Squalus acutipinnis Regan, 1908
|
publication ID |
https://doi.org/ 10.1643/Ci-14-217 |
|
DOI |
https://doi.org/10.5281/zenodo.6062764 |
|
persistent identifier |
https://treatment.plazi.org/id/734687A2-5E13-B07E-FCE1-0AE7FD43FD40 |
|
treatment provided by |
Plazi |
|
scientific name |
Squalus acutipinnis Regan, 1908 |
| status |
|
Squalus acutipinnis Regan, 1908 View in CoL
Stompneus-penhaai (Afrikaans); Bluntnose spiny dogfish (English) Figures 1–9; Tables 1–2 View Table 1 View Table 2
Squalus acutipinnis Regan 1908a:241 View in CoL , 248, pl. XXXVII (original description, illustrated; type locality: Kwazulu-Natal, South Africa [not NE of Bird Island as stated on p. 241]; 40 ftm/ 73.152 m; lectotype: BMNH 1905.6.8.8 [see below]).– Regan, 1908b:45, 47 (cited; South Africa, Mauritius).– Regan, 1921:412 (listed; Kwazulu-Natal, South Africa).– Krefft, 1968 (in part):34, 51–54, pl. III A (cited, description, designation of lectotype; South Africa).– Merrett, 1973:94, 104, 108, 109 (cited; South Africa).– Kondyurin and Myagkov, 1979 (revision, South Africa [not seen, cited in Myagkov and Kondyurin, 1986]).– Cadenat and Blache, 1981:47, 51, 52, fig. 31f–g (cited; South Africa).– Myagkov and Kondyurin, 1986:8 –10, 15 (description; Southern Africa).– Last et al., 2007:11 (cited; Southwest Indian Ocean).– Ebert et al., 2010:22, 23 (cited; Southern Africa).– Ebert and Stehmann, 2013 (in part):66 (cited as possibly valid; Southern Africa).
Squalus fernandinus View in CoL not Molina, 1782.— Garman, 1913:195 – 196 (synonymy, description).– Bigelow and Schroeder, 1948:480 (cited, South Africa).– Bigelow and Schroeder, 1957:32, 35 (cited, South Africa).– Smith, 1961:59, 61 (brief account, South Africa).
Squalus megalops View in CoL (not MacLeay, 1881).— Bass et al., 1976 (in part):11, 9–13, 16–18, 60; fig. 11; pl. 3 (description, illustrated; Southern Africa).– Compagno, 1984 (in part):118, 119 (description, illustrated; South Africa).– Bass et al., 1986 (in part):61, 62, fig. 5.26 (description; Southern Africa).– Parin, 1988:49 (cited; Southeastern Atlantic Ocean).– Muñoz-Chápuli and Ramos, 1989:6, 11, 18, 19 (diagnosis, illustrated, Eastern Atlantic).– Compagno et al., 1989 (in part):22 (account, illustrated).– Compagno and Niem, 1999 (in part):1230 (cited; South Africa to Mozambique).– Ebert et al., 2013 (in part):91 (general account on S. megalops View in CoL ).
Squalus cf. megalops View in CoL .— Naylor et al., 2012a (in part):58, 148, fig. 42 (cited, molecular systematic analysis; South Africa, Indian Ocean).– Naylor et al., 2012b (in part):fig. 2.7 (molecular systematic analysis; South Africa).
Lectotype.— BMNH 1905.6.8.8 , juvenile female, 578 mm TL, Western Indian Ocean, South Africa, Kwazulu-Natal, donated by Dr. E. Warren. Designated by Krefft (1968).
Paralectotypes.— (3 specimens) BMNH 1859.5.7.68 (not Squalus acutipinnis ), neonate female, 190 mm TL, Southeastern Atlantic Ocean, South Africa, Cape of Good Hope, donated by Sir Andrew Smith; BMNH 1881.3.11.2, adult female, 852 mm TL, stuffed specimen, Western Indian Ocean, Mauritius, donated by M. De Robillard; BMNH 1900. 11.6.14 (not Squalus acutipinnis ), adult female, 565 mm TL, Southeastern Atlantic Ocean, South Africa, Table Bay, donated by Dr. J. D. F. Gilchrist.
Non-type material.— (29 specimens) SAIAB 7829, adult female, 575 mm TL, South Africa, Port Elizabeth, 33.96°S, 25.60°E; SAIAB 10443, 3 neonate females, 200 mm TL each, neonate male, 200 mm TL, adult male, 410 mm TL, South Africa, Cape Town, 33.91°S, 18.41°E; SAIAB 19863, adult female, 570 mm TL, South Africa, Port Alfred, 34.26°S, 27.58°E; SAIAB 21933, juvenile female, 252 mm TL, South Africa, off Gansbaai, 35.03°S, 19.43°E; SAIAB 25360, adult female, 530 mm TL, South Africa, Algoa Bay, 34.0 3°S, 25.70°E; SAIAB 25361, juvenile female, 460 mm TL, South Africa, Algoa Bay, 34.03°S, 25.70°E; SAIAB 25369, juvenile female, 375 mm TL, adult female, 450 mm TL, South Africa, Algoa Bay, 34.03°S, 25.70°E; SAIAB 25390, juvenile female, 460 mm TL, South Africa, Algoa Bay, 34.03°S, 25.70°E; SAIAB 25394, adult female, 525 mm TL, South Africa, Algoa Bay, 34.03°S, 25.70°E; SAIAB 26639, adult female, 485 mm TL, unknown locality; SAIAB 34576, adult female, 550 mm TL, South Africa, Bushmans River Mouth, 33.68°S, 26.66°E; SAM 12986, neonate female, 185 mm TL, South Africa, unknown precise locality; SAM 12996, neonate male, 205 mm TL, 2 neonate females, 202, 205 mm TL, South Africa, St. Francis Bay, 34.06°S, 25.13°E; SAM 28638, 2 juvenile females, 365, 375 mm TL, South Africa, False Bay, 34.18°S, 18.61°E; SAM 32550, adult female, 695 mm TL, unspecified locality, South Africa, Eastern Cape, 34.44°S, 26.01°E; SAM 32894, neonate female, 273 mm TL, 3 juvenile males, 240, 310, 318 mm TL, South Africa, off Mossel Bay, 34.56°S, 22.53°E; SAM 34217, juvenile male, 395 mm TL, South Africa, Eastern Cape, unspecified locality, 34.13°S, 26.36°E; ZMB 19151, adult female, 675 mm TL, South Africa, Simonstown.
Diagnosis.— A species of Squalus mostly from the Western Indian Ocean diagnosed by the following combination of characters: snout rounded and short (preorbital snout length 6.3% for lectotype, 6.5–7.1% TL for non-type material), prenarial snout length 0.8 (0.9–1.0) times inner nostril-labial furrow space; first dorsal fin upright, triangular in shape and low (8.2%, 8.0–9.3% TL); pectoral fins markedly broad with posterior margin conspicuously greater than trunk height when adpressed on body; and caudal fin with narrow dorsal lobe and continuous caudal fork between lobes. Squalus acutipinnis can be distinguished from all species of the S. megalops-cubensis group (except from S. megalops , S. brevirostris , and S. crassispinus ) by fewer total (108, 107–111 [mode 109, n ¼ 10] vs. 113–125), precaudal (81, 81–83 [mode 82, n ¼ 10] vs. 84–96), and monospondylous vertebrae (41, 37–42 [mode 40–41, n ¼ 10] vs. 41–49). Squalus acutipinnis differs from S. megalops from Southern Australia by having a rounded snout (vs. pointed snout), L-shaped free rear tips of pectoral fins and pectoral fins not lobe-like (vs. evidently rounded and lobe-like pectoral fins), triangular first dorsal fin (vs. rounded first dorsal fin at tip), pectoral fins transcending trunk height when adpressed on body (vs. pectoral fins, never reaching trunk height), shorter prespiracular length (11.6%, 7.7–12.1% TL vs. 13.5%, 11.6–13.4% TL), more elongated first dorsal spine (3.5–4.3% TL vs. 1.8–3.6% TL), dermal denticles broad at crown with length equal to width (vs. dermal denticles slender at crown with length much greater than width), and margins of pectoral and caudal fins white but not uniform (vs. evident uniform white margins). Squalus acutipinnis further differs from S. brevirostris from Japan by having pectoral fins with L-shaped free rear tips and not lobe-like, and with straight posterior margin (vs. pectoral fins triangular and with pointed free rear tips, lobe-like and with strongly concave posterior margin), length of pectoralfin anterior margin 1.8 (1.5–1.9) times greater than length of its inner margin (vs. length of pectoral-fin anterior margin 1.4, 1.2–1.5 times greater than its inner margin length), and length of first dorsal-fin posterior margin relatively greater 9.6%, 8.8–10.3% TL (vs. 7.7%, 8.3–8.8% TL). The Southern African species is also clearly distinct from S. crassispinus by narrower first and second dorsal spines (first dorsal spine base width 0.6%, 0.6–0.9% of TL vs. 1.3%, 1.2–1.3% of TL; second dorsal spine base width 0.6%, 0.7–1.0% of TL vs. 1.5%, 1.3– 1.4% of TL).
