Rhynchotalona latens ( Sarmaja-Korjonen, Hakojärvi & Korhola, 2000 ) Damme & Nevalainen, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4613.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:0E6BDEF5-E83B-4051-B4B0-C2F90DA11C0D |
|
persistent identifier |
https://treatment.plazi.org/id/7B2387F0-FFA5-AD40-0AB9-5E1FFE49639B |
|
treatment provided by |
Plazi |
|
scientific name |
Rhynchotalona latens ( Sarmaja-Korjonen, Hakojärvi & Korhola, 2000 ) |
| status |
comb. nov. |
Rhynchotalona latens ( Sarmaja-Korjonen, Hakojärvi & Korhola, 2000) comb. nov.
Unapertura latens in Sarmaja-Korjonen et al. (2000 : Fig. 2 View FIGURE 2 ); Szeroczyńska & Sarmaja-Korjonen (2007); Nevalainen (2008: Figs 11–12); Luoto et al. (2013); Nevalainen et al. (2013, 2018); potentially Unapertura spp. in Sweetman & Sarmaja- Korjonen (2017), however the identity of the latter could not be confirmed here without intact specimens, only that it falls within the R. latens -group.
Holotype. Intact adult parthenogenetic female from Kalatoin near Nuuksio National Park , coordinates N60°20'26.0'' E 24°37'39.1'', southern Finland, coll. by L. Nevalainen in 2006 