Thor dicaprio, Anker & Baeza, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5039.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:FE3CBE11-8E0E-4DB7-B122-1A0B47DFC87E |
|
persistent identifier |
https://treatment.plazi.org/id/7C51094E-195A-FFF5-AEBC-FB17501453E8 |
|
treatment provided by |
Plazi |
|
scientific name |
Thor dicaprio |
| status |
sp. nov. |
Thor dicaprio sp. nov.
( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 , 6A View FIGURE 6 )
Thor amboinensis . — Chace 1972: 130, figs. 55, 56; Herrnkind et al. 1976: 65; Criales et al. 1984: 313; Sterrer 1986: 327, pl. 9, fig. 11 (colour photograph); Wicksten 2005a: 32; Wicksten 2005b: 104, fig. 4. pl. 1, fig. B; Kuiter & Debelius 2009: 127 (part., colour photographs from the western Atlantic); Felder et al. 2009: 1059; Baeza & Piantoni 2010: 152, fig. 1b–d; Briones-Fourzán et al. 2012: 61; Humann et al. 2013: 115 (colour photograph); De Grave & Anker 2017: 33; Colombara et al. 2017: 3; Poupin 2018: 126 [not Thor amboinensis ( De Man, 1888) ].
(?) Thor paschalis . — Schmitt 1924a: 70; Schmitt 1924b: 82; Schmitt 1935: 151; Schmitt 1936: 370 [not Thor paschalis ( Heller, 1862) ].
Type material. Holotype: ovig. female (pocl 3.4 mm), FLMNH UF 32256 , French Antilles , Saint Martin, Rocher Créole (Creole Rock), 18°07’02.3”N, 63°03’25.7”W, shallow coral reef, depth less than 5 m, leg. A. Anker, J. Slapcinski, J.P. Maréchal, G. Paulay, A. Bemis and F. Michonneau, 18.04.2012 [fcn BSTM-1118] GoogleMaps . Paratypes: 1 male (pocl 2.25 mm), USNM 136412 About USNM , Trinidad and Tobago, Tobago, Buccoo Reef, Smithsonian-Bredin Caribbean Expedition, 04.04.1959 [not examined, see above]; 1 ovig. female (pocl 3.5 mm), OUMNH. ZC. 2011-06-051, Mexico, Quintana Roo, Isla Cozumel , 1 km south of Playa Corona , 20°25’48.05”N, 87°00’27.09”W, shallow sand flat with sea grass, algae, overgrown corals, some fan corals and abundant rubble, depth less than 1.5 m, leg. A. Anker & J. Duarte-Gutiérrez, 09.07.2010 [fcn COZ3-012] GoogleMaps .
Additional material. 2 males (pocl 1.3, 1.8 mm), 2 ovig. females (pocl 2.1, 2.4 mm), USNM, Belize, Carrie Bow Cay , depth 1–3 m, associated with sea anemone Stichodactyla helianthus , leg. J.A. Baeza , 09.2009.
Description. Body plump, bulkier in ovigerous females ( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 ). Rostrum slightly descendent, falling short of distal margin of first (basal) article of antennular peduncle; dorsal margin armed with 3–4 teeth, posterior-most tooth usually smaller and situated slightly posterior to posterior margin of orbit; ventral margin bearing subdistal tooth, latter small or as large as apical tooth, forming bifid rostral tip ( Figs. 1B View FIGURE 1 , 2B View FIGURE 2 ). Supraorbital tooth absent; antennal tooth well marked, distinctly separated from, and overreaching, ventral angle of orbit; pterygostomial margin angular, often with minute tooth ( Figs 1A, B View FIGURE 1 , 2B View FIGURE 2 ).
Pleura of first to third pleonites broadly rounded ventrally; third pleuron forming low hump, its posteromedian margin being distinctly higher than proximal surface of fourth pleuron, however, not produced posteriorly and not overhanging it; fourth pleuron with distoventral angle sharp or produced into small acute tooth; fifth pleuron with distoventral angle produced into small acute tooth; sixth somite about 1.7 times as long as fifth, distinctly shorter than telson, with sharp or subacute distolateral and distoventral angles ( Figs. 1C View FIGURE 1 , 2B View FIGURE 2 ). Telson slender, markedly tapering distally; dorsal surface armed with 4 pairs of cuspidate setae situated between 0.4 and 0.8 of telson length; distal margin with small median point and 3–4 (typically 4) pairs of spiniform setae, intermediate pairs stoutest and longest ( Fig. 1D, E View FIGURE 1 ).
Eyes with cornea very large, broader than eyestalk ( Figs. 1A View FIGURE 1 , 2 View FIGURE 2 , 3 View FIGURE 3 ).
Antennular peduncle stout; stylocerite well developed, distally acute, reaching well beyond distal margin of second article, with distinctly protruding, blunt tooth on proximolateral margin; first article with small tooth on ventromesial margin; second article with sharp distolateral tooth reaching distal third of third (distal) article; dorsal scale of third article subtriangular, acute distally, without denticle on lateral margin; dorsal flagellum swollen, proximal 8–13 subdivisions with dense row of aesthetascs ( Figs. 1A, F View FIGURE 1 , 2B View FIGURE 2 ).