Description.— Measurements and meristic data are summarized in Tables 1 View Table 1 and 2 View Table 2 . Single values are for lectotype, while ranges represent all non-type specimens from which data were taken.
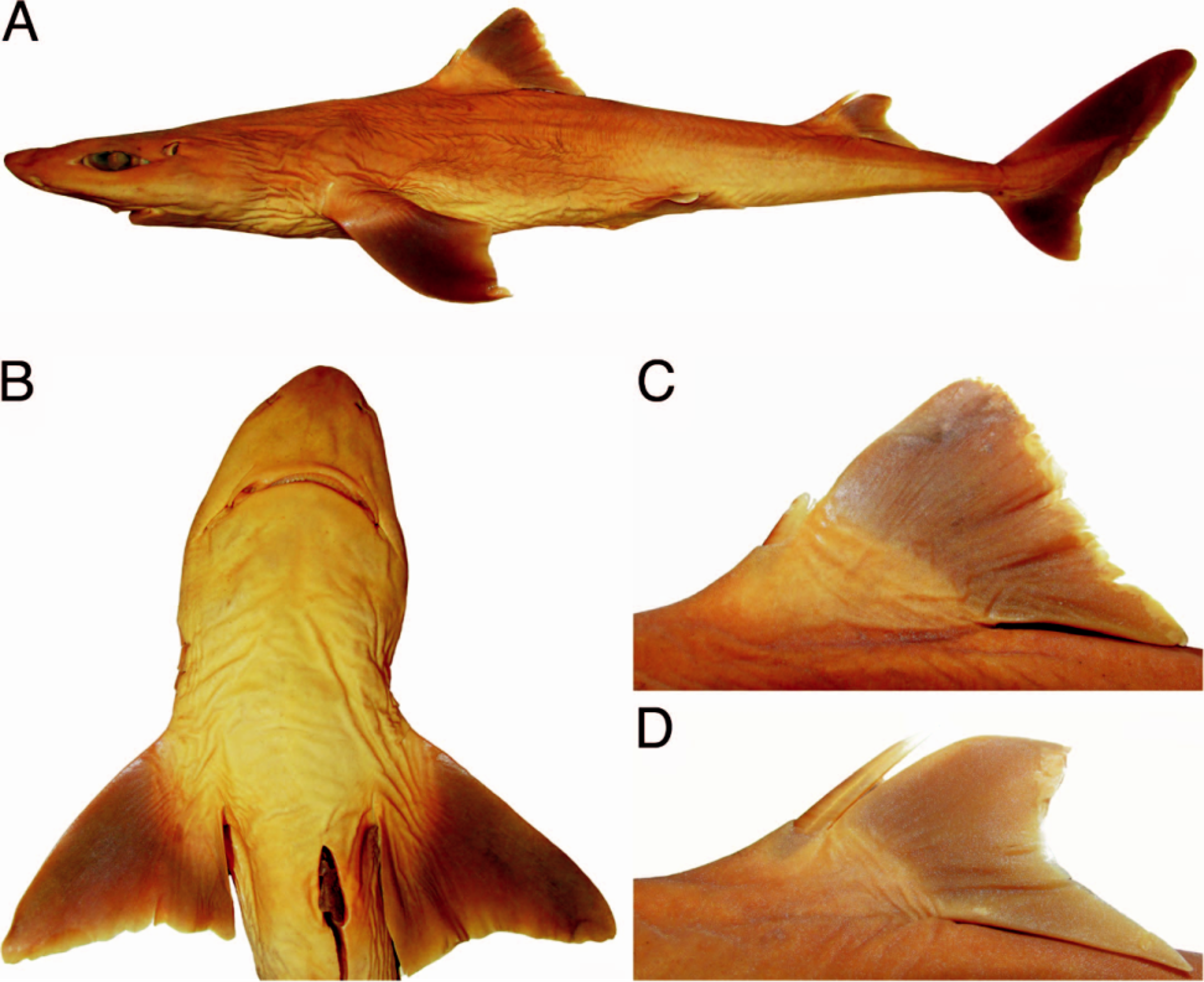
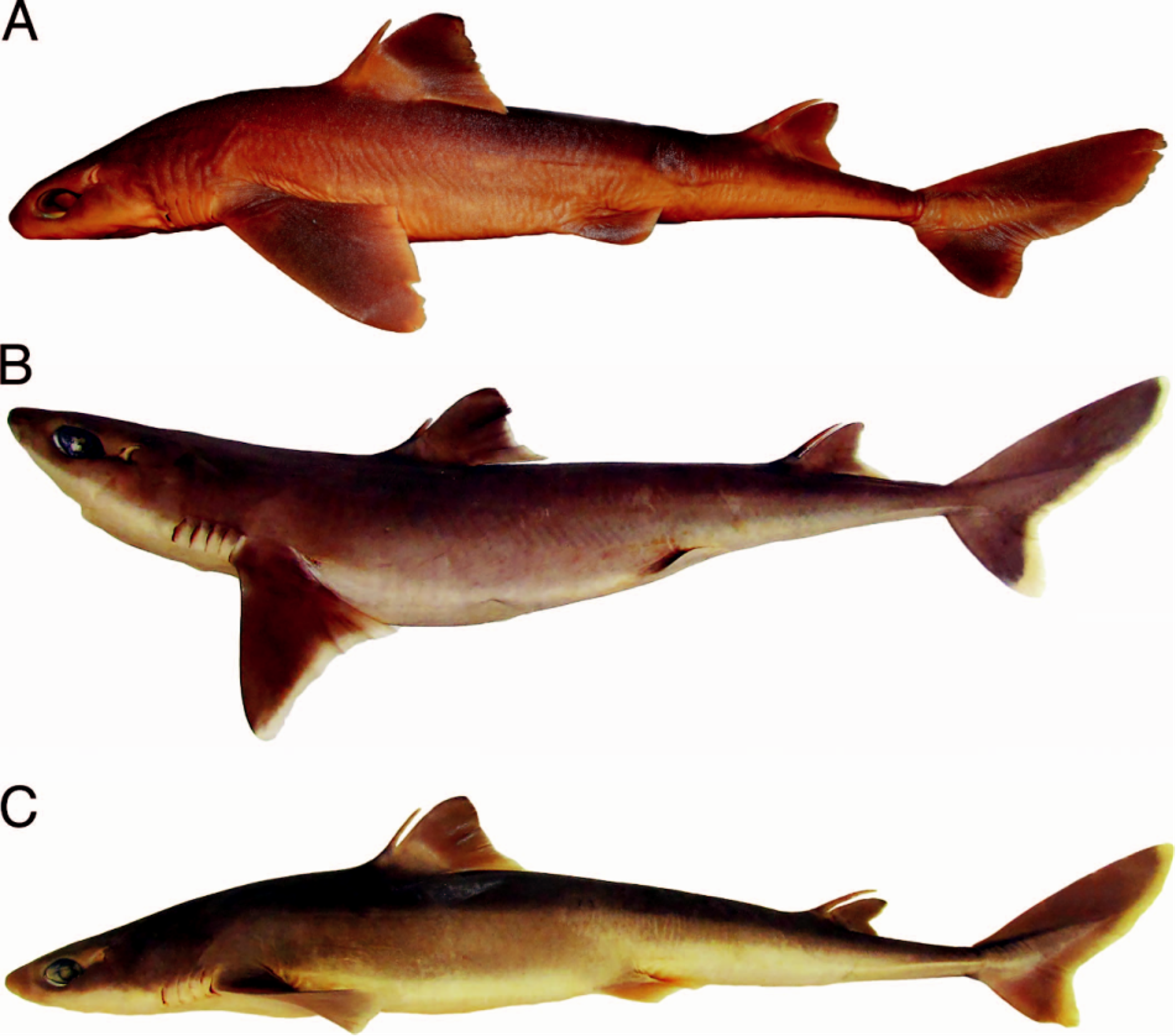
External morphology.— Body fusiform ( Fig. 1 View Fig. 1 ), arched from posterior margin of eye to vertical through pelvic-fin origin, and very deep at trunk with height 12.0% TL in lectotype (9.2–12.7% TL for non-type specimens). Head very small, its length 23.1% (21.4–24.3%) TL, depressed anteriorly; head width 1.2 (1.1–1.4) times trunk width, and 1.7 (1.4–1.7) times abdomen width. Snout short 4.0% (3.9–4.4%) TL, obtuse, and rounded at tip; nasal apertures oblique and located laterally with strongly bilobed anterior margin of nostrils; prenarial length 0.8 (0.9–1.0) times eye length and one-half preoral length; prenarial length 0.8 (0.9–1.0) times distance between nostril and upper labial furrow. Eyes large (5.1%, 4.3–5.1% TL) and horizontally oval, with concave anterior margin and acute posterior margin; eye length 3.5 (2.0–2.8) times its height, positioned nearer to snout tip than to first gill slit. Prespiracular length about one-half prepectoral length; spiracles half-moon shaped, small (1.5%, 1.2– 2.2% TL), located above and posterior to posterior eye margin. Fifth gill slit height 1.5 (1.0–1.4) times first gill slit height.
