Forsterygion maryannae (Hardy, 1987)
|
publication ID |
https://doi.org/ 10.1080/00222930802256842 |
|
persistent identifier |
https://treatment.plazi.org/id/7C749926-7358-FF82-FE60-DDAFFD2BFBA3 |
|
treatment provided by |
Carolina |
|
scientific name |
Forsterygion maryannae (Hardy, 1987) |
| status |
|
Forsterygion maryannae (Hardy, 1987) View in CoL
Oblique-swimming triplefin
Tripterygion View in CoL sp. C Doak 1972, p. 105; Grace 1973, p. 17; Grace 1974, p. 23; Grace 1975, p. 98; Grace 1976, p. 104; Doak 1978, p. 105, Fig.; Housely 1980, p. 87; Nicholson and Roberts 1980, p. 141.
Tripterygion View in CoL n.sp. 6 Nicholson 1979, p. 137; Willan et al. 1979, p. 452.
Forsterygion View in CoL sp. 6 Anderson 1973, p. 22; Housely, Riddell and Grace 1981, p. 39. Forsterygion View in CoL sp. C Thompson 1981, p. 237–238, Fig.
Tripterygion View in CoL sp. 4 Ayling 1982, p. 281, pl. 40.
Tripterygion View in CoL sp. Paulin and Stewart 1985, p. 51; Roberts et al. 1986, p. 358. Tripterygiidae View in CoL g. and sp. Indet Hardy 1986b, p. 31;
Gen. and sp. Indet. (Ayling sp. 4) Hardy et al. 1987, p. 246.
Obliquichthys maryannae Hardy 1987, p. 53 –58, Figs 2–4 View Figure 2 View Figure 3 View Figure 4 ; Francis 1979, p. 50, pl. 129; Paulin et al., 1989, p. 220; Hardy 1990, p. 13–14.
Diagnosis
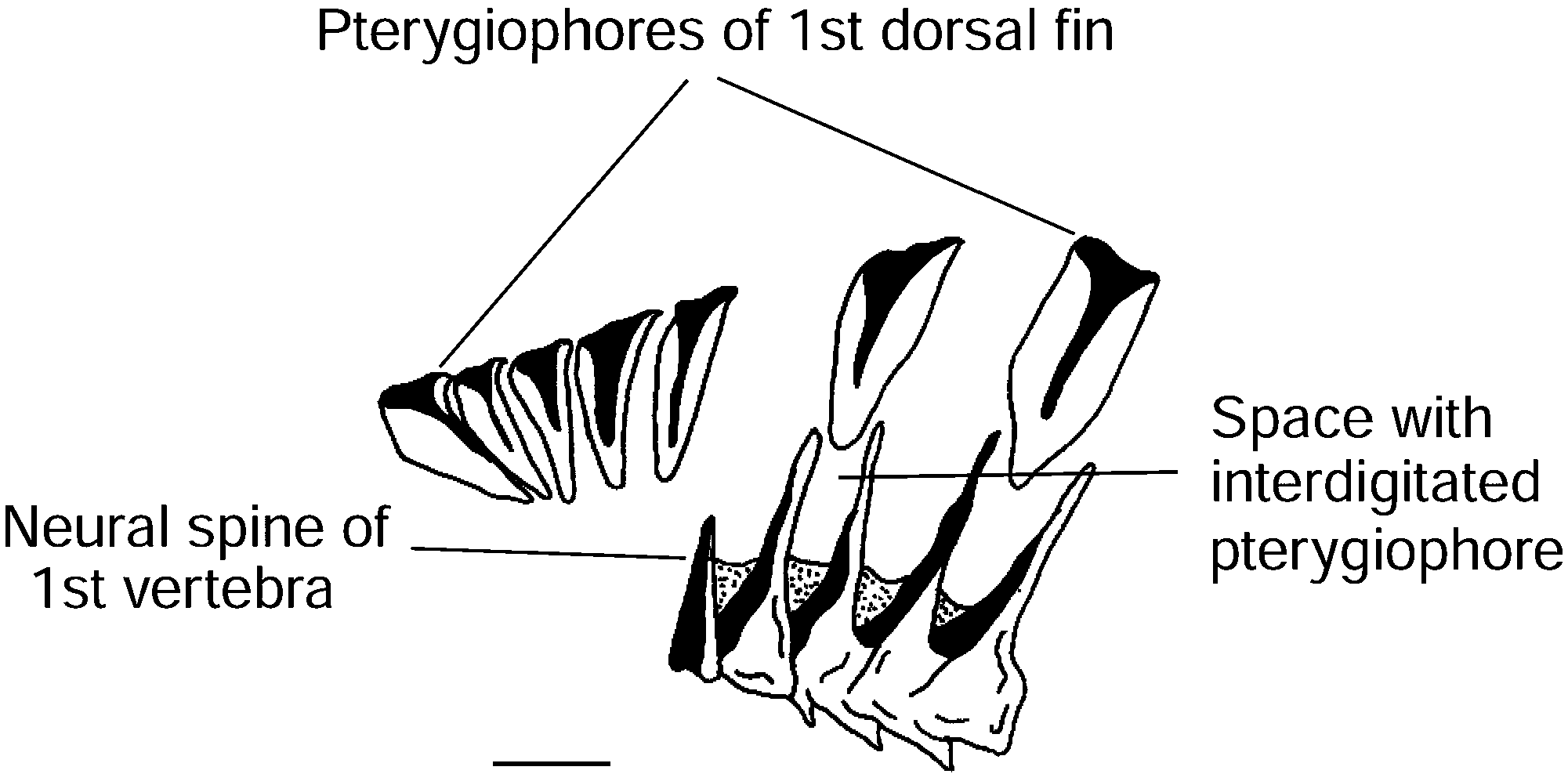
A moderately small tripterygiid fish compared to other New Zealand species, with the following set of characters: Body elongated, laterally compressed posteriorly; mouth strongly angled upward; notched scales absent; short, distally complex gill rakers, body yellow with a broad, black stripe extending along upper side of body.Discontinuous lateral line with 16–22 tubular scales. Anterior two spines of first dorsal fin shorter than remainder, which are of equal length. No sensory papillae on frontal, temporal and upper part of otic areas. Post-temporal partially serrated. Dorsal fin formula V-0N-0-1- 0-1 (33). Ten procurrent rays in both upper and lower caudal lobes; in the upper lobe, one procurrent ray between upper lobe