Antenna well developed, moderately robust; basicerite distally armed with strong ventrolateral tooth and small dorsolateral tooth; carpocerite short, reaching to about mid-length of scaphocerite; scaphocerite overreaching antennular peduncle by one third to almost one half of its length, subrectangular, about 2.2 times as long as maximal width; lateral margin nearly straight, distolateral tooth falling short of strongly produced distomesial angle of blade ( Figs. 1A, G View FIGURE 1 , 2A View FIGURE 2 ).
Mouthparts typical for genus ( Fig. 1H–N View FIGURE 1 ). Mandibles slightly asymmetrical; incisor process narrow, distal margin obliquely truncate, armed with 4–5 very small teeth; molar process well developed, swollen ( Fig. 1H, I View FIGURE 1 ). Maxillule with distally bilobed palp; dorsal lobe with slender seta; ventral lobe with stouter, stiff seta ( Fig. 1J View FIGURE 1 ). Maxilla with basial endite (“mesial lacinia”) bearing deep cleft; endopod (palp) bipartite but not subdivided; scaphognathite rather broad ( Fig. 1K View FIGURE 1 ). First maxilliped with modestly developed caridean lobe and subdivided endopod (palp) ( Fig. 1L View FIGURE 1 ). Second maxilliped with small podobranch on epipod ( Fig. 1M View FIGURE 1 ). Third maxilliped slightly overreaching antennal scale, exopod reaching distal sixth of antepenultimate article ( Fig. 1N View FIGURE 1 ).
First pereiopod not particularly elongate or enlarged in males, reaching almost as far as distolateral tooth of antennal scaphocerite; ischium slightly more than half-length of merus, with some spiniform setae on ventral margin, including at least 1 distoventral spiniform seta; merus subrectangular, armed with 2 spiniform setae on dorsal margin, one proximal and one distal, and 2 adjacent spiniform setae in proximal third of ventral margin; carpus about 0.7–0.8 times as long as merus, shorter and distally conspicuously widening, cup-shaped in larger males, more slender, cylindrical in smaller males and females; carpo-propodal brush present; chela not swollen; palm as long as merus; fingers slightly more than half as long as palm ( Figs. 1O View FIGURE 1 , 2B View FIGURE 2 ).
Second pereiopod slender, overreaching scaphocerite in full extension; ischium almost as long as merus, with two stiff curved setae on ventral margin; merus about 0.6 times as long as carpus (all carpal subarticles combined); carpus with 6 subarticles with ratio approximately equal to 1.5–2: 1: 3: 2: 1–1.5: 2.3; chela shorter than 3 mostdistal carpal subarticles combined, with fingers distinctly shorter than palm ( Figs. 1P View FIGURE 1 , 2B View FIGURE 2 ).
Ambulatory pereiopods slender, similar in length and general shape; proportions somewhat variable in males, possibly influenced by age and/or hierarchy, or host association ( Figs. 1Q–V View FIGURE 1 , 2B View FIGURE 2 ; see also Chace 1972: fig. 56q–v). Third pereiopod of male prehensile or subprehensile, subcheliform; ischium about 1.6–1.9 times as long as wide; unarmed; merus 5.0–5.5 times as long as wide, with 1 stout cuspidate seta on subdistal ventrolateral margin; carpus slightly less than half-length of merus and about half-length of propodus; propodus armed with 3–6 small, widely spaced spinules on ventral (flexor) margin; ventral margin slightly widening at about distal third, then noticeably converging towards dorsal (extensor) margin; distal third of ventral margin densely furnished with row of slender spiniform setae, in addition to 1 stout spiniform seta near propodo-dactylar articulation; dactylus about 0.25 length of propodus, varying from robust to slender; ventral (flexor) margin with 5 (in more robust dactylus) to 10 or so (in more slender dactylus), closely appressed, accessory spinules, and 1 subdistal spine, considerably longer and stouter than proximal spinules, but shorter than stout terminal unguis; subdistal spine and terminal unguis very slightly diverging ( Fig. 1Q, R View FIGURE 1 ; see also Chace 1972: fig. 56q, r). Third pereiopod of female not prehensile, similar to fourth pereiopod (see below), however, without microscopic comb-like spinule at distal end of propodus.
Fourth pereiopod not prehensile in either sex; ischium, merus and carpus similar to those of third pereiopod, more slender; merus with 1 stout cuspidate seta on subdistal ventrolateral margin; propodus armed with about 6 widely and subequally spaced spinules on ventral margin, 2–4 more closely inserted spiniform setae on distal fifth and 1 microscopic comb-like spinule near articulation with dactylus; dactylus about 0.2 length of propodus, varying from robust to moderately slender; ventral (flexor) margin with 2–4 non-appressed, accessory spinules and 1 subdistal spine, considerably longer and stouter than proximal spinules, but shorter than stout terminal unguis; subdistal spine and terminal unguis feebly diverging ( Fig. 1S, T View FIGURE 1 ; see also Chace 1972: figs. 56s, t).