and deposited at the Department of Geology, University of Helsinki, Helsinki, Finland ( Nevalainen, 2008). No paratypes exist, yet additional photos of specimens from the type locality are included in this study. GoogleMaps
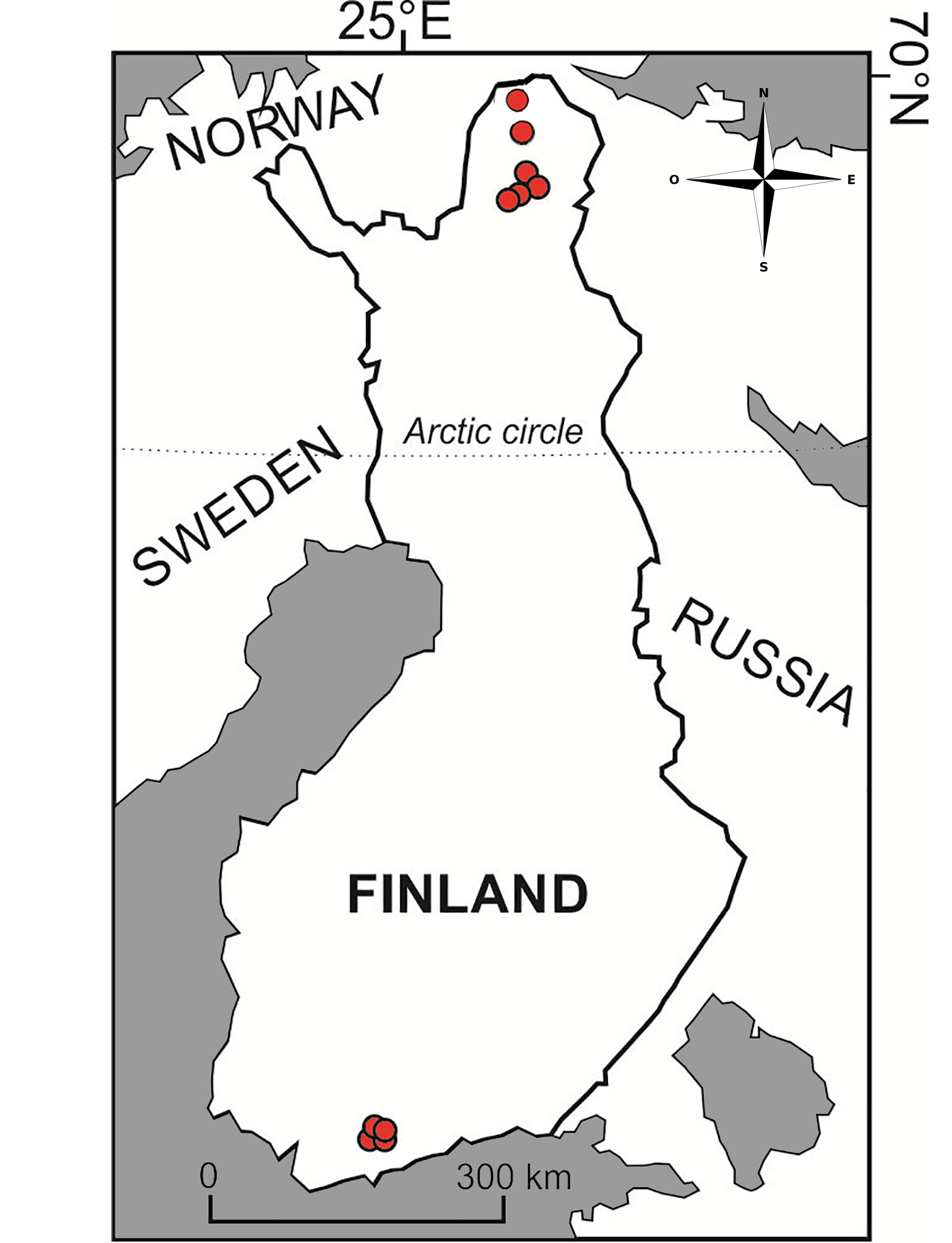
Material examined. One adult parthenogenetic female, intact specimen from an unnamed small lake (0.35 ha; Figs 1 View FIGURE 1 E–F) at an altitude of 180 m a.s.l. near Kenesjärvi of the Utsjoki River, Tenojoki tributary, Lapland, northern Finland, coordinates N 69°41'9.4'', E 27°3'50.9'', collected by technical research staff of the Kevo Subarctic Research Institute, 30.VII.2012.
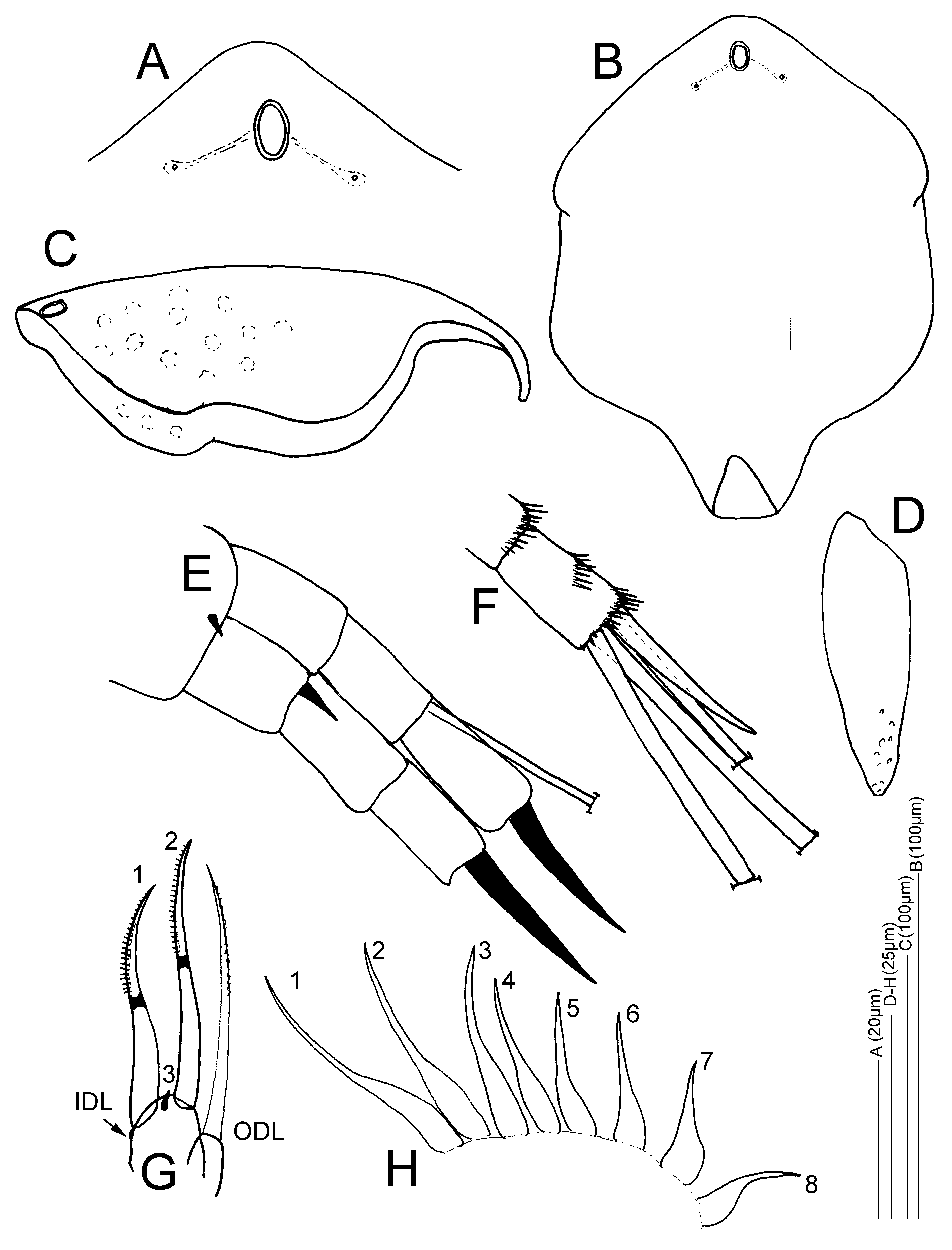
Partial description of the adult parthenogenetic female. General Habitus and carapace. . Habitus oval in lateral view, with an evenly curved, convex dorsal margin, continuing smoothly into a convex posterior carapace region ( Figs 1 View FIGURE 1 A–B, 2A). There is no pronounced posterodorsal corner, and the ventral margin of the body is straight. Carapace ( Figs 1 View FIGURE 1 A–C, 2A, 2C–D) with faint longitudinal striation ( Fig. 1C View FIGURE 1 ) or with verrucae arranged along rows ( Fig. 1B View FIGURE 1 ). Valve setae 40–50 ( Figs 2A, C View FIGURE 2 ), in three distinct groups: a medium-sized anterior group, a short middle group, and a posterior group of about 20 conspicuous longer setae. The posterior group contains the longest setae on the carapace margin and these setae increase in size towards the posteroventral corner ( Figs 2A, C View FIGURE 2 ). The posteriormost seta is about the same length as the postabdominal terminal claw. These setae are implanted with fine setulae on the anterior and the posterior margins and alternated by small setulae on the carapace ( Figs 2 View FIGURE 2 C–D); the posteroventral corner is without a notch and bears no denticles, yet has groups of minute setulae, each group separated by a relatively thicker longer setule ( Fig. 2D View FIGURE 2 ). The ventral margin of the valves is straight, with no embayments, and the anterior edge of the carapace has no notches or protrusions ( Figs 1 View FIGURE 1 A–B, 2A,C). In anterior and in dorsal view, the carapace clearly shows a moderately developed dorsal keel in the median part of the body.