Mouth highly arched and broad, its width 8.5% (7.7–9.0%) TL and 2.2 (1.9–2.2) times distance between nostrils; upper labial furrow elongated 2.8% (2.5–2.9%) TL and slim. Unicuspid teeth similar in both jaws, labial-lingually flattened and alternate, broad at crown; teeth broader in lower jaw than in upper jaw; cusp robust and slightly elongated, diagonal and upwardly directed; mesial cutting edge concave; distal heel markedly rounded; slender mesial heel; apron short in upper teeth and more elongated in lower teeth; lectotype with three series of functional teeth in upper jaw and two series in lower jaw; tooth row formula 13/11 ( Fig. 2 View Fig. 2 ; Table 2 View Table 2 ).
Pre-first dorsal length 1.4 (1.3–1.5) times prepectoral length. Triangular first dorsal fin with convex anterior margin, straight posterior margin, slightly slender at apex, very low with height 8.2% (8.0–9.3%) TL, corresponding to 1.5 (1.4–1.5) times its inner margin length; wide at its base with base length 8.3% (7.5–8.5%) TL; first dorsal fin length 1.2 (1.1–1.3) times second dorsal fin length. First dorsal spine short (1.9%, 3.5–4.3% TL), not reaching fin apex. Interdorsal space 1.0 (1.0–1.3) times prepectoral length, and 1.9 (1.7–2.4) times dorsal-caudal distance. Second dorsal fin also low, its height 6.1% (5.1–6.7%) TL, corresponding to 1.2 (1.0–1.4) times its inner margin length; wide at its base (6.5%, 5.7– 7.8% TL base length); second dorsal fin with convex anterior margin and concave posterior margin, fairly falcate. Second dorsal spine slender and elongate (3.8%, 4.5–6.3% TL), not reaching fin apex; second dorsal spine length 2.0 (1.1–1.6) times first dorsal spine length.
Pectoral fins conspicuously broad with convex anterior margin, forming an acute angle with its posterior margin; inner margin convex; posterior margin somewhat straight; both apex and free rear tip rounded, although the latter Lshaped and not lobe-like; pectoral fin apex slightly transcending its free rear tip; length of pectoral-fin anterior margin 1.8 (1.5–1.9) times greater than its inner margin length; length of pectoral-fin posterior margin conspicuously transcending trunk height when adpressed on body; length of pectoral-fin posterior margin 1.1 (1.0–1.4) times trunk height. Pectoral-pelvic distance 0.7 (0.7–0.9) times pelviccaudal distance; pre-pelvic length 1.5 (1.3–1.6) times distance between dorsal fins; pelvic fin located at mid-distance between first and second dorsal fins in lectotype, but in non-type specimens pelvic fin slightly nearer to second dorsal fin than to first dorsal.
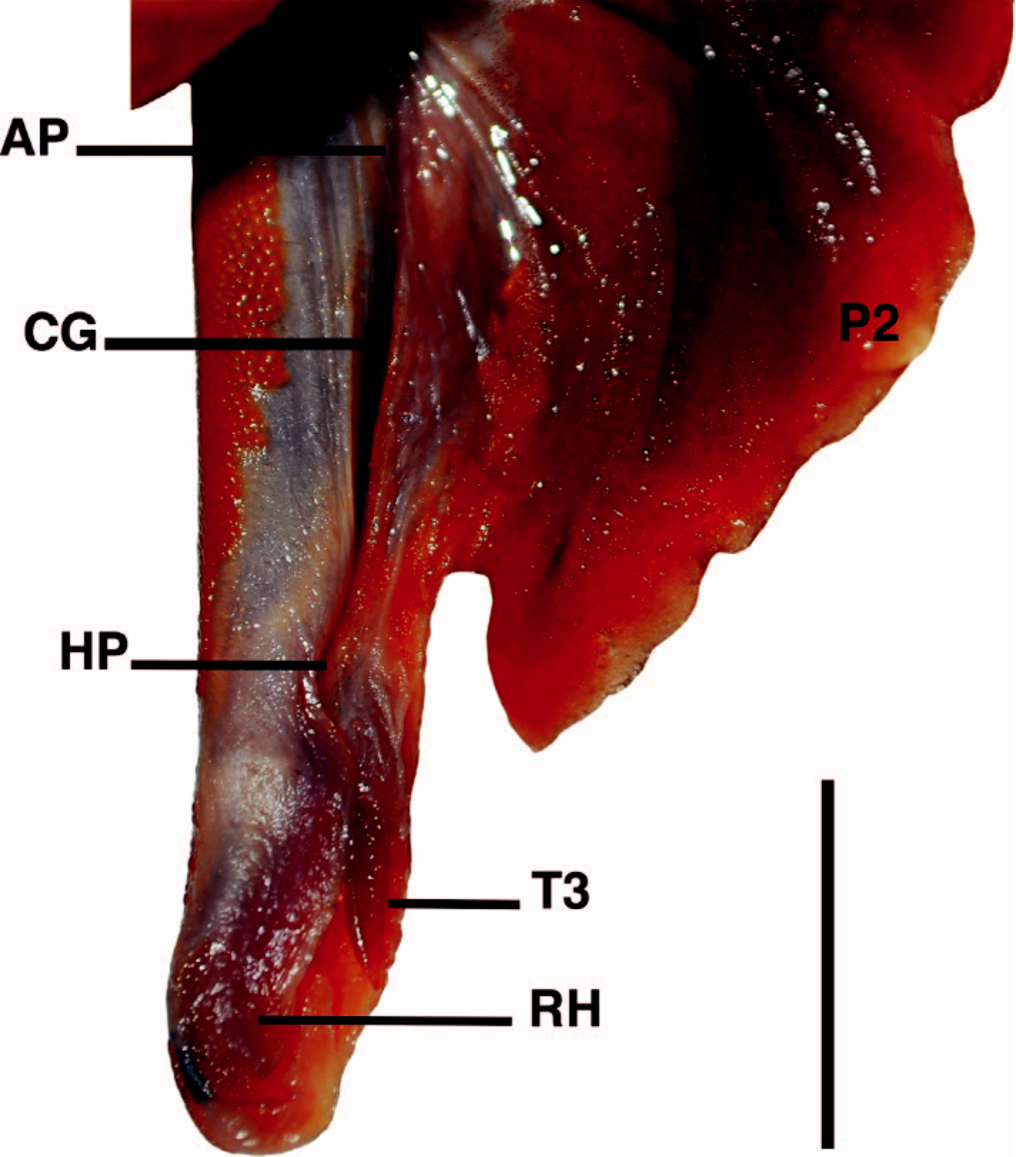
Pelvic fins pentagonal with slightly convex anterior and posterior margins, and free rear tips pointed and triangular; pelvic fin length 10.8% (9.8–11.6%) TL; pelvic inner margin length 5.1% (4.2–6.4%) TL. Clasper in adult males slightly transcending pelvic fin free rear tips, with clasper outer length 1.8% TL; siphon prominent, short, and located ventrally from the posterior half of the basipterygium to intermediate cartilages of pelvic fins; clasper groove longitudinal, positioned dorsally; apopyle with broad aperture, anteriorly situated in clasper groove; hypopyle with narrow aperture, posteriorly in clasper groove and anterior to rhipidion; rhipidion large, flap-like with its free edge laterally positioned, internally supported by dorsal terminal 2 cartilage, and located at posterior end of clasper ( Fig. 3 View Fig. 3 ).