and posterior epural, three rays opposite posterior epural, one ray between posterior and anterior epurals. Two opposite anterior epural, one opposite neural spine of second preural vertebra; in lower lobe, three rays opposite haemal spine of second preural vertebra, four rays between haemal spines of second and third preural vertebra, two rays opposite haemal spine of third preural vertebra and one anterior haemal spine of third preural vertebra.
Description
The following counts and proportions (given as times in standard length unless otherwise stated) are based on 62 non-type specimens of 27–53mm SL. F. maryannae differs from F. varium in the following aspects: Inter orbit slightly convex. Eyes dorso-laterally oriented. Snout blunt, rounded. Supraorbital tentacles small. Mouth strongly angled upwards, with symphysis of upper jaws level with midpoint of eye. Posterior margin of anterior nostril with small bifid flap. Moderately small species compared to other New Zealand species, but not to triplefins in general. Body somewhat elongate and becoming laterally compressed posteriorly. Anterior two spines of first dorsal fin shorter than remainder, which are of equal length.
First dorsal fin spines VI–VII; second dorsal fin spines XVIII–XXII; third dorsal fin rays 12–15; pectoral fin rays 16–18; anal fin with 0–1 spine and 24–27 soft rays. Discontinuous lateral line with 16–22 tubular scales and no notched scales. Total vertebral number 42–46.
Head length 4.0–4.3; predorsal length 4.9–5.9; preanal length 2.1–2.3; peduncle length 9.4–11.6; peduncle depth 11.1–12.5; pectoral fin length 4.0–4.5; eye diameter 2.8–3.2 in HL; upper jaw length 2.2–2.3 in HL; snout length 3.3–4.1 in HL.
Head sensory canals. Supratemporal canal with few small sensory pores. Preoperculo-mandibular canal with single sensory pore line. No sensory papillae on the frontal, temporal and upper part of otic areas. Ventral part of the
preoperculo-mandibular canal with six pores, three at lower corner of preoperculum, two at tip of upper jaw, one at lower jaw symphysis.