Fifth pereiopod generally similar to fourth pereiopod, shorter, slightly more slender; merus with or without 1 cuspidate seta on subdistal ventrolateral margin; propodus with 7–9 small spinules on ventral margin, including 2 larger ones near propodo-dactylar articulation, without rows of serrulate setae on distolateral surface; dactylus similar to that of fourth pereiopod ( Fig. 1U, V View FIGURE 1 , see also Chace 1972: figs. 56u, v).
First pleopod of male with endopod about 0.6 length of exopod, fringed by spaced, moderately long and robust setae on mesial margin ( Fig. 1W, X View FIGURE 1 ; see also Chace 1972: figs. 56w, x). Second pleopod of male with appendix masculina more than twice as long as appendix interna, not reaching to distal margin of endopod, swollen, with dense covering of long subspiniform setae ( Fig. 1Y, Z View FIGURE 1 ; see also Chace 1972: figs. 56y, z, x).
Uropod with rami subequal in length; protopod with lateral lobe ending in sharp tooth; exopod with stout distolateral spiniform seta adjacent to acute distolateral tooth, both at some distance from distal margin; diaeresis distinct only in its lateral section; endopod without specific features ( Fig. 1D View FIGURE 1 ).
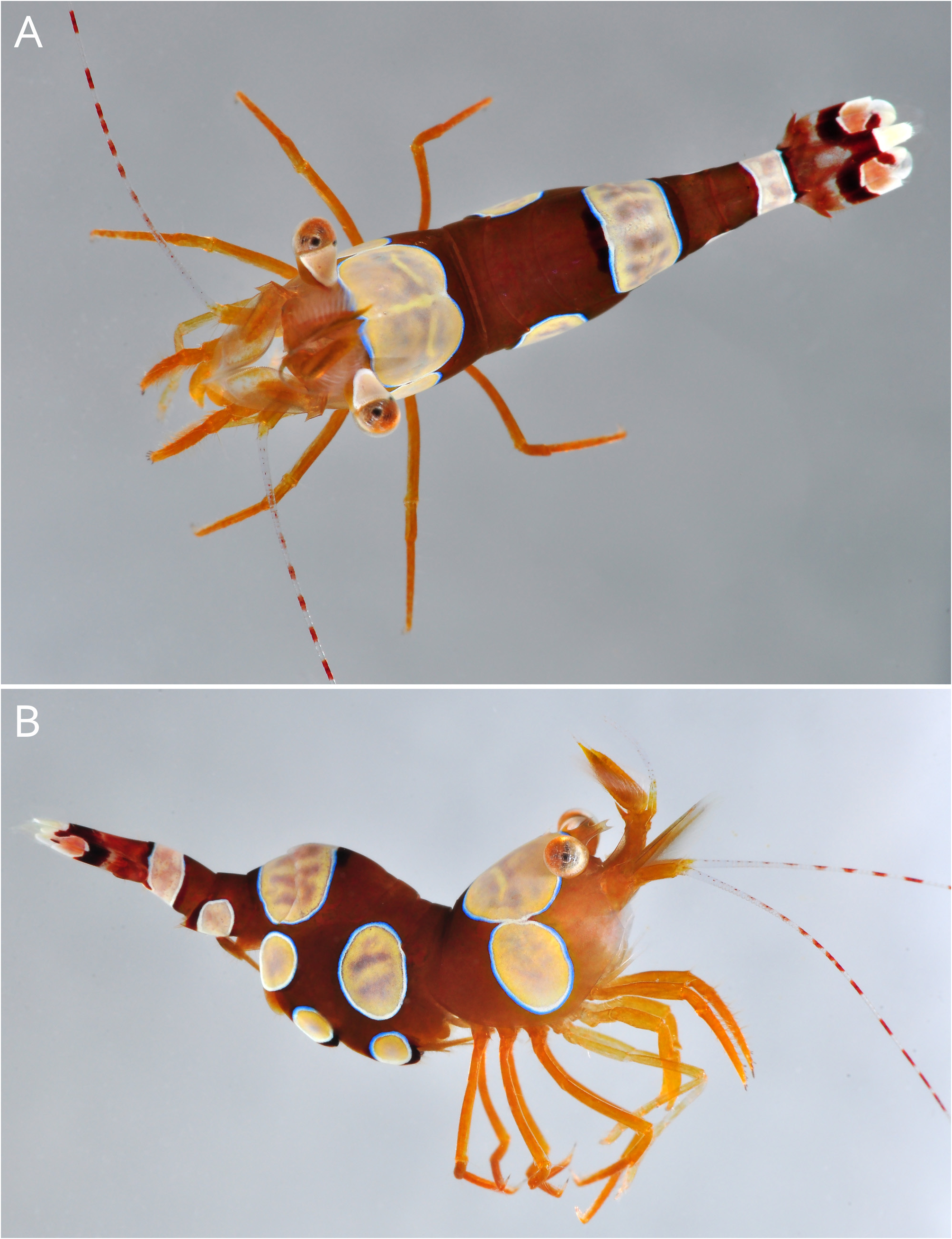
Colour pattern. “Sexy shrimp pattern” consisting of large, rounded or ovoid, highly contrasting spots of mainly white and pale yellow colour, with bluish and grey tones, most delimited by narrow pale blue circle, on brown dominated background. Body background varying from chocolate brown to brown-orange or brown-greenish, much paler and more yellow-greenish or bluish at night. Carapace with (i) one very large, white or white-grey-yellow marbled dorsal patch, reminiscent of apple in dorsal view and occupying most of dorsal surface, however, not reaching posterior margin (hereafter DPC, dorsal patch of carapace), and (ii) two smaller white or white-grey-yellow marbled, ovoid (oval-circular), lateral patches, one on each carapace flank, tangential to large dorsal patch (hereafter LPC, lateral patch of carapace); all three patches (DPC and 2 LPC) delimited by white internal ring and sky-blue external ring, latter particularly well marked on anterior margin of DPC. Pleon with several white or white-grey-yellow marbled patches, distributed as following: (i) two large more or less ovoid lateral patches, one on each side, shared between posterior area of first pleuron and large portion of lateral surface of second pleuron (LPP1-2, lateral patch of pleonites 1 and 2); (ii) two smaller ovoid lateral patches, one on each side, on distoventral area of third pleuron (LPP3, lateral patch of pleonite 3); (iii) two even smaller and less regularly shaped, more or less oval or subcircular lateral patches, one on each side, on ventrolateral surface of fifth pleuron (LPP5, lateral patch of pleonite 5); (iv) one very large patch, subrectangular in dorsal view, more oval-shaped in lateral view, occupying most of posterior area of third pleonite and large portion of fourth pleonite (DPP3-4, dorsal patch of pleonites 3 and 4); and (v) one smaller patch forming full transverse band or white ring across posterior half of sixth pleonite (TBP6 