Caudal peduncle with evident lateral keels, originating behind insertion of second dorsal fin; upper and lower precaudal furrows well developed; caudal fin somewhat rectangular with elongated upper caudal lobe (20.7%, 19.7– 22.8% TL), slightly slender at tip dorsal to vertebral column; upper caudal lobe length 0.9 (0.8–1.1) times head length, and 2.0 (1.8–2.0) times lower caudal lobe length; short lower caudal lobe (10.5%, 10.3–12.0% TL) with straight preventral margin; very discrete caudal fork between lobes, its width 7.2% (6.9–7.5%) TL.
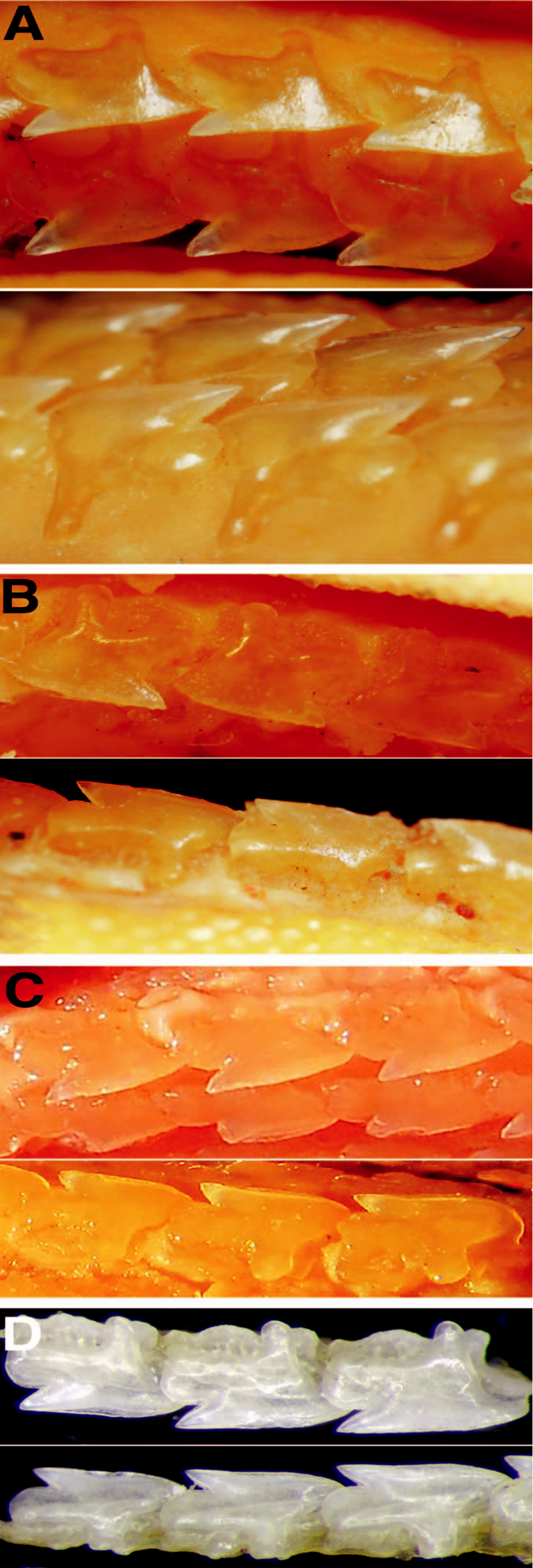
Dermal denticles.— Denticles lanceolate and unicuspid ( Fig. 4 View Fig. 4 ), somewhat broad at crown; denticles close together but not imbricated; denticle length slightly greater than width; median ridge elongated, extending far anteriorly from crown base; lateral ridges shorter than median ridge, asymmetrical with irregular lengths; both median and lateral ridges bifurcate distally. Juveniles differ from adults by having somewhat more slender denticles, very sparsely grouped, relatively far from each other; median ridge prominent, not bifurcated; lateral expansions inconspicuous.
Coloration.— Body brownish dorsally ( Figs. 8 View Fig. 8 , 9 View Fig. 9 ) and pale laterally from dorsal to pelvic fins to just anterior to upper caudal furrow, and extending ventrally. Dorsal fins brown, whitish at fin base, with posterior margin discretely white although not extending to apex on first dorsal fin; dorsal-fin spines light brown and whitish at tips. Pectoral fins brown, pale at base, with posterior margins white but not uniform. Pelvic fins light gray, pale dorsally and ventrally at base, and whitish on inner and posterior margins. Caudal fin predominantly dark brown with post-ventral margins not uniformly white, and slightly broad white apex on ventral lobe.
Clasper skeletal morphology.— Intermediate segment single ( Fig. 5 View Fig. 5 ), barrel-shaped and small, connecting pelvic fin basipterygium to axial cartilage of clasper; beta cartilage single, cylindrical and slender, located dorsal to intermediate segment; short and sinuous axial cartilage; end-style slender with constricted distal extremity; dorsal marginal cartilage elongated, sinuous laterally, dorsal to axial cartilage; ventral marginal cartilage broad distally, concave on inner side, forming a folded plate at contact with accessory terminal cartilage (or spur); accessory terminal cartilage exposed externally, slender, thorn-like, somewhat elongated, with evident lateral furrows on each side, and partially attached to ventral terminal cartilage; dorsal terminal cartilage short and hook-like, slightly concave at tip, located distally to dorsal marginal cartilage; dorsal terminal 2 cartilage leaf-like, sharp on lateral edge, attached medially to dorsal terminal cartilage and anteriorly to dorsal marginal cartilage; ventral terminal cartilage somewhat spoon-like with rounded posterior tip.
Vertebral counts.— Precaudal vertebrae 81 for lectotype (81–83 [mode 82] for non-type specimens; Table 2 View Table 2 ); caudal vertebrae 27 (25–29 [mode 27–28]); total vertebrae 108 (107–111 [mode 109]); monospondylous vertebrae 41 (37–42 [mode 40–41]); diplospondylous vertebrae 67 (66–71 [mode 68–71]).
Geographic distribution.— Squalus acutipinnis is originally described from Kwazulu-Natal, South Africa, and Mauritius ( Fig. 6). It is more common in the Western Indian Ocean, frequently occurring from Kwazulu-Natal to Algoa Bay in the Eastern Cape ( South Africa). There are few records from the Atlantic side of South Africa but without occurrences to the north-northwest of Cape Town.
Etymology.— The epithet acutipinnis is from acutus (¼acute) and pinna (¼fin) in Latin, and probably concerns to the acute angle between anterior and posterior margins of the pectoral fin, which was used to characterize this species (etymology not provided in original description).
Remarks.— Squalus acutipinnis was first described by Regan (1908a) based on four specimens from different localities in the Eastern Atlantic and Western Indian Oceans surrounding South Africa. All specimens were mentioned in the original description and again in Regan (1908b), in which he formally stated that the syntypes were deposited in the Natural History Museum in London. Later, Krefft (1968) designated a lectotype for S. acutipinnis that corresponds to the specimen figured in the original description and to specific information provided by Regan (1908a).