Cephalic structures. The ocellus and the eye are relatively small. Both are of similar size; the diameter of the compound eye is larger than which of the ocellus by only 10–15% ( Figs 1A View FIGURE 1 , 2 View FIGURE 2 A–B). The distance between the ocellus and the tip of the rostrum is two times the distance between the centres of the eye and ocellus ( Fig. 2B View FIGURE 2 ). The rostrum is well developed, in lateral view evenly curved; the tip curved downwards to posteriorly ( Figs 1 View FIGURE 1 A–B, 2A–B). The rostrum is two times the length of the first antenna, which is implanted at its base ( Figs 1A View FIGURE 1 , 2B View FIGURE 2 ) and the aesthetascs do not reach beyond the apex of the rostrum. One oval-shaped major headpore, clearly visible in lateral view as a large fenestra with a chitineous ring ( Fig. 2B View FIGURE 2 ); the main pore is about two times as long as wide along the midline, and situated about 1–1.5x its length from the posterior margin of the headshield ( Figs 2B View FIGURE 2 , 3 View FIGURE 3 A–B). Two small pores, situated laterally and just anteriorly of the main pore, at a distance; the distance between the midline of the headshield and the lateral pores is about three times the width (minor axis) of the main pore, or 1.5x time the length (major axis) of the main pore ( Figs. 3A View FIGURE 3 ). Between the main pore and the lateral pores, connecting darker fields or lines are visible ( Figs 3 View FIGURE 3 A–B). The headshield is about 1.5x as long as wide when pressed flat entirely ( Fig. 3B View FIGURE 3 ), and may be ornamented with verrucae ( Fig. 3C View FIGURE 3 ). Its total length is about 145–195µm ( Sarmaja-Korjonen et al. 2000). Fornices well developed ( Fig. 3B View FIGURE 3 ). For more details and photos of the separated headshield of Finnish specimens of this species, see Sarmaja-Korjonen et al. (2000) and Sweetman & Sarmaja-Korjonen (2017).
Labrum ( Fig. 2E View FIGURE 2 ). Small labral keel ( Figs 1 View FIGURE 1 A–B, 2A,B) with a moderately convex smooth ventral margin, narrowing towards the posterior apex and with a small lip and a slight indentation; the apex is blunted, not acute ( Fig. 2E View FIGURE 2 ).
Antennules/A1. Antennal body ( Fig. 3D View FIGURE 3 ) long, about three to four times as long as wide, and narrowing towards the apex; nine aesthetascs. Sensory seta and setulation not studied.
Second antennae/A2 ( Figs 3 View FIGURE 3 E–F). Relatively short second antennae with stout segments. Coxal spine short and small. Spinal formula 001/101, setal formula 013/003. Exopod and endopod of similar lengths. Spine on the first exopod segment small, about one third the length of the second exopod segment. Terminal spines robust, very large and of similar lengths, about 1.5– 2x as long as the last antennal segment on which they are implanted. Seta on the second endopod segment present, about as long as the three segments of the endopod together. Well developed spinule groups present on the antennal segments ( Fig. 3F View FIGURE 3 ).
Six pairs of thoracic limbs. First five limbs with epipodites, all with elongated finger-like projection.
First limb/P1 with anterior setule groups. ODL with one long slender seta with fine setulation in the distal half, and just longer than the longest IDL seta; IDL with two developed setae of similar size with a chitinous ring and distal unilateral setulation, with one reduced element between their bases ( Fig. 3G View FIGURE 3 ).
Second limb/P2 with eight scrapers gradually decreasing in size between exopodite and gnathobase. Third scraper long and slender, of similar size as the second scraper, and markedly longer than scraper four ( Fig. 3H View FIGURE 3 ).
Third limb/P3 with seven exopodite setae.
Fourth limb/P4 with six exopodite setae.
Fifth limb/P4 with four exopodite setae.
Sixth limb/P6 a simple setulated lobe. Epipodites of P3–P5 visible in the habitus in lateral view ( Fig. 2A View FIGURE 2 ).
Postabdomen ( Figs 2 View FIGURE 2 G–F) widest at the preanal corner, which is pronounced. Length of postabdomen about 2.5x as long as wide, relatively short and robust in relation to the body ( Figs 1A,C View FIGURE 1 , 2A View FIGURE 2 ). Ventral margin straight; dorsodistal postanal margin straight to slightly convex; postanal portion of the postabdomen slightly tapering distally. Dorsodistal embayment (at the basis of the terminal claw) closed ( Figs 2 View FIGURE 2 G–F). Preanal margin convex, anal margin straight and just longer than the postanal margin. Postanal corner pronounced.
