Heteragrion Selys, 1862
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5356.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:F3CE1E00-45BB-44C8-8911-1A355BFD223C |
|
DOI |
https://doi.org/10.5281/zenodo.10028260 |
|
persistent identifier |
https://treatment.plazi.org/id/73444D3A-FFEF-9112-6AD7-FD67AE25A0D3 |
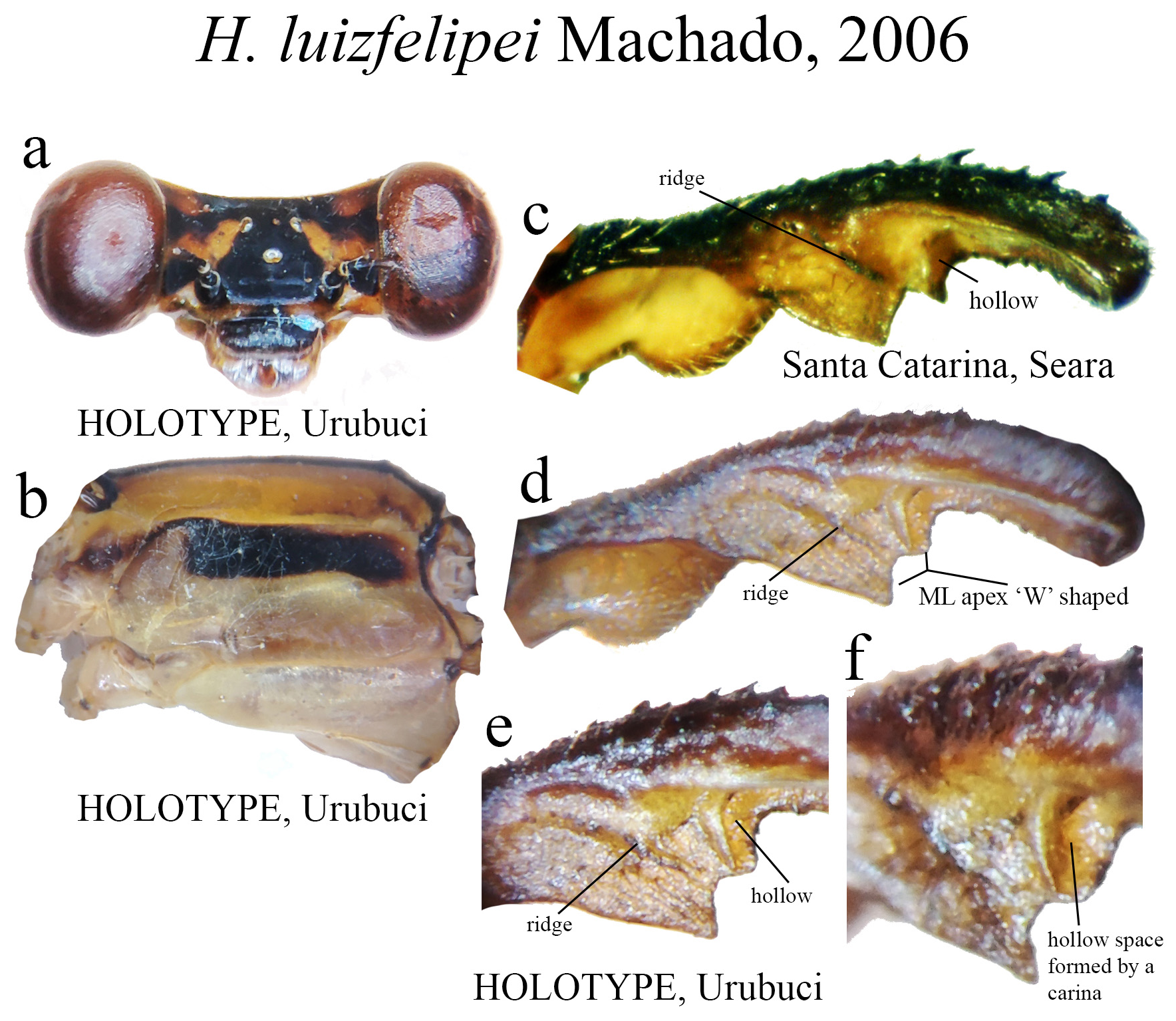
|
treatment provided by |
Plazi |
|
scientific name |
Heteragrion Selys, 1862 |
| status |
|
Heteragrion Selys, 1862 View in CoL
Type species: Heteragrion flavovittatum Selys, 1862: 18 , by original designation.