Many morphological characters provided by Regan (1908a) are congruent with our observations; for example, the short snout, large pectoral fins, and caudal fin with continuous caudal fork between upper and lower postventral margins, as well as some proportional dimensions (e.g., pectoral fin length and its distance to the fifth gill slit). However, some supposedly diagnostic characters of the species, such as prenarial length, inner nostril to labial furrow space, and length and height of first dorsal fin, are not congruent among all type specimens of S. acutipinnis . This discrepancy is explained by the heterogeneous nature of the type series of S. acutipinnis (see discussion below). The present redescription is based on the lectotype and new (non-type) material from South Africa.
Doubts also exist concerning the precise type locality of S. acutipinnis as Regan (1908a:241) cited two different localities, one as Bird Island near Algoa Bay in South Africa and the second as Kwazulu-Natal, South Africa. Both localities are written on the label of the type specimens. Some previous authors have mentioned the type locality as being either Algoa Bay, Bird Island or Kwazulu-Natal, such as Merrett (1973) who considered the former locality as being that of the lectotype. Kwazulu-Natal is clearly the type locality of S. acutipinnis as indicated in the original description as well as being the locality of the lectotype later designated by Krefft (1968), whereas Bird Island should probably be considered an error.
DISCUSSION
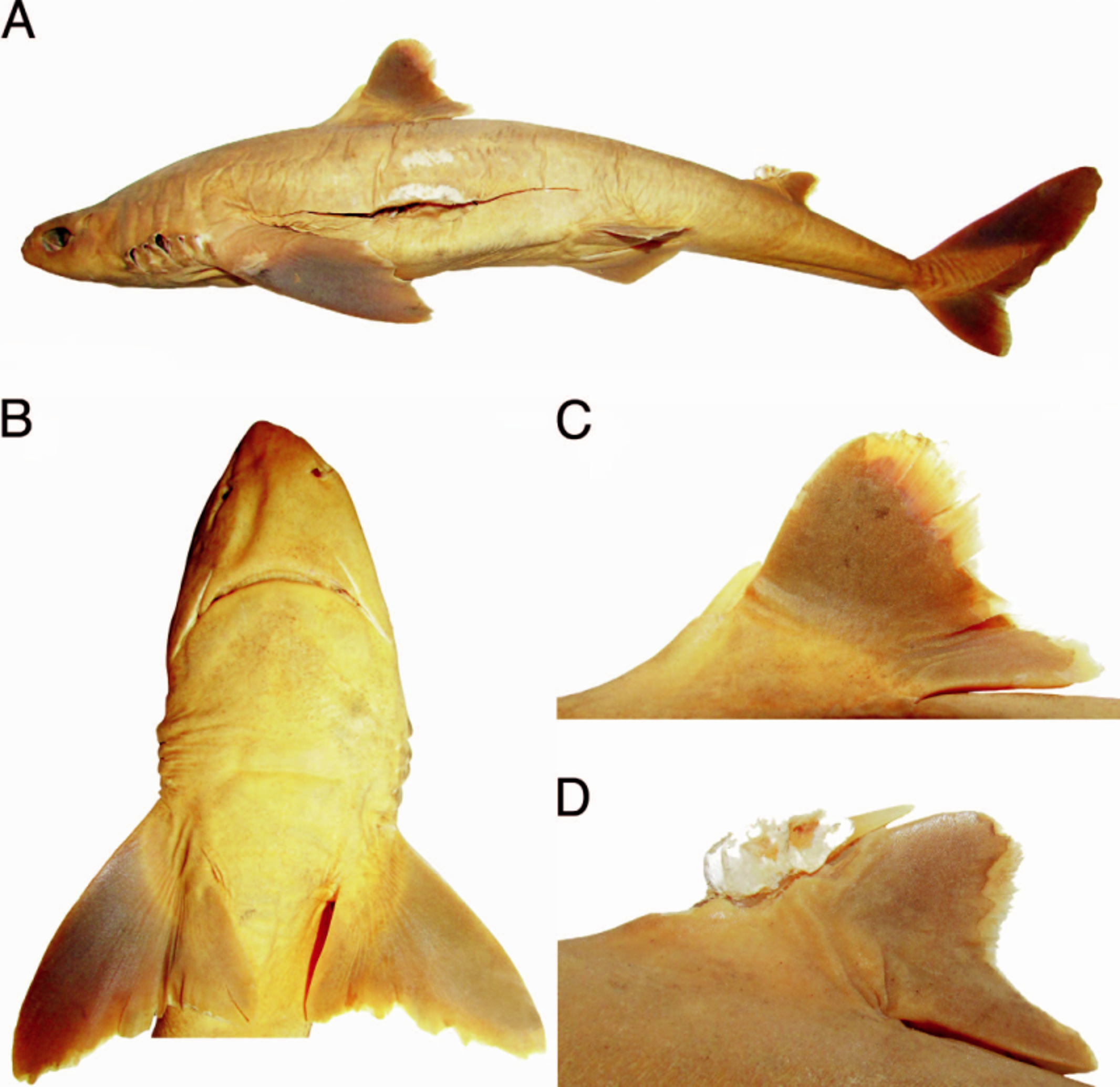
Analysis of the type series of S. acutipinnis revealed relevant morphological variation between the lectotype and paralectotypes ( Figs. 1 View Fig. 1 , 7 View Fig. 7 ): the proportional distance between first dorsal-fin origin and pectoral-fin origin 1.4 times preorbital length in the lectotype vs. 0.7–1.1 times in the paralectotypes; first dorsal-fin base length 1.3 times preorbital length vs. 0.9–1.2 times; fifth gill slit 1.5 times longer than first gill slit vs. 1.1–1.2 times; dermal denticles more slender at crown base, their length somewhat equal to width, and with asymmetrical lateral expansions in the lectotype vs. denticles broader at crown base with their length greater than width, and symmetrical lateral expansions; and continuous caudal fork between lobes vs. evident discontinuous caudal fork. The types are also very distinct in dentition: upper teeth wide in lectotype vs. more slender in paralectotypes; lower teeth with thick and slightly elongated cusp, directed upwardly vs. lower teeth wider and depressed, with cusp very short and somewhat oblique; concave mesial cutting edge vs. straight mesial cutting edge; pointed mesial heel vs. rounded mesial heel; apron shorter in upper teeth than in lower teeth vs. very short apron in both jaws ( Fig. 2 View Fig. 2 ). Vertebral counts also show variation in the types of S. acutipinnis , with greater disparity in the precaudal vertebrae (81 in lectotype vs. 84) and caudal vertebrae (27 vs. 25; Table 2 View Table 2 ).
Our analysis of the type specimens of S. acutipinnis , therefore, clearly indicates that Regan (1908a) based the original description on a combination of two different species that are not sympatric and clearly separable from each other by external morphology and measurements. Morphological variation in the type series of S. acutipinnis has not been previously reported and has doubtlessly contributed to the taxonomic confusion concerning the identity and validity of this species. One of the species of the type series is illustrated in the original description as the representative of S. acutipinnis , which refers to the lectotype (BMNH 1905.6.8.8, female, 578 mm TL) from Kwazulu-Natal ( South Africa) as well as to a stuffed paralectotype (BMNH 1881. 3.11.2, female, 852 mm TL) from Mauritius (previously considered missing by Krefft, 1968). The other two remaining paralectotypes of S. acutipinnis ( Fig. 7 View Fig. 7 ) represent an undescribed species of Squalus that is under investigation as part of a taxonomic revision of all species of Squalus (Viana and Carvalho, unpubl.).
Another factor that may have contributed to the confusion concerning the validity of S. acutipinnis is the attempt by some authors to recognize only species groups of Squalus in the region (e.g., Bass et al., 1976; Compagno, 1984) rather than attempt to better define morphologically the species present off South Africa. This may have, unintentionally, obscured relevant diagnostic characters of S. acutipinnis , contributing to its status as a synonym of other nominal species that have wide geographical distribution such as S. megalops and S. mitsukurii (e.g., Chen et al., 1979; Compagno, 1984).
