micropholcommatinae , although there is still much to be determined for the 15 known genera. The biology of the Taphiassinae is now moderately well-known ( Fig. 223 View Figure 223 ), although webs of Taphiassa are still undescribed. The Gigiellinae and the Patelliellini are the least understood biologically of any Micropholcommatidae .
Web morphology and prey capture. The Micropholcommatidae are derived Araneoidea with a sheet- or tangle-web building ecology ( Hickman 1944; Forster 1959) – an observation supported by spinneret spigot morphology. All studied species of Textricellini build very small, horizontal, platform sheet-webs, often between leaflets of moss, on top of which they sit and wait for prey (M. Rix, pers. obs.; Hickman 1945; Forster 1959). Species of Rayforstia and Raveniella will readily build such webs in captivity (Fig. 221D), and the silk appears to be sticky (M. Rix, pers. obs.). Species of Micropholcomma , in contrast, have been shown to build irregular, three-dimensional tangle-webs, not unlike those constructed by certain Theridiidae (M. Rix, pers. obs.; Hickman 1944), and both textricellin and micropholcommatin species have been fed on small Collembola in captivity ( Hickman 1944, 1945). Taphiassine webs are poorlyknown, and only those of Olgania excavata have been described (see Taxonomy, above) ( Fig. 223B View Figure 223 ). Webs of Gigiellinae and Patelliellini are unknown.
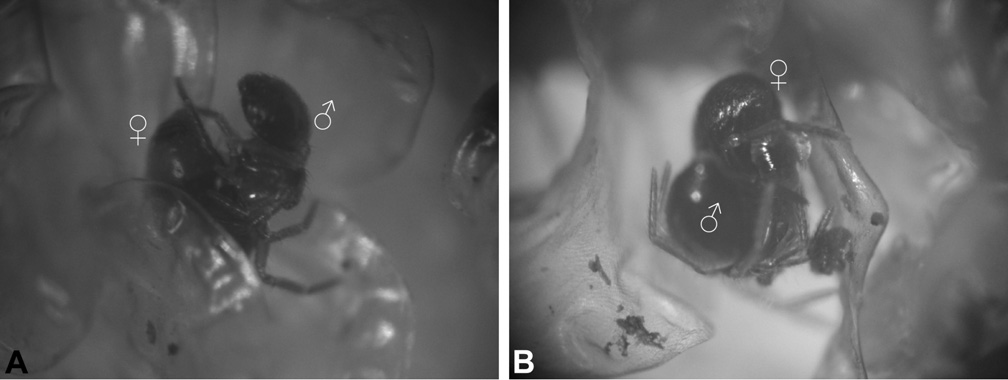
Courtship, mating and egg sacs. Knowledge of micropholcommatid reproductive biology is fragmentary, with only a few egg sacs and mating behaviours recorded. Courtship has never been described in any Micropholcommatidae , although it is highly likely that male stridulation plays an important role in most or all micropholcommatine species. Eterosonycha alpina has been observed mating in captivity: a male was photographed ‘suspended’ from a female by his inserted left pedipalp, which pulsated periodically as haemolymph was pumped under pressure (M. Rix, pers. obs.). The male hung motionless from the female throughout the mating process, even while the female walked over and through leaflets of moss ( Fig. 222 View Figure 222 ).
Egg sacs have been previously described for species of Micropholcomma and Raveniella , and egg sacs of Taphiassinae are newly-described. Micropholcomma parmatum builds a small, white, “pill-box shaped” egg sac which is attached to leaflets of moss ( Hickman 1944), and R. luteola also builds a small, white, “biconvex lens shaped” egg sac which is likewise attached to the substrate ( Hickman 1945); in Western Australia, similar egg sacs probably belonging to R. peckorum have been found attached to leaves in humus and to the underside of logs (M. Rix, pers. obs.). Two spiderlings hatched out of an egg sac made by M. parmatum (after 57 days), and egg sacs of R. luteola had three or four eggs inside ( Hickman 1944, 1945). In M. parmatum , females possess vestigial pedipalpal nubbins on the maxillae, and Hickman (1944) recorded that one of two spiderlings that hatched also had vestigial pedipalps, suggesting that micropholcommatid species with vestigial female pedipalps can be sexed at any age. Egg sacs are now known for species of Taphiassa and Olgania , with both taxa possessing a similar egg sac morphology ( Figs 223A, 223D View Figure 223 ). Taphiassine egg sacs are small and spherical, and composed of a thin layer of fine, loosely-woven elastic silk through which the eggs are usually visible. They are suspended by horizontal supporting-threads which are attached to the egg sac by drawn-out tufts of silk, and are hung either above the female’s sheet-web or nearby. Egg sacs seem to contain only a very small number of eggs (one in O. excavata and two in T. globosa ) ( Figs 223A, 223D View Figure 223 ), although females of O. excavata have been seen with multiple hanging egg sacs (M. Rix, pers. obs.).
Life cycle and general biology. Based on collection records, micropholcommatid spiders appear to be strictly seasonal, annual breeders in most of south-western Western Australia, where adult specimens are most easily found in the wet winter months of May to September (M. Rix, pers. obs.). Elsewhere in south-eastern Australia and New Zealand micropholcommatids seem less seasonal, with adults often present throughout the year ( Forster 1959). The generation-time of most Micropholcommatidae is unknown, although in south-eastern Australia and New Zealand it is likely that there is a strong overlap in generations. Many micropholcommatid populations seem loosely colonial, and specimens are often aggregated within small, favourable microhabitats (M. Rix, pers. obs.). Aerial ballooning has never been observed in any micropholcommatid taxon, and most species seem highly susceptible to desiccation; specimens of Raveniella peckorum , once removed from leaf litter, can die in an un-hydrated vial within several minutes (M. Rix, pers. obs.).
| AMNH |
American Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Micropholcommatinae |
|
Tribe |
Micropholcommatini |
|
Genus |