Squamation. Head naked and base of pectoral fin naked. Posterior part of belly covered with small cycloid scales. Two to three scale rows between anterior end of second dorsal fin and lateral line. Three scales between second and third dorsal fins. Twelve scales around caudal peduncle. Undulate scale margin. Number of radii in different body regions ranging between eight and twelve. One group of ctenii in scales 1–5 and the last scale, two groups in scales 6–21 of the lateral line scales. Denticles on inter-radial circuli, short, blunt and spaced. Pear-shaped focus with smooth area surrounded by incomplete circulus.
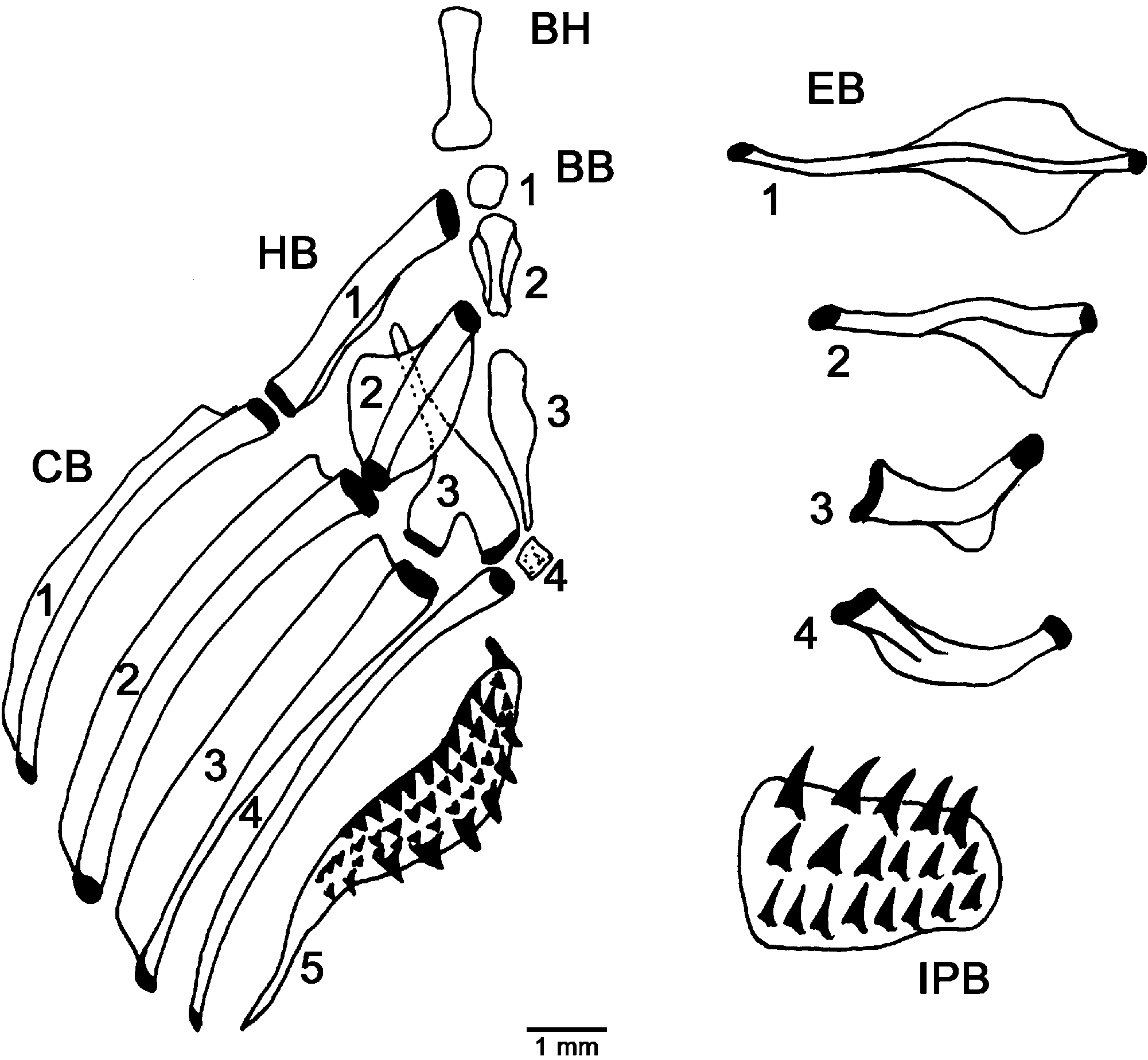
Osteology. Post-temporal smooth, partially exposed. Cartilaginous, incomplete interorbital septum. Third hypobranchial with broad and unseparated legs. Gill rakers on the anterior side of the first ceratobranchial. Epibranchials straight, first epibranchial with phalanges directed anteriorly and posteriorly. Adze-shaped urohyal (lateral view). Undeveloped basibranchial attachment. Pterygiophore supporting first segmented ray of third dorsal fin anterior to neural spine of twenty-sixth vertebra. One pterygiophore between second and third dorsal fins. Dorsal fin formula V-0N-0-1-0-1 (33). Short neural spine of second preural vertebra. Short, broad, pointed haemal spine of second preural vertebra. Ten procurrent rays in both upper and lower lobes; in the upper lobe, one procurrent ray between upper lobe and posterior epural, three rays opposite posterior epural, one ray between posterior and anterior epurals. Two opposite anterior epural, one opposite neural spine of second preural vertebra; in lower lobe, three rays opposite haemal spine of second preural vertebra, four rays between haemal spines of second and third preural vertebra, two rays opposite haemal spine of third preural vertebra and one anterior haemal spine of third preural vertebra.