Regan (1908b) emphasized that S. acutipinnis was clearly separable from S. megalops (type locality: Port Jackson, New South Wales, Australia) from Southern Australia and Tasmania by not having acutely pointed pectoral fin free rear tips and straight pectoral posterior margin, which reaches the level of the first dorsal fin insertion, features corroborated as diagnostic in our study. These species clearly differ from each other by pectoral fin shape, which is also consistently narrower in S. megalops than in S. acutipinnis ( Figs. 8 View Fig. 8 , 9 View Fig. 9 ). The former has conspicuously rounded and lobe-like free rear tips, pectoral fin apex transcending its free rear tip, and pectoral-fin posterior margin never transcending trunk height when adpressed on body, confirming the observation by previous authors that S. acutipinnis has longer pectoral fins than S. megalops (e.g., Krefft, 1968). Squalus acutipinnis is also very distinct from the holotype of S. megalops by having an arched mouth (vs. relatively straight mouth), continuous caudal fork between lobes (vs. discontinuous caudal fork), and pelvic fins with pointed and triangular free rear tips (vs. pelvic fins with very rounded and lobe-like free rear tips).
Specimens of S. acutipinnis further differ from S. megalops from Southern Australia by external measurements ( Table 1 View Table 1 [single value for S. megalops is from holotype]): smaller prespiracular length (11.6%, 7.7–12.1% TL vs. 13.5%, 11.6– 13.4% TL, respectively); smaller distance between inner margin of nostril and upper labial furrow (4.7%, 4.3–4.9% TL vs. 5.3%, 4.6–5.3% TL); smaller interorbital space (7.8%, 7.4–8.6% TL vs. 8.7%, 8.3–10.0% TL); greater length of first dorsal spine (3.5–4.3% TL vs. 1.8–3.6% TL) corresponding to at least half of first dorsal fin height (vs. less than half of first dorsal fin height); and second dorsal spine length 0.6 (0.8– 1.0) times second dorsal fin height (vs. 0.4, 0.5–0.8 in S. megalops ). Despite slightly overlapping, morphometric characters have recently proven useful for distinguishing closely related species of Squalus ( Last et al., 2007) .
Last et al. (2007) reported intraspecific variation in S. megalops from different regions of Australia based mainly on external measurements. Squalus acutipinnis differs significantly from the S. megalops of these authors by the following characters: greater labial furrow length (2.8%, 2.5–2.9% TL for S. acutipinnis vs. 2.1–2.4% TL and 2.3–2.5% TL for S. megalops from Southeastern Australia and Queensland, respectively); smaller internarial space (3.8%, 3.6–4.3% TL vs. 4.3–4.7% TL and 4.6–4.9% TL for S. megalops from Southeastern Australia and Queensland, respectively); higher first dorsal fin (8.2%, 8.0–9.3% TL for S. acutipinnis vs. 6.1– 7.4% TL, 6.2–6.6% TL and 7.0–7.5% TL for S. megalops from Southeastern Australia, Queensland, and Western Australia, respectively); greater posterior margin of first dorsal fin (9.6%, 8.8–10.3% TL for S. acutipinnis vs. 7.6–8.1% TL and 7.5–8.1% TL for S. megalops from Queensland and Western Australia, respectively); higher second dorsal fin (6.1%, 5.1– 6.7% TL for S. acutipinnis vs. 3.6–4.6% TL, 3.2–4.0% TL, and 3.7–4.3% TL for S. megalops from Southeastern Australia, Queensland, and Western Australia, respectively); greater length of pectoral anterior margin (17.7%, 15.1–18.1% TL for S. acutipinnis vs. 13.6–14.9% TL, 12.3–12.6% TL, and 13.7– 15.1% TL for S. megalops from Southeastern Australia, Queensland, and Western Australia, respectively), and greater length of pectoral inner margin (9.8%, 9.5–11.8% TL for S. acutipinnis vs. 7.4–9.2% TL and 7.7–8.8% TL for S. megalops from Southeastern Australia and Queensland, respectively).
Measurements of the lectotype of S. acutipinnis are clearly distinct from those provided by Garrick (1960) for S. megalops from Southeastern Australia, with the exception of preoral distance (8.9% TL vs. 8.5–9.2% TL), dorsal caudal margin length (20.7% TL vs. 19.6–22.1% TL), and precaudal distance (79.9% TL vs. 79.0–82.0% TL). These observations are congruent with the results given for S. megalops by Bass et al. (1976) from South Africa, indicating its conspecificity with S. acutipinnis . These authors probably took into account their definition of the S. megalops group for evaluating the taxonomic status of the South African species, rather than the strong differences in measurements apparent between these two species. The synonymy of these two species proposed by Bass et al. (1976) is unwarranted according to our results that support the validity of S. acutipinnis .
The Southern African species is also easily distinct from its regional congeners S. acanthias , S. blainvillei (sensu Bass et al., 1976) , and S. mitsukurii (sensu Compagno, 1984) by: short and rounded snout (vs. elongate and pointed snout); broad pectoral fins (vs. narrow pectoral fins); lanceolate dermal denticles (vs. non-lanceolate unicuspid in S. acanthias , and tricuspid in specimens identified as S. blainvillei and S.
mitsukurii from South Africa). Additionally, S. acutipinnis differs from S. acanthias by lacking white spots dorsally on the body (vs. presence of conspicuous white spots) and having a greater number of precaudal vertebrae (81, 81–83 vs. 74–81, respectively). It is also distinct from S. acanthias by having anterior margin of nostrils bilobate (vs. anterior margin of nostrils unilobate), first dorsal-fin origin anterior to pectoral free rear tips (vs. first dorsal-fin origin posterior to pectoral free rear tips).
More recently, Naylor et al. (2012a, 2012b), based on mitochondrial genes, indicated that a possible new species might occur in South Africa that is distinct from S. megalops from Australia and more closely related to S. brevirostris from Japan. Our current study shows that S. acutipinnis is clearly distinct from the Japanese species by ( Figs. 8 View Fig. 8 , 9 View Fig. 9 ): pectoral fin with rounded free rear tips, not lobe-like, and straight posterior margin (vs. triangular and pointed free rear tips, lobe-like, and strongly concave posterior margin); length of pectoral anterior margin 1.8, 1.5–1.9 times length of pectoral fin inner margin (vs. length of pectoral anterior margin 1.4, 1.2–1.5 times length of pectoral inner margin); pre-first branchial length 1.6, 1.5–2.5 times prespiracular length (vs. pre-first branchial length 1.5, 1.4–1.5 times prespiracular length); and length of posterior margin of first dorsal fin greater in S. acutipinnis than in S. brevirostris (9.6%, 8.8– 10.3% TL vs. 7.7%, 8.3–8.8% TL, respectively). The pelvic fin of the lectotype of S. acutipinnis is located closer to the midline between both dorsal fins, whereas in the holotype of S. brevirostris it is closer to the first dorsal fin.
Squalus cf. megalops (sensu Naylor et al., 2012 a, 2012 b) also refers to a new species of Squalus from South African waters that was probably also included in the analysis of Bass et al. (1976) when defining the S. megalops group in the region, an act that further contributed to the taxonomic confusion concerning S. acutipinnis . Current investigation of this apparent new species indicates it exhibits more morphological similarities with S. megalops from Australia and S. brevirostris , rather than with S. acutipinnis .
Concerning dentition, the middle tooth is absent in S. acutipinnis , contrary to what was reported by Bass et al. (1976) and Myagkov and Kondyurin (1986) for distinguishing Southern African species of Squalus . This character is rarely efficient in separating species of the genus (e.g., Chen et al., 1979; Mũnoz-Chápuli and Ramos, 1989; Last et al., 2007), as presently noticed for S. acutipinnis , S. megalops , and S. brevirostris .