transverse band of pleonite 6); at least two largest patches, e.g. LPP1-2 and DPP3-4, typically delimited by white internal ring and skyblue external ring; anterior and posterior areas near DPP3-4, and areas posterior to LPP5 and TBP6 much darker, almost black; LPP3 and DPP3-4 distinct and usually well separated from each other in females, but fused into single large band transverse running across most of third pleonite and anterior portion of fourth pleonite in males. Telson reddish brown dorsally, with two longitudinal dark lines in its central portion and bright white distal third. Eye peduncles orange ventrally, white dorsally; cornea silvery white with some yellowish tinge. Antennular and antennal peduncles, including scaphocerite, golden-orange; dorsal antennular flagellum golden-orange; ventral antennular flagellum transparent with minute red spots; antennal flagellum with conspicuous red and white banding, however, red rings starting somewhat more distally, most proximal 10 or so units of flagellum being transparent with small red spots. Third maxillipeds, chelipeds and ambulatory pereiopods uniform golden-orange; second pereipods pale orange-yellow. Pleopods orange-brown; ovigerous females with two large yellow-marbled spots bordered by blue and black ring on protopods of second and third pleopods. Each uropod with very distinctive pattern for most part shared by both rami, as following: proximal third brown-red, followed by some semi-transparent area; central area with dark chocolate-brown to almost black, somewhat oblique band; distal half white, with pale orange-pink patch on both exopod and endopod, and dark brown patch on endopod only ( Figs. 2–5 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 ).
Etymology. This beautiful and charismatic new species of Thor is named after the well-known actor and environmental activist Leonardo DiCaprio for his continuous engagement in the protection of biodiversity (including coral reefs and other tropical marine habitats) through various conservation projects, and for bringing more attention to the catastrophic effects of the global climate change. Used as a noun in apposition.
Common names suggested. Leonardo DiCaprio’s sexy shrimp; banded-antennae squat shrimp.
Type locality. Saint Martin, French Antilles (collection locality of the holotype) .
Distribution. West Atlantic: throughout Caribbean Sea, with material and/or photographic records from Colombia (Santa Marta), Panama (Bocas del Toro), Honduras (Utila), Belize, Mexico (e.g. Cozumel), Cuba (Isla de la Juventud), Cayman Islands, US Virgin Islands, Antigua, Saint Martin, Martinique, Dominica, Guadeloupe, Barbados, Tobago, Bonaire and Curaçao; Gulf of Mexico (Flower Garden Banks off Texas); southern Florida (including Florida Keys); Bahamas (Grand Bahama); Bermuda; Brazil (North Chain, Fernando de Noronha Chain, Trindade e Martim Vaz) (e.g. Chace 1972; Herrnkind et al. 1976; Criales 1984; Sterrer 1986; Wicksten 2005a, 2005b; Coelho Filho 2006; Felder et al. 2009; Baeza & Piantoni 2010; Briones-Fourzán et al. 2012; De Grave & Anker 2017; Tavares et al. 2017; Titus et al. 2018; Poupin 2018 and references therein; all as T. amboinensis ; present study).