Otolith. Posterior end rounded. Lateral surface smooth. Antirostrum broad. Crista superior absent.
Colour. Mid-lateral region of body with broad, black stripe extending from above pectoral fin base to the anterior half of caudal fin. Main body colour yellowish, sometimes faintly mottled, above lateral stripe; brownish-yellow with up to two or three faint and irregularly mottled longitudinal stripes below lateral stripe. Head, lips and mouth yellowish, belly very pale. Opercular region brownish. Eye black.
First dorsal fin mainly black with slight reddish-brown colouration just above base. Second dorsal fin base yellow. Third dorsal fin faintly reddish-orange on base, the remaining colourless. Anal fin similar to third dorsal fin. Pectoral fins yellowish or colourless. Pelvic fins colourless. Caudal fin with black posterior end.
Size. 58mm SL.
Distribution
Around the North and South Islands , from the Three Kings Islands to Stewart Island, including Snares Islands. Usually found over rocky reefs at depths of 1–50m. Usually seen schooling up to 5m above substratum, although often seen resting in crevices .
Material examined
Auckland University specimens (n 562): 5, 38– 41mm SL , North Cape, 28 Feb 1999 ; 18, 27– 47mm SL, Three Kings Islands , 1 Mar 1999; 21, 28– 54mm SL, Mokohinau Islands , 9 Dec 1997; 1, 35mm SL, Hen and Chicken Islands , 6 Feb 1997; 1, 51mm SL, Catherine Bay, Great Barrier Island , 4 Sept 1997; 6, 36– 50mm SL, Great Barrier Island , 4 Sept 1997; 8, 44– 53mm SL, Kaikoura , 15 Oct 1997; 2, 35– 51mm SL, Breaker Bay , Wellington , 9 Feb 1998.
Forsterygion nigripenne Valenciennes (In Cuvier and Valenciennes (1836)) Estuarine triplefin
Tripterygion nigripenne Valenciennes View in CoL in Cuvier and Valenciennes (1836, p. 413–414, pl. 339); Hutton 1904, p. 46 ( New Zealand); Waite 1907, p. 32; Darby 1966, p. 192– 195; McDowall 1990, p. 292, Fig.17.1, pl. 67.
Tripterygion nigripenne Richardson 1844, p. 211 View in CoL .
Tripterygion nigripenne Gunther 1861, p. 277 View in CoL .
Tripterygion nigripenne View in CoL (non Valenciennes in Cuvier and Valenciennes (1836)) Hutton 1872, p. 31, 354.
Tripterygion varium View in CoL (non Forster in Bloch and Schneider (1801)) Waite 1913, p. 7 (part). Enneapterygius varius Rendahl 1926, p. 10 View in CoL .
Tripterygion View in CoL sp. Woods 1963, p. 47.
Forsterygion nigripenne Anderson 1973, p. 3 View in CoL .
Forsterygion View in CoL sp. Paulin et al., 1989, p. 220.
Forsterygion nigripenne Paulin and Roberts, 1992, p. 91 View in CoL –92, Figs 44a, b, pl. 16 A–C. Grahamina nigripenne Fricke and Roberts 1993, p. 14 –16, Fig. 8.