Other species included: Heteragrion aequatoriale Selys, 1886 ; H. albifrons Ris, 1918 ; H. alienum Williamson, 1919 ; H. angustipenne Selys, 1886 ; H. archon De Marmels, 2008 ; H. aurantiacum Selys, 1862 ; H. azulum Dunkle, 1989 ; H. bariai De Marmels, 1989 ; H. beschkii Hagen in Selys, 1862; H. bickorum Daigle, 2005 ; H. breweri De Marmels, 1989 ; H. brianmayi Lencioni, 2013 ; H. calafatiensis Mendoza-Penagos, Juen & Vilela, 2022 ; H. calendulum Williamson, 1919 ; H. cauei Ávila-Jr., Lencioni & Carneiro, 2017; H. chlorotaeniatum De Marmels, 1989 ; H. chrysops Hagen in Selys, 1862; H. cinnamomeum Selys, 1862 ; H. consors Hagen in Selys, 1862; H. cooki Daigle & Tennessen, 2000 ; H. corderoi sp. nov.; H. cyane Machado & Souza, 2014 ; H. demarmelsi Stand-Pérez, Bota-Sierra & Pérez-Gutiérrez, 2019 ; H. denisye Vilela, Koroiva & Guillermo-Ferreira, 2019 ; H. dorsale Selys, 1862 ; H. eboratum Donnelly, 1965 ; H.erythrogastrum Selys, 1886 ; H. flavidorsum Calvert, 1909 ; H. freddiemercuryi Lencioni, 2013 ; H. gorbi Cezário & Guillermo-Ferreira, 2021 ; H. gracile Machado, 2006 ; H. ictericum Williamson, 1919 ; H. icterops Selys, 1862 ; H. inca Calvert, 1909 ; H. itacolomii Ávila-Jr., Lencioni & Carneiro, 2020; H. johndeaconi Lencioni, 2013 ; H. lencionii Vilela, Farias & Santos, 2021 ; H. luizfelipei Machado, 2006 ; H. majus Selys, 1886 ; H. makiritare De Marmels, 2004 ; H. mantiqueirae Machado, 2006 ; H. mitratum Williamson, 1919 H. muryense Costa & Santos, 2000; H. obsoletum Selys, 1886 ; H. ochraceum Hagen in Selys, 1862; H. ovatum Selys, 1862 ; H. palmichale Hartung, 2002 ; H. pemon De Marmels, 1987 ; H. peregrinum Williamson, 1919 ; H. petiense Machado, 1988 ; H. rogertaylori Lencioni, 2013 ; H. roquei Vilela, Rodrigues & Lencioni, 2022 ; H. rubrifulvum Donnelly, 1992 ; H. silvarum Sj ̂stedt, 1918; H. simulatum Williamson, 1919 ; H. tatama Bota-Sierra & Novelo-Gutiérrez, 2017 ; H. thais Machado, 2015 ; H. tiradentense Machado & Bedê, 2006 ; H. triangulare Hagen in Selys, 1862; H. tricellulare Calvert, 1901 ; H. valgum Donnelly, 1992 . From now on, authors’ names and publication dates will be omitted.
Generic characterization and diagnosis (distinct features from other heteragrionids in squared brackets). Medium to large (36–60mm) heteragrionids [ Dimeragrion Calvert, 1913 30–52mm; Heteropodagrion Selys, 1885 and Oxystigma 35–50mm]. Head varying from entirely black, black dorsally and yellow ventrally, reddish, and irregular patterns of black, blue, grey and orange [black and blue in Oxystigma ; brown to black with pale details in Dimeragrion and Heteropodagrion ]; angulated frons (shared with other heteragrionids). Posterolateral margins and hind lobe of prothorax rounded (shared with Oxystigma ). Pterothorax often bearing a black humeral stripe; overall coloration black/brown, with pale and vivid yellow/orange areas in most species, others with shades of grey and blue [ Dimeragrion with black and pale colors, bearing some dorsal pruinescence in some species; Heteropodagrion with reddish tones]; hind femur almost reaching S2 [reaching mid S 2 in Dimeragrion , Heteropodagrion and Oxystigma ]; no accessory veins basal or distal to CuP [with 1 or more basal veins in Dimeragrion ]; CuP closer to antenodal 2 than to 1 [closer to antenodal 1 in Heteropodagrion ; closer to antenodal 1 or midway between 1 and 2 in Oxystigma ]; two cells between quadrangle and vein descending from subnodus in HW discoidal field [1 cell in Oxystigma ; 1 or 2 in Heteropodagrion ]; RP 3 recessed at subnodus or proximal to nodus [recessed well before subnodus in Dimeragrion and Heteropodagrion ; at subnodus in Oxystigma ]. Abdomen with no tubercles in ventral S1 [a pair of tubercles in Dimeragrion ]; in most species yellow/orange, dark brown or black dorsally with pale apical rings, S8–10 largely orange, with some species presenting lively red or blue/black colors [largely black in Oxystigma ; bearing pruinosity in the last segments in Dimeragrion ]; distal segment of genital ligula with lateral flagella, lacking inner fold [well-developed inner fold in Dimeragrion and Heteropodagrion ]. Cerci elongated, forcipate or spear-like, robust compared to the length of S10, with a well-developed medial lobe [poorly developed on Heteropodagrion ]; Group B species with well-developed paraprocts, which vary in size and shape.