The clasper of the three species compared here is very conservative in relation to the number of terminal cartilages and the shapes of the beta cartilage, intermediate segment, axial cartilage, and marginal cartilages. Squalus acutipinnis is, however, more similar to S. brevirostris than to S. megalops concerning the terminal cartilages. The former two species have accessory terminal cartilage (spur) thicker and shorter as well as dorsal terminal cartilage (claw) thicker, shorter, and conspicuously concave in its distal portion when compared to S. megalops from Australia. The internal morphology of the clasper may be useful for distinction at species level in Squalus as shown by descriptions of terminal clasper components in species from other regions ( Muñoz-Chápuli and Ramos, 1989).
Vertebral counts are greatly useful for distinguishing many species of the genus (e.g., Springer and Garrick, 1964; Last et al., 2007; Table 2 View Table 2 ). Squalus acutipinnis , S. megalops from Southern Australia, and S. brevirostris present overlap in numbers of vertebrae, even though the holotype of S. megalops has more precaudal vertebrae than the lectotype of S. acutipinnis and specimens of S. brevirostris . On the other hand, S. acutipinnis can be easily distinguished from other congeners such as S. bucephalus , S. raoulensis , S. albifrons , S. altipinnis , and S. notocaudatus by monospondylous, precaudal, and total vertebrae.
According to Last et al. (2007), some specimens of the S. megalops group can be placed in the ‘‘highfin megalops subgroup,’’ characterized by tricuspid dermal denticles, small snout (6.0–7.0% TL preoral length), high and upright first dorsal fin (7.7–9.4% TL height), and heavy dorsal-fin spines (0.7–1.1% TL exposed base length). These characters are present in S. acutipinnis , except for the shape of the dermal denticles (lanceolate as in some other species of the Squalus megalops group) and shape of the dorsal fins. Due to the overlap of characters between the species complexes of Squalus , we prefer not to allocate S. acutipinnis to a species group. Current investigation of the species of Squalus in South African waters (Viana and Carvalho, unpubl.) indicates that the diversity of the genus in both the Atlantic and Indian Oceans is greater than previously recognized.
Table 1. External measurements expressed as percentage of the total length (% TL) for the type specimens of Squalus acutipinnis, Squalus megalops, and Squalus brevirostris. Ranges for other specimens are also provided. Total length is expressed in millimeters. A: BMNH 1881.3.11.2; B: BMNH 1859.5.7.68; C: BMNH 1900.11.6.14; L: lectotype; H: holotype; n: number of specimens; x: mean; SD: standard deviation.
| Squalus acutipinnis | Squalus megalops | Squalus brevirostris | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Character | L | A | B C n Range | x | SD | H | n Range x | SD | H | n Range x | SD |
| TL (mm) | 578 | 852 | 190 565 8 310-695 | 525.6 | 110.8 | 550 | 10 220–625 442.2 | 139.3 | 426 | 8 275–578 431 | 90.3 |
| PCL | 79.9 | 81.2 | 76.8 80.2 8 75.8–80 | 78.6 | 1.3 | 90.4 | 10 75–80.3 78.5 | 1.4 | 79.8 | 8 78.9–81.3 79.5 | 0.8 |
| PD2 | 61.1 | 64.9 | 55.4 61.1 8 58.1–63.5 | 61.4 | 1.7 | 71.6 | 10 58.9–62.7 61.3 | 1.5 | 61.7 | 8 60.6–63.8 62.1 | 1.1 |
| PD1 | 31.3 | 27.9 | 29.6 28 8 28.6–31.3 | 29.9 | 1 | 34.5 | 10 28.7–31.4 29.9 | 0.9 | 29.6 | 8 28.5–31 29.5 | 0.8 |
| SVL | 49.8 | 53.4 | 46.1 48.1 8 45.8–50.4 | 48.5 | 1.7 | 57.8 | 10 44–51.8 47.9 | 2.3 | 47.7 | 8 45.4–49.5 46.8 | 1.5 |
| PP2 | 45.8 | 49.6 | 43.8 45.3 8 42.6–48.7 | 46.1 | 2.3 | 54.9 | 10 43.2–50.1 45.9 | 2.2 | 43.7 | 8 42.4–46.9 44.2 | 1.5 |
| PP1 | 22.5 | 19.4 | 22.1 22.7 8 19.4–23.6 | 21.6 | 1.4 | 23.7 | 10 20.2–24 21.8 | 1.2 | 21.7 | 8 19–22.9 21.1 | 1.2 |
| HDL | 23.1 | 20.2 | 24.3 23 8 21.4–24.3 | 23.1 | 0.9 | 24.7 | 10 21–24.8 22.5 | 1.4 | 21.9 | 8 20–23.5 22 | 1.1 |
| PG1 | 18.8 | 16.2 | 19.1 19.1 8 17.7–19.3 | 18.7 | 0.6 | 22.3 | 10 17.4–20.3 18.9 | 1 | 17.9 | 8 16.8–19.1 18.1 | 0.7 |
| PSP | 11.6 | 11.2 | 13.1 11.9 8 7.7–12.1 | 11.2 | 1.5 | 13.5 | 10 11.6–13.4 12.6 | 0.7 | 12.1 | 8 11.3–12.9 12.2 | 0.5 |
| POB | 6.3 | 7.1 | 6.9 7.1 8 6.5–7.1 | 6.8 | 0.2 | 7.5 | 10 6.6–7.7 7.1 | 0.4 | 6.7 | 8 6.6–7.5 7.2 | 0.2 |
| PRN | 4 | 4.4 | 3.5 4.7 8 3.9–4.4 | 4.2 | 0.2 | 4.2 | 10 3.9–5.4 4.5 | 0.4 | 4 | 8 3.8–4.4 4.2 | 0.2 |
| POR | 8.9 | 8.4 | 10.1 9.5 8 8.4–9.2 | 8.9 | 0.3 | 9.7 | 10 8.8–10.7 9.5 | 0.7 | 9.1 | 8 8.4–10 9.1 | 0.5 |
| INLF | 4.7 | 3.7 | 4.8 4.9 8 4.3–4.9 | 4.5 | 0.2 | 5.3 | 10 4.6–5.3 5.1 | 0.3 | 5 | 8 4.7–5.5 5 | 0.3 |
| MOW | 8.5 | 6.8 | 7.5 8.5 8 7.7–9 | 8.3 | 0.4 | 9.6 | 10 7.8–9.4 8.5 | 0.5 | 8.1 | 8 7.5–8.1 7.8 | 0.2 |
| ULA | 2.8 | 1.8 | 2.4 2.4 8 2.5–2.9 | 2.6 | 0.2 | 2.7 | 10 2.2–2.8 2.5 | 0.2 | 2.6 | 8 2–2.7 2.3 | 0.3 |
| INW | 3.8 | 4.5 | 3.7 3.8 8 3.6–4.3 | 4 | 0.2 | 4.5 | 10 3.9–4.8 4.3 | 0.3 | 3.6 | 8 3.4–3.9 3.6 | 0.2 |