Diagnosis
A species of Forsterygion with the following set of characters: Spines of first dorsal fin high, often as high as second dorsal, with posterior spines longer than anterior Supraorbital tentacles simple. No sensory papillae on frontal, temporal and upper part of otic areas. Dorsal fin formula V-0N-0-1-0-1 (34). Ten procurrent rays in upper caudal lobe, nine in lower lobe; in upper lobe, one procurrent ray between upper lobe and posterior epural, five opposite epurals, one between anterior epural and neural spine of second preural, two anterior to neural spine of third preural vertebra; in lower lobe, eight procurrent rays opposite haemal spine of second preural vertebra, one opposite haemal spine of third vertebra.
Description
The following counts and proportions (given as times in standard length unless otherwise stated) are based upon 29 non-type specimens of 33–86mm SL. F. nigripenne differs from F. varium in the following combination of characters: supraorbital tentacles simple. First dorsal high, often as high as second dorsal, with posterior spines longer than anterior.
First dorsal fin spines VI; second dorsal fin spines XVIII–XXI; third dorsal fin rays 11–13; pectoral fin rays 16–19; anal fin rays II, 24–26. Discontinuous lateral line with 14–18 tubular scales, 20–25 notched scales. Total vertebral number 42–44.
Head length 4.2–4.7; predorsal length 4.7–6.1; preanal length 2.2–2.6; peduncle length 1.6–1.8; peduncle depth 9.6–10.3; pectoral fin length 3.2–3.4; eye diameter 2.7–3.6 in HL; upper jaw length 2.6–3.5 in HL; snout length 3.1–3.4 in HL.
Head sensory canals. Otic canal extends posteriorly to operculum. Supratemporal canal with few small sensory pores. Preoperculo-mandibular canal with single sensory pore line. No sensory papillae on frontal, temporal and upper part of otic areas. Ventral part of the preoperculo-mandibular canal with nine pores, four at base of preoperculum, four at tip of upper jaw and one at lower jaw symphysis.
Squamation. Nape and base of pectoral fin naked. Areas around anus and genital pores surrounded with small cycloid scales. Five to six scale rows between anterior end of second dorsal fin and lateral line. Eight to nine scales between last pored scale and second dorsal fin. Two scales between second and third dorsal fins. Twenty to twenty-one scales around caudal peduncle. Scales with crenate anterior margin. Secondary radii present in scales from all body regions. Eight to thirteen radii in scales from caudal peduncle. Long ctenii in scales from area below lateral line and under pectoral fin. Lateral line canal, short, constricted. Two separated groups of ctenii on posterior side of lateral line scales. Denticles with narrow base. Tongue area filled with circuli. Oval focus with fine granules.
Osteology. Post-temporal smooth, not exposed. Club-shaped basihyal with straight anterior end. First basibranchial with two lobes close to each other. First hypobranchial curved, second not flared. Third hypobranchial separated by shallow gap. Phalanges on lateral end of first epibranchial directed anteriorly and posteriorly. Gill rakers oppositely arranged. Third epibranchial straight. Heartshaped urohyal (ventral view), bird’s head-shape (lateral view). Poorly developed, non-elevated basibranchial attachment. Pterygiophore supporting first segmented ray of third dorsal fin anterior to neural spine of twenty sixth vertebra. One pterygiophore between second and third dorsal fins. Dorsal fin formula V-0N-0-1-0- 1 (34). Straight ventral edge of upper hypural and ventral edge of lower hypural. Parhypural with rounded end. Small, rounded hole at junction of lower hypural base with parhypural and urostyle. Short, broad, neural spine of second preural vertebra. Similar width epurals. Fourteen soft rays in caudal fin, one unbranched and six branched (none multibranched) rays in both upper and lower lobes. Ten procurrent rays in upper caudal lobe, nine in lower lobe; in upper lobe, one procurrent ray between upper lobe and posterior epural, five opposite epurals, one between anterior epural and neural spine of second preural, two anterior to neural spine of third preural vertebra; in lower lobe, eight procurrent rays opposite haemal spine of second preural vertebra, one opposite haemal spine of third vertebra.
Otolith. Dorsal depression absent. Pointed rostrum. Broad, long antirostrum.