Ecology and life history. There is no evidence that T. dicaprio sp. nov. is a “cleaner shrimp”, despite its bright colours (including the conspicuous, red-white banded antennal flagella). In more than 16 hours of diving and in situ observation (see below), one of us (JAB) never saw this species cleaning a fish. Thor dicaprio sp. nov. and other members of the T. amboinensis complex have been reported to live with a wide variety of coelenterate hosts ( Chace 1972; Suzuki & Hayashi 1977; Sarver 1979; Guo et al. 1996; Wirtz 1997; Khan et al. 2004; Tavares et al. 2017 and references therein) or dwelling in small rock cracks, apparently without a host ( Wirtz 1997; Tavares et al. 2017 and references therein). Records of associations with non-coelenterate hosts, e.g. crinoids ( Criales 1984) or sponges ( Fig. 7A, B View FIGURE 7 ), are uncommon and most likely incidental. The most common hosts of these shrimps are various true sea anemones of the order Actiniaria , especially in the Atlantic Ocean ( Chace 1972; Wicksten & Hernández 2000; Baeza & Piantoni 2010; Tavares et al. 2017).
Thor dicaprio sp. nov. can be considered a symbiotic generalist, as previously suggested by Chace (1972), although with a clear preference for several species of sea anemones (see Table 1). In the Bahamas (Grand Bahama), the occurrence of T. dicaprio sp. nov. on sea anemones is rather low, at 20% and 13% in Lebrunia neglecta and Bartholomea annulata , respectively, and usually with only one or two, but no more than two shrimps per anemone ( Herrnkind et al. 1976; Stanton 1977). In sharp contrast to the Bahamas, in Belize, the occurrence of T. dicaprio sp. nov. on the large and structurally heterogeneous sea anemone Stichodactyla helianthus (Ellis, 1768) is high (~66%). Shrimps inhabit this anemone in large groups; as many as 11 individuals have been observed on a single host individual ( Baeza & Piantoni 2010). Groups exhibit no particularly complex social structure and feature female-biased sex ratios more frequently than expected by chance alone. The overall population sex ratio is also male-biased ( Baeza & Piantoni 2010).
Thor dicaprio sp. nov. can exploit various micro-environments of the host. Most often, shrimps remain on the substrate close to the periphery of the host’s tentacles or well protected near the anemone’s column, underneath the tentacles, as in the case of B. annulata , C. gigantea , and S. helianthus ( Nizinski 1989; Baeza & Piantoni 2010). However, T. dicaprio sp. nov. can also be found in direct contact with its anemone host, near the centre of the oral disc or perched to the column right beneath the crown of tentacles, for instance, of S. helianthus ( Baeza & Piantoni 2010) . On a few occasions, T. dicaprio sp. nov. was seen climbing down among the fronds of L. neglecta ( Herrnkind et al. 1976; Stanton 1977), indicating that the shrimps may be to some degree protected from the anemone’s nematocysts, perhaps after a period of acclimation. Similarly, for the eastern Atlantic T. amboinensis s. lat., Wirtz (1997) noted that the “contact between the shrimp and the tentacles did not elicit feeding reactions of the anemone”.
The posture and resting behaviour of T. dicaprio sp. nov. are essentially the same as those observed for most other members of the T. amboinensis complex. The pleon is maintained flexed upwards (dorsally) and is almost constantly waved upwards / downwards or circularly ( Herrnkind et al. 1976; Baeza & Piantoni 2010), a curious behaviour that led to its popular names, “sexy shrimp” and “dancing shrimp” (see also Video 1).
Thor dicaprio sp. nov. is a protandric hermaphrodite, i.e. all shrimps first mature as males and change their sex into females later in life. Preliminary data suggest that T. dicaprio sp. nov. exhibits “pure search promiscuity” as mating system, but this inference needs to be confirmed experimentally ( Baeza & Piantoni 2010). In Belize, unusually large males are sometimes parasitised by an undescribed species of isopod (family Entoniscidae ). Infestation rates were similarly low between the sexes, approximately 11–12% ( Baeza & Piantoni 2010).
In Carrie Bow Cay, Belize (hereafter CBC), a total of 16 hours of scuba diving during sampling of T. dicaprio sp. nov. allowed one of the authors (JAB) to observe the species’ in situ behaviour. Additionally, 8 (non-continuous) hours of free diving were allocated exclusively for observation of shrimps without disturbing them. Special care was taken to observe and record shrimps (with a photographic camera) from a distance that did not affect their natural behaviour. Preliminary observations indicate that staying 0.5–0.9 m away from the host anemone does not perturb the shrimps. However, it was possible to reduce this distance with time, especially when sea anemones were approached quietly and slowly.