These features have been described and compiled based on previous studies by Geijskes (1976), De Marmels (1987, 1989), von Ellenrieder & Garrison (2007), Garrison et al. (2010), Lencioni (2013), and Garrison (2014).
Morphological comments
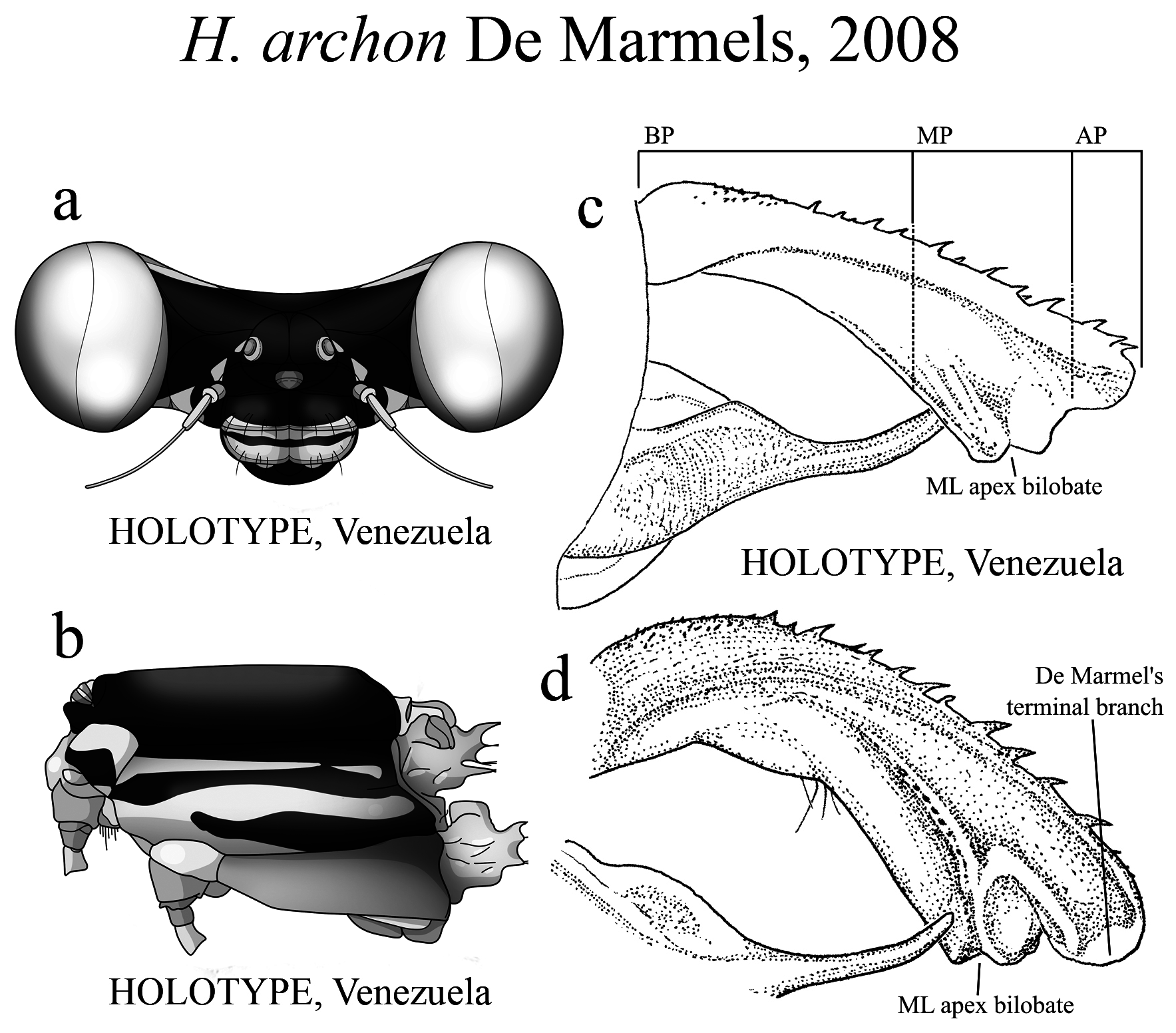
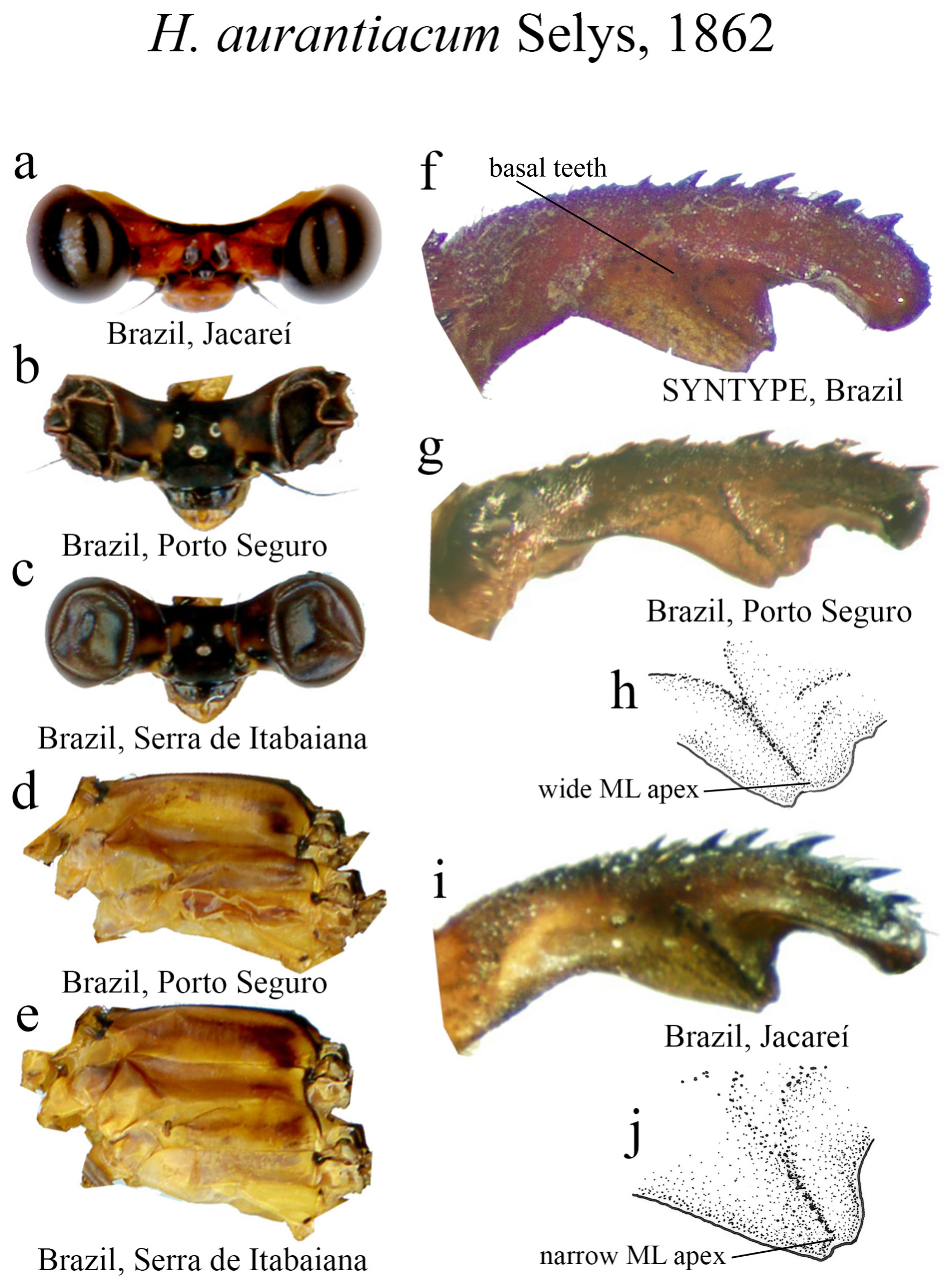