| INO | 7.8 | 8.4 | 9.9 8.4 8 7.4–8.6 | 8.2 | 0.4 | 8.7 | 10 8.3–10 8.9 | 0.4 | 8.6 | 8 8–9 8.3 | 0.3 |
| EYL | 5.1 | 3.8 | 5.9 4 8 4.3–5.1 | 4.6 | 0.3 | 5.2 | 10 4.2–6.5 5 | 0.7 | 5 | 8 4.2–5.1 4.6 | 0.3 |
| EYH | 1.5 | 2.5 | 1.7 2 8 1.6–2.4 | 2 | 0.2 | 2.3 | 10 1.9–2.6 2.3 | 0.2 | 2.2 | 8 2–2.6 2.2 | 0.2 |
| SPL | 1.5 | 1.3 | 1.5 1.4 8 1.2–2.2 | 1.6 | 0.3 | 1.1 | 10 1.3–2 1.6 | 0.2 | 1.7 | 8 1.4–1.8 1.6 | 0.2 |
| GS1 | 1.8 | 1.1 | 2.4 2.3 8 1.7–2.4 | 2 | 0.2 | 2 | 10 1.2–2.5 2 | 0.3 | 2.1 | 8 1.5–2.6 2.1 | 0.4 |
| GS5 | 2.7 | 2 | 2.7 2.6 8 2.1–2.6 | 2.3 | 0.2 | 3 | 10 2–2.6 2.4 | 0.2 | 2.3 | 8 2.1–2.6 2.3 | 0.2 |
| IDS | 22.1 | 28.9 | 21.2 25.3 8 20.1–25.4 | 24 | 1.9 | 27.3 | 10 21.3–27.2 24.5 | 1.9 | 24.9 | 8 23.3–27.2 25 | 1.3 |
| DCS | 11.5 | 10.8 | 11.3 11.9 8 10.3–12.3 | 11.1 | 0.7 | 11 | 10 9.6–11.7 10.6 | 0.8 | 10.5 | 8 10–10.6 10.4 | 0.2 |
| PPS | 18 | 23.5 | 18.7 22.3 8 17.8–22.6 | 20.7 | 1.9 | 26.9 | 10 16.8–24.2 20.9 | 2.4 | 21.4 | 8 17.2–22.8 20.3 | 2 |
| PCA | 25.3 | 27.6 | 24.1 25.3 8 24.8–27.2 | 25.7 | 0.7 | 26.4 | 10 22.9–28.1 26.1 | 1.9 | 28.1 | 8 24.6–29.4 27.8 | 1.5 |
| D1L | 13.9 | 13.7 | 12.2 13.3 8 13.4–14.2 | 13.8 | 0.3 | 15.1 | 10 10.9–15.1 13.6 | 1.2 | 13 | 8 13.3–14.7 13.9 | 0.4 |
| D1A | 11.1 | 9.9 | 12.9 10.1 8 11.1–11.8 | 11.4 | 0.3 | 8.9 | 10 10.3–13.3 11.8 | 1 | 10.3 | 8 7.9–12 10.8 | 1.3 |
| D1B | 8.3 | 8.2 | 6.4 8.6 8 7.5–8.5 | 7.9 | 0.4 | 8.5 | 10 7.3–8.8 8.1 | 0.5 | 7.3 | 8 7.4–9.2 8 | 0.6 |
| D1H | 8.2 | 7.1 | 10.2 8 8 8–9.3 | 8.7 | 0.4 | 5.3 | 10 8.1–10.4 8.7 | 0.7 | 7.6 | 8 7.3–8.6 8 | 0.4 |
| D1I | 5.6 | 5.2 | 6.9 4.8 8 5.5–6.5 | 6 | 0.3 | 6.9 | 10 3.6–6.4 5.9 | 0.8 | 5.8 | 8 5.4–6.8 6.1 | 0.5 |
| D1P | 9.6 | 9.2 | 8.5 8.8 8 8.8–10.3 | 9.4 | 0.5 | 8.9 | 10 7.5–9.9 8.8 | 0.7 | 7.7 | 8 8.3–8.8 8.6 | 0.2 |
| D1ES | 1.9 | 4.9 | 2.2 2.4 8 3.5–4.3 | 3.8 | 0.3 | — | 10 1.8–3.6 2.8 | 0.6 | — | 7 2.6–4.2 3.5 | 0.5 |
| D1BS | 0.6 | 0.9 | 0.6 0.7 8 0.6–0.9 | 0.7 | 0.1 | 0.8 | 10 0.5–0.8 0.7 | 0.1 | 0.7 | 8 0.5–0.7 0.6 | 0.1 |
| D2L | 11.6 | 10 | 13.1 11.2 8 10.5–13 | 11.6 | 1 | 10.9 | 10 10.4–13.2 12 | 0.9 | 11.6 | 8 11.2–13.2 12.2 | 0.6 |
| D2A | 9.5 | 7.8 | 10.6 9.6 8 8.9–11.3 | 9.6 | 0.8 | 9.5 | 10 8.8–11.5 10.3 | 0.8 | 9.8 | 8 10.3–11.6 10.7 | 0.4 |
| D2B | 6.5 | 6 | 7.1 7.1 8 5.7–7.8 | 6.6 | 0.7 | 7 | 10 5.6–7.9 6.8 | 0.7 | 6.6 | 8 6.1–7.9 7 | 0.5 |
| D2H | 6.1 | 4.6 | 9.1 4.6 8 5.1–6.7 | 5.8 | 0.5 | 5.7 | 10 5.3–7.6 6.2 | 0.6 | 6 | 8 5.7–7 6.2 | 0.5 |
| D2I | 5.1 | 3.9 | 6 4.2 8 4.1–5.7 | 5 | 0.6 | 4.3 | 10 4.3–5.9 5.3 | 0.5 | 5 | 8 4.8–5.6 5.2 | 0.3 |
| D2P | 5.9 | 4.4 | 4.9 4.7 8 4.5–6.2 | 5.4 | 0.6 | 3.9 | 10 4.1–6 5 | 0.6 | 4.3 | 8 4.3–5.3 4.9 | 0.3 |
Table 2. Meristic data for Squalus acutipinnis, S. megalops, and S. brevirostris. Summarized values for other nominal species of Squalus are also provided. A: BMNH 1881.3.11.2; B: BMNH 1900.11.6.14; n: number of specimens; SD: standard deviation.
| Squalus acutipinnis | Squalus megalops | Squalus brevirostris | ||||
|---|---|---|---|---|---|---|
| Paralectotypes | ||||||
| Character | Lectotype | A B n Range Mean SD | Holotype | n Range Mean SD | Holotype | n Range Mean SD |
| Precaudal | ||||||
| vertebrae | 81 | — 84 10 81–83 82 0 | 86 | 8 79–83 81 1 | — | 3 80–83 82 2 |
| Caudal | ||||||
| vertebrae | 27 | — 25 10 25–29 27 1 | — | 8 26–30 28 1 | — | 3 26–30 28 2 |
| Total | ||||||
| vertebrae | 108 | — 109 10 107–111 109 1 | — | 8 107–111 109 2 | — | 3 106–112 109 3 |
| Monospondylous | ||||||
| vertebrae | 41 | — 42 10 37–42 40 1 | 40 | 8 39–41 40 1 | — | 3 39–40 40 1 |
| Diplospondylous | ||||||
| vertebrae | 67 | — 67 10 66–71 69 2 | — | 8 66–72 68 2 | — | 3 66–72 70 3 |
| Upper tooth | ||||||
| rows (right) | 13 | 13 13 4 12–14 13 1 | 13 | 6 13–14 13 0 | 12 | 3 12–14 13 1 |
| Upper tooth | ||||||
| rows (left) | 13 | 12 13 4 11–14 13 1 | 13 | 6 12–13 13 1 | 13 | 3 12–13 12 1 |
| Lower tooth | ||||||
| rows (right) | 11 | — 11 4 10–12 11 1 | 12 | 6 11–12 12 1 | 10 | 3 10–10 10 0 |
| Lower tooth | ||||||
| rows (left) | 11 | — 11 4 10–13 11 1 | 12 | 6 10–12 12 1 | 10 | 3 10–11 11 1 |
| Upper tooth | ||||||
| series | 3 | 2 2 4 2–2 2 0 | 2 | 8 2–2 2 0 | 3 | 3 2–2 2 0 |
| Lower tooth | ||||||
| series | 2 | 2 2 4 2–3 2 1 | 2 | 8 2–2 2 0 | 2 | 3 1–2 2 1 |
| Source | present study | present study | present study |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Squalus acutipinnis Regan, 1908
| Sarah T. de F. Viana & Marcelo R. de Carvalho 2016 |
Squalus fernandinus
| Smith, J. L. B. 1961: 59 |
| Bigelow, H. B. & W. C. Schroeder 1957: 32 |
| Bigelow, H. B. & W. C. Schroeder 1948: 480 |
| Garman, S. 1913: 195 |
Squalus acutipinnis
| Ebert, D. A. & W. T. White & K. J. Goldmann & L. J. V. Compagno & T. S. Daly-Engel & R. D. Ward 2010: 22 |
| Last, P. R. & W. T. White & J. J. Pogonoski 2007: 11 |
| Myagkov, N. A. & V. V. Kondyurin 1986: 8 |
| Cadenat, J. & J. Blache 1981: 47 |
| Merrett, N. F. 1973: 94 |
| Regan, C. T. 1921: 412 |
| Regan, C. T. 1908: 241 |
| Regan, C. T. 1908: 45 |