In CBC, T. dicaprio sp. nov. was most commonly observed within only a few centimetres from the periphery of the tentacles of its host sea anemone, S. helianthus . Whenever small blades of sea grass appeared in the immediate vicinity of the anemone, the shrimps did not hesitate to perch on them. Occasionally, they were seen on the tentacles, column or near the mouth of the anemone, or perching on the rock surface near the anemone’s column. Most of the time, shrimps were seen feeding, picking food particles from the nearby rocks or encrusting coralline algae (Videos 1–3). On one occasion, when the current was relatively strong, a shrimp was seen catching a small piece of floating algae that passed nearby (Video 1). An apparent dominance hierarchy existed within the observed groups of T. dicaprio sp. nov., with large (usually female) individuals being dominant over smaller (male or smaller female) ones. For example, when larger shrimps were grazing and approached other, smaller shrimp, the latter moved away either rapidly or slowly from the path of the large individual (Video 2). However, no agonistic encounters (e.g. fights) were observed.
Other crustaceans associated with S. helianthus in CBC include the arrow crab Stenorhyncus seticornis (Herbst, 1788) , the spider crab Mithrax cinctimanus (Stimpson, 1860) , and the shrimp Periclimenes rathbunae Schmitt, 1924 . However, interactions between them and T. dicaprio sp. nov. were infrequent. On one occasion, a specimen of P. rathbunae attempted to grasp with its claws one small male feeding some centimetres away from the anemone’s tentacles. However, this interaction seemed territorial rather than predatory and resulted in the smaller shrimp, i.e. T. dicaprio sp. nov., moving slightly away from the anemone (Video 3). Shrimps can move, i.e. walk very slowly (during grazing) or very quickly. For example, during sampling attempts, some shrimps promptly escaped towards the tentacles or the base of the column of the anemone, using the classic caridean “escape response”, a backward tail flipping that rapidly propel the shrimp away from danger. On a few occasions, shrimps fled from the anemone to the adjacent sea grass or swiftly entered small rock crevices near the anemone. They then remained away from their hosts for some minutes or returned relatively quickly, following a trajectory different from that originally used for reaching the off-host refuge.
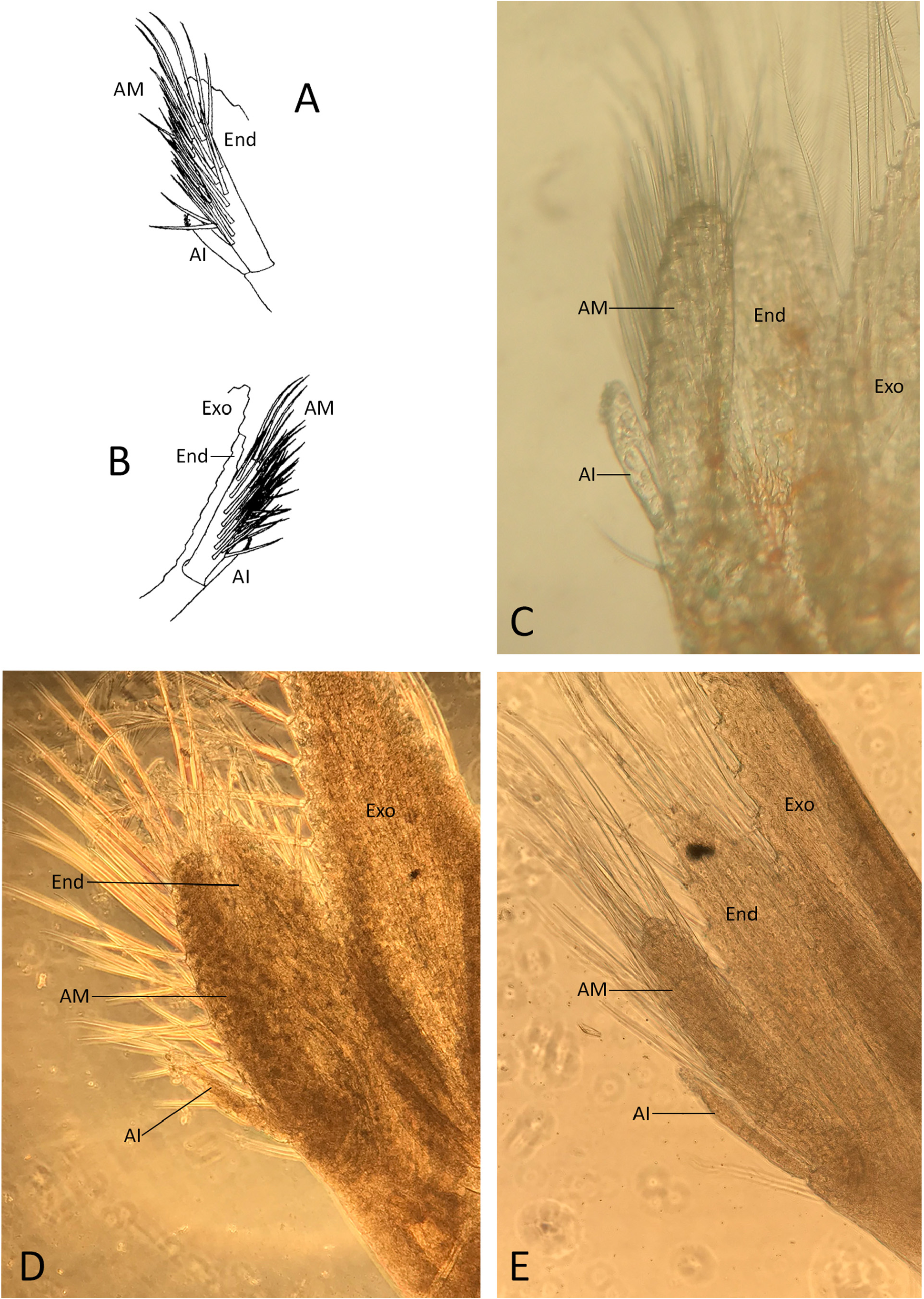
Taxonomic remarks. Thor dicaprio sp. nov. differs from all other species of Thor , including all specimens from outside of the western Atlantic currently identified as T. amboinensis [s. lat.], by the highly diagnostic “sexy shrimp pattern” with a largely undivided dorsal patch of the carapace (DPC) and the red-white banded antennal flagella ( Figs. 2–5 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 ). The new species corresponds to the well-defined and genetically homogenous TWA clade of Titus et al. (2018), which is distinctive at least from the Indo-West Pacific clades of the T. amboinensis complex, with the pairwise COI genetic distances between T. dicaprio sp. nov. and the other clades being 8.8–19.2% (see Titus et al. 2018: Table S4). On the other hand, T. dicaprio sp. nov. cannot be easily separated from the Indo-West Pacific, eastern Atlantic and eastern Pacific specimens of T. amboinensis [s. lat.] based on morphological grounds alone and therefore represents a true cryptic species. The development and