Colour. Body grey-brown to grey-green with series of yellow blotches running along the central part of body. Darker blotches dorsolaterally, sometimes extending to base of dorsal fins. Head with yellowish mottling extending from dorsal surface and covering the upper lip. Preoperculum greenish with yellow edge. Operculum reddishbrown with pale posterior edge. First dorsal fin with black edge and yellowish broad band at the middle, reddish-brown at the base; fin rays pale to yellow. Second dorsal fin similar in colouration to first dorsal, sometimes darker lacking yellowish colour, fin rays transparent or yellow. Third dorsal fin dusky distally, sometimes with black and yellow spots at base or covering two-thirds length of fin rays. Pectoral fin with black spots and dark arc at base. Anal fin with white edge, membrane white, sometimes dusky especially distally. Breeding males may be very dark.
Size. 86mm SL.
Distribution
Throughout New Zealand, from Northland to Stewart Island , including Chatham Islands. It is common in estuaries and tidal lower reaches of rivers and streams where there is cover from rocks, submerged trees, etc .
Material examined
Museum specimens (n 529): NMNZ P. 1327, 5, 55–84mm SL., Wanganui River Estuary, Apr 1950 ; NMNZ P. 27830, 10, 40–69mm SL., Mill Creek, Half moon Bay , Stewart Island , 3 Mar 1992 . Auckland University specimens: 2, both 55mm SL, Pataua Estuary, Ngunguru Bay , 3 Aug 1999 ; 12, 33– 86mm SL, Whangateau Wharf, Whangateau Estuary , 13 Dec 1999 .
Morphological variation within F. capito and the status of T. jenningsi
Among the 16 fish specimens collected by E. Jennings from the Auckland Islands, Hutton (1879) described six specimens as Tripterygium jenningsi ( Jenyns, 1841) . Although he did not indicate where the type specimens were deposited, they were most likely originally in the Otago Museum. Hardy (1989) discussed the identity of Hutton’s specimen of T. jenningsi and recognized it as belonging to a new genus. Fricke and Roberts (1993) suggested that the common Auckland Islands Grahamina belonged to the same species as the New Zealand mainland G. capito , and therefore referred it to their new genus Grahamina , designating NMNZ P. 20546 as the lectotype ( Russell 1996).
Specimens of F. capito collected by K. D. Clements, Auckland University from Lyttleton and Akaroa Harbours, Banks Peninsula in August 2001 appeared to be morphologically distinct in both the ventral head profile and the presence of a ridge at the base of the first two dorsal fins. These Banks Peninsula specimens were found at that time to be genetically distinct from populations of F. capito from other parts of the South Island and North Islands ( Hickey and Clements 2005), raising the question of their relationship to Hutton’s specimens of T. jenningsi from the Auckland Islands. These observations led us to undertake a detailed examination of F. capito populations, with a particular focus on specimens from southern New Zealand. This led to the identification of three groups that were more or less distinct morphologically. Group I included specimens from the Auckland Islands, Antipodes Islands and some specimens from Akaroa. Group II included other Akaroa specimens and specimens from Lyttletton and Akaroa Harbours, Banks Peninsula, and Portobello, Otago Harbour. Group III included all of the other F. capito populations from New Zealand.
Several morphological characters were used in the comparison of the three population groups: body proportions; anal fin position relative to caudal fin; upper jaw extension relative to eye; nape squamation; presence or absence of a dorsal ridge, arising under the origin of the first dorsal fin and extending to the second dorsal fin; otolith morphology and some osteological characters such as shape of the operculum and urohyal. Some of the above mentioned characters allowed the separation of a few specimens of the three population groups, but these morphological differences tended to be variable and inconsistent within the three groups of F. capito . Specimens of group II could be distinguished reliably from group III by the presence of the dorsal ridge in the former. However, since a dorsal ridge also occurred in a few group I specimens this character could not be used to distinguish between groups I and II.
These observations raised the possibility that G. capito could consist of two or more species. However, all adult specimens examined from the Auckland Islands and Antipodes Islands were long-term museum material with faded colour patterns, precluding the used of colour in comparing them to the remaining F. capito populations. More specimens are required, especially from the former locations, to investigate properly the relationships between these populations of F. capito in terms of both colour pattern and genetic isolation. At the time of writing, only three juvenile F. capito specimens suitable for DNA sequencing have been obtained from the Auckland Islands (collected by B. Doak April 2002). This low number of specimens makes it very difficult to reach robust conclusions about the genetic relationships of these populations, other than group I (represented by three specimens) and group II being distinct from group III, but not from each other. Morphologically, the three groups cannot be consistently and reliably separated. Therefore, resolving the taxonomic status of these populations of F. capito awaits further investigation when more specimens become available.