Postanal dorsal margin with five to six large, singular, robust marginal teeth, increasing in size distally ( Figs 1A,C View FIGURE 1 , 2A View FIGURE 2 ,G–F). The most robust spine is followed distally by a narrow marginal spine ( Fig. 2F View FIGURE 2 ). Lateral spines strongly developed, only the distal spines clearly arranged in groups, otherwise seemingly a continuous row of lateral spines of similar size (not arranged clearly into lateral fascicles) and continuing into the lateral anal spines while gradually decreasing in size ( Fig. 2G View FIGURE 2 ). Anal margin with two singular proximal teeth and one median group of seven to eight small spinules ( Fig. 2G View FIGURE 2 ).
Terminal claw about the same length as the anal margin; claw rather straight. Basal spine on the terminal claw robust, about a third of the terminal claw length, accompanied by four-five short basal spinules ( Fig. 2G View FIGURE 2 ). The length of the postabdomen and claw together measure ca. 100µm ( Nevalainen, 2008).
Ephippial female and ephippium. Ephippium ( Fig. 1D View FIGURE 1 ) briefly described in Nevalainen (2008): a round single-egged ephippium with thickened dorsal and postero-dorsal margins, size ca. 200µm.
Size 0.25–0.3 mm, length/width ratio 1.4–1.55
Male. Unknown.
Diagnosis. Rhychotalona latens comb. nov. can be distinguished from the rest of the species of Rhynchotalona by the presence of a continuous row of five to six marginal teeth on the postabdomen, increasing in size distally. This feature is unique for the genus; all other Rhynchotalona have two to three large marginal teeth on the postabdomen. In addition, R. latens has a row of longer ventral setae on the posterior valve margin, as in the Nearctic R. longiseta Sinev & Kotov, 2014 (see Sinev & Kotov 2014), but in comparison to the latter, R. latens has a shorter rostrum. The unrevised R. kistarae also has relatively long posterior ventral valve setae, although not as pronounced as in R. latens , and the latter species differs in the armature of the postabdomen. R. latens also differs from its congeners in the position of the lateral pores in the headshield, which are located at some distance and anteriorly to the main pore. In all other species in the genus, the lateral pores are adjacent to the main pore and not situated anteriorly. The adult parthenogenetic female of R. latens is further separated by its small size, just 0.25 mm in length; only adult females of R. kistarae fall within this size range (0.23–0.32mm), the other species in the genus are larger, at least 0.4mm ( Sinev & Kotov, 2014). There are no denticles on the posteroventral corner of the valves in R. latens .
Beyond Rhynchotalona , in general habitus and in postabdomen, R. latens could be superficially confused with other smaller Alona- like and Alonella- like chydorids. The potential presence of verrucae on the carapace and the general shape of the postabdomen, may lead to lumping with species of the Alona guttata Sars, 1862 or Coronatella rectangula (Sars, 1862) (former A. rectangula ) complexes. In comparison to the latter taxa however, R. latens is much smaller, the rostrum is relatively longer, and the marginal spines on the postabdomen are long and singular, not serrated (as in A. guttata ) or in groups (as in C. rectangula ). In addition, the relatively long posterior valve setae in R. latens are not found in any Alona -like species in Europe (unless one considers R. kistarae ).
Distribution and ecology. In contemporary waters, intact specimens of R. latens have only been found so far in a few localities in Finland ( Fig. 4 View FIGURE 4 ): southern Finland (lakes Hauklampi, Iso Majaslampi, Iso Lehmälampi and Kalatoin, Nuuksio district; Nevalainen 2008); northern Finland (lakes Gárggoláttu, Lasâjáávráŝ, Sylvilampi, Vaaranpäällyslampi and Taivaljärvi, Inari–Utsjoki region, Lapland in Nevalainen 2018; small lake near Utsjoki River, Tenojoki tributary, Utsjoki region, Lapland; this study; Figs 1 View FIGURE 1 E–F). Complete specimens have been recovered from oligotrophic acidic clear and brown water lakes and ponds, bordered with Sphagnum and rich in submerged vegetation ( Nevalainen 2008). According to Sweetman & Sarmaja-Korjonen (2017), this species is “ not so sensitive to pH, but instead simply prefers oligotrophic waters”. Type locality, Kalatoin (Nuuksio district, southern Finland) is a “ very acidic and dystrophic small lake (about 1 ha) lake with paludified shores”, fish-free and surrounded with bog vegetation ( Nevalainen 2008). In Nevalainen et al. (2018), where R. latens was discovered in surface sediment and intact in modern cladoceran communities of northern Finnish Lapland, it thrives in transparent or brown-water low pH lakes. The current subarctic study site near Utsjoki River in northern Finland is surrounded by a rim of wetland; the catchment vegetation consisting of mixed pine and mountain birch woodland ( Figs 1 View FIGURE 1 E–F). Based on this, R. latens seems to occur also in wetland lakes with habitats rich in vegetation (e.g. Sphagnum, Carex ) and soft gyttja sediments, in common with Nevalainen et al. (2018). However, more surveys are needed to understand its ecology. The true contemporary distribution is unknown. Headshield and postabdomen remains have been suggested during the analysis of recent sediments of lakes in the Tatrá Mountains ( Sienkiewicz & Gąsiorowski 2016), the Alps ( Bigler et al. 2006, Milan et al. 2017) and in the central Canadian treeline region in the Northwest Territories in Canada, which may indicate a wider Palaearctic and potential Holarctic distribution for this species ( Sweetman et al. 2010, Sweetman & Sarmaja-Korjonen 2017) or species complex.
In the Polish Tatrá Mountains, subfossils of the species appear briefly in the sediment record of an oligotrophic fishless freshwater lake Wielski Staw Polski (alt. 1665m) around the years 1850 and again in 1950, but not later ( Sienkiewicz & Gąsiorowski 2016); Bigler et al. (2006) mention it from recent remains in surface sediments (0–3 cm) of two out of 30 oligotrophic mountain lakes studied (Lej Pitschen, alt. 2223m and Lej Marsch, alt. 1812m) in the Swiss Alps; Milan et al. (2017) mention the species from a core in the littoral zone of the Bardolino Basin at the subalpine Lake Garda (alt. 65m) in Italy, but provide no date for the age of occurrence; Sweetman & Sarmaja- Korjonen (2017) found subfossils from recent surface sediments in two oligotrophic lowland lakes (named TK-18 and TK-32) in the central Canadian Arctic Treeline region, NE of Great Slave Lake, Northwest Territories, Canada. Intact specimens from the Nearctic are yet to be found.
Based on analysis of cladoceran remains in surface sediments, representative of modern cladoceran communities, the taxon appears to be relatively common across Finland, although mostly not an abundant part of the cladoceran communities. It was present as fossils in 26 of 73 lakes studied across Finland from 60 to 70°N ( Nevalainen et al. 2013) and five of 31 lakes studied in north eastern Finnish Lapland ( Nevalainen et al. 2018), where it also occurred in modern communities of five lakes (two lakes being the same with the fossil distribution). We may expect the species in other regions of the northern Palaearctic as well. Kattel et al. (2015) mention recent remains of R. latens (as Unapertura ) from Zhangdu Lake in Hubei Province, central China, however the identifications could not be confirmed here.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Aloninae |
|
Genus |
Rhynchotalona latens ( Sarmaja-Korjonen, Hakojärvi & Korhola, 2000 )
| Damme, Kay Van & Nevalainen, Liisa 2019 |
Unapertura latens
| in Sarmaja-Korjonen 2000 |