armature of the appendix masculina may reveal as a potential diagnostic character of T. dicaprio sp. nov. In the western Atlantic specimens, the appendix masculina appears to be somewhat more swollen and more densely furnished with numerous stiff subspiniform setae ( Fig. 6A–C View FIGURE 6 ; see also Baeza & Piantoni 2010: fig. 2a). In the Indo-West Pacific specimens, the appendix masculina is less inflated and possesses less numerous spiniform setae ( Fig. 6D, E View FIGURE 6 ; see also Kemp 1916: fig. 1f, although not clearly shown). However, more specimens need to be examined by SEM, especially from the eastern Pacific and eastern Atlantic, to confirm the validity of this possible distinguishing character.
Chace (1972) was somewhat surprised by the morphological variation among his western Atlantic male specimens of T. amboinensis , which correspond to the herein described T. dicaprio sp. nov. This variation affects the armature of the posterior margin of the telson (3 vs. 4 spiniform setae); the shape of the carpus of the first pereiopod (robust and short, cup-shaped vs. more slender and elongate, cylindrical); the proportions and prehensile condition of the third pereiopod; and the armature on the ventral (flexor) margin of the dactyli of the fourth and fifth pereiopods (2 or 3 vs. 4 accessory spinules). In addition, it seems that the merus of the fifth pereiopod may be either armed with a stout cuspidate seta or unarmed. However, it must be noted that the two males illustrated by Chace (1972) are quite different in size. The male from Tobago associated with a “tufted anemone”, i.e. the paratype of T. dicaprio sp. nov. ( Chace 1972: fig. 55; see also Fig. 1 View FIGURE 1 ), is significantly larger at pocl 2.25 mm than the non-type male from Antigua associated with a “thick-tentacled anemone” ( Chace 1972: fig. 56) at pocl 1.6 mm. The paratype also has generally more robust pereiopods, including the carpus of the first pereiopod and the dactyli of the ambulatory pereiopods. Since Chace (1972) himself came to the conclusion that all these characters are variable in the western Atlantic material, they must be considered as part of normal intraspecific variation of the species. This view is corroborated by the analysis of the COI gene in Titus et al. (2018: fig. S1), who recovered a genetically homogenous TWA clade. It is highly likely that the morphological variation observed in T. dicaprio sp. nov. is directly linked to sexual system of the species, including protandric hermaphroditism ( Baeza & Piantoni 2010; see also above).
The rostral formula is variable in T. dicaprio sp. nov. (see above) and also in the Indo-West Pacific material herein temporarily identified T. amboinensis [s. lat.]. For instance, in the specimens from the Mozambique Channel, Red Sea and French Polynesia, the number of the dorsal teeth on the rostrum varies from two to four, whereas the rostral tip may be simple or bifid, i.e. the ventral tooth is more distal and as large as the main apical tooth. Several specimens have a small subdistal ventral tooth or a trace of it in the form of a blunt angle, whilst in others, the ventral margin is almost perfectly straight. This variation is hardly surprising though, considering that several cryptic species are involved in the Indo-West Pacific ( Titus et al. 2018), and therefore, will need a further scrutiny. The rostral formulae are generally variable in species of Thor and are usually unreliable as distinguishing characters.