F. capito–F. varium hybrids
Among samples of triplefin fishes collected from Doubtful Sound (Fiordland), the Catlins coast (Southland), Portobello (Otago Harbour) and Bluff wharf were found a number of specimens that exhibited characters intermediate between F. capito and F. varium . These samples also included F. capito , but not F. varium , although the latter species was observed at Bluff Wharf.
Although hybridization and introgression among fish species are well documented ( Verspoor and Hammar 1991; Krupp et al. 1992; Leary et al. 1995), no previous case of triplefin hybridization has been fully documented. Fricke (1994) suggested that F. capito and G. signata hybridized at the Chatham Islands, but a reexamination by Clements et al. (2000) suggested that all of the specimens involved were F. capito .
A total of 15 specimens of the apparent F. varium / F. capito hybrid were collected and preserved in 70% ethanol. Their hybrid status was first recognized genetically by their anomalous mitochondrial control region sequences ( Hickey and Clements 2005).
The hybrids are moderately large (44.8–67.3mm SL), with the following characteristics: Snout non-steep, angled (30 °). Dorsal fin spines V–VII; second dorsal fin spines XX–XXI; third dorsal fin rays 14–15; pectoral fin rays 16–17; anal fin rays I, 26–28; total vertebral number 43–46. Discontinuous lateral line with 18–20 tubular scales, 13–23 notched scales. Head naked. Membrane between first and second dorsal fins completely incised. First dorsal fin low. Spines of first dorsal fin of similar thickness and length to each other. Lateral line arched over pectoral fin, extending to membrane between 16–17 spines of second dorsal fin. Supraorbital tentacles with two branches (rarely simple). Upper jaw sloped (25 °), extending to below anterior one-third of eye. Preserved specimens of the apparent hybrids look darker than F. capito , especially the first dorsal fin, anterior half of the second dorsal fin and the dorsal aspect of the head.
Putative hybrids were compared to 137 specimens of F. capito and 68 specimens of F. varium . Seven meristic and nine morphometric characters were considered in addition to the head sensory canal pattern. F. capito and F. varium showed differences in the range of two meristic characters (second dorsal fin spines, lateral line tubular scales) and overlapped in five other characters ( Tables 9–13). In morphometric characters, these two species overlapped in seven out of nine characters studied ( Table 14). The hybrid characters showed a range of variation between F. capito and F. varium . This is also the case in hybrids of other fish species, where characters are not necessarily systematically distributed between the parent species ( Krupp et al. 1992). In the meristic characters, F. capito overlapped with the hybrid in four characters, two were intermediate between the two parental species, and one was similar to both parental species ( Tables 9–13). In morphometric characters, the hybrid overlapped with F. varium in two characters, overlapped with F. capito in two characters, overlapped with both parental species in two characters, and was distinct from both parental species in three characters ( Table 14). In general appearance, on the basis of the pattern of head sensory canals, five out of six otolith characters and five osteological characters, the hybrid is closer to F. capito . The hybrid specimens are highly unlikely to represent extreme examples of either F. capito or F. varium , since several characters were outside the normal range for these two species. The morphological characters together with the molecular results provide strong evidence of a hybrid origin.
| NMNZ |
Museum of New Zealand Te Papa Tongarewa |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Forsterygion maryannae (Hardy, 1987)
| Jawad, Laith A. 2008 |
Tripterygion
| Jawad 2008 |
Forsterygion
| Jawad 2008 |
Forsterygion
| Jawad 2008 |
Tripterygion
| Jawad 2008 |
Tripterygion
| Jawad 2008 |
Tripterygion
| Jawad 2008 |
Forsterygion
| Jawad 2008 |
Grahamina nigripenne
| Fricke and Roberts 1993: 14 |
Forsterygion nigripenne
| Paulin and Roberts 1992: 91 |
Obliquichthys maryannae
| Hardy 1987: 53 |
Forsterygion nigripenne
| Anderson 1973: 3 |
Tripterygiidae
| Whitley 1931 |
Enneapterygius varius
| Rendahl 1926: 10 |
Tripterygion nigripenne
| Gunther 1861: 277 |
Tripterygion nigripenne
| Gunther 1861 |
Tripterygion nigripenne
| Richardson 1844: 211 |