The material of T. amboinensis [s. lat.] from the Indo-West Pacific, eastern Pacific, as well as eastern and central Atlantic, will be studied elsewhere. One of us (AA) performed a preliminary analysis of the colour patterns of individuals referred to as T. amboinensis from outside of the range of the TWA clade (mainly eastern Atlantic and Indo-West Pacific), using hundreds of colour photographs published in articles, books, as well as photographs available on internet and from personal archive, including numerous high-resolution photographs sent by underwater photographers, and found some differences that could be important in the differentiation between the putative species of this challenging species complex. For instance, it was noted that in most individuals from Christmas Island, Indonesia (e.g. Sulawesi, Lombok, Bali, West Papua), Philippines, Palau, southern Japan (Ryukyu Islands), as well as some individuals from French Polynesia (Moorea) and eastern Australia (Queensland, New South Wales), each of the dorsal (upper) antennular flagella displays a very conspicuous white band just proximal to the deep red tip ( Fig. 7C, D View FIGURE 7 ; see also Kuiter & Debelius 2009; Hoeksema & Fransen 2011; Scott et al. 2014), a feature never seen, for instance, in the individuals from the eastern Atlantic (Madeira, Canary Islands) ( Fig. 6 View FIGURE 6 ; see also Kuiter & Debelius 2009), western Indian Ocean (Red Sea, Oman, Maldives, Egypt) ( Fig. 7A View FIGURE 7 ; see also Kuiter & Debelius 2009; von Jaffa & Khalaf 2017), south-eastern Australia (New South Wales) ( Fig. 7B View FIGURE 7 ) and Hawaii (A. Anker, pers. obs., based on photographs from Maui and Hawai’i by Jess Rickard and David Fleetham, respectively). Therefore, it cannot be excluded that T. discosomatis from the Andaman Islands will turn out to be a valid species, since Kemp (1916: 389) stated that in his specimens, “all the other appendages are reddish brown” and that possibly includes the antennules. As was already noted above, in most specimens from the eastern Atlantic and Indo-West Pacific, the DPC has a shape of an opened clam in dorsal observation of the shrimps, i.e. with deep incisions on both posterior and anterior margins, the two halves being connected in the middle ( Figs. 7 View FIGURE 7 , 8 View FIGURE 8 ). In contrast, in the specimens from the eastern Pacific, the DPC is almost completely divided into two halves by a deep cleft reaching almost to its anterior margin ( Fig. 9 View FIGURE 9 ). It must be noted, however, that the rarity of in situ photographs from the eastern Pacific currently does not allow us to evaluate the consistency of this last character. There are some other differences in the colour pattern of T. amboinensis [s. lat.] that are possibly of taxonomic significance, but these will be discussed elsewhere.
In addition to T. discosomatis of Kemp (1916), at least two other nominal species of Thor will need to be considered in an eventual future revision of the Indo-Pacific material of T. amboinensis [s. lat.]. Thor hainanensis Xu & Li, 2014 , described based on the material collected intertidally in Hainan, China ( Xu & Li 2014), and T. cocoensis Wicksten & Vargas, 2001 , described from Cocos Island off Costa Rica and Galápagos ( Wicksten & Vargas 2001). Both species are lacking supraorbital teeth and were originally described without information on their colour pattern. However, the record of T. hainanensis by Madhavan et al. (2019), illustrating the colour pattern of a specimen from Lakshadweep, India, shows that this taxon, if correctly identified, may be part of the T. amboinensis complex, possibly representing one of the clades in Titus et al. (2018). Since T. hainanensis is morphologically very close to T. cocoensis ( Xu & Li 2014) , the latter species may represent the eastern Pacific clade of the T. amboinensis complex. However, both of these hypotheses will need to be confirmed by examination of new photo-vouchered material.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Thor dicaprio
| Anker, Arthur & Baeza, J. Antonio 2021 |
Thor amboinensis
| Poupin, J. 2018: 126 |
| De Grave, S. & Anker, A. 2017: 33 |
| Colombara, A. M. & Quinn, D. & Chadwick, N. E. 2017: 3 |
| Humann, P. & Deloach, N. & Wilk, L. 2013: 115 |
| Briones-Fourzan, P. & Perez-Ortiz, M. & Negrete-Soto, F. & Barradas-Ortiz, C. & Lozano-Alvarez, E. 2012: 61 |
| Baeza, A. J. & Piantoni, C. 2010: 152 |
| Kuiter, R. H. & Debelius, H. 2009: 127 |
| Felder, D. L. & Alvarez, F. & Goy, J. W. & Lemaitre, R. 2009: 1059 |
| Wicksten, M. K. 2005: 32 |
| Wicksten, M. K. 2005: 104 |
| Sterrer, W. 1986: 327 |
| Criales, M. M. 1984: 313 |
| Herrnkind, W. & Stanton, G. & Conklin, E. 1976: 65 |
| Chace, F. A. Jr. 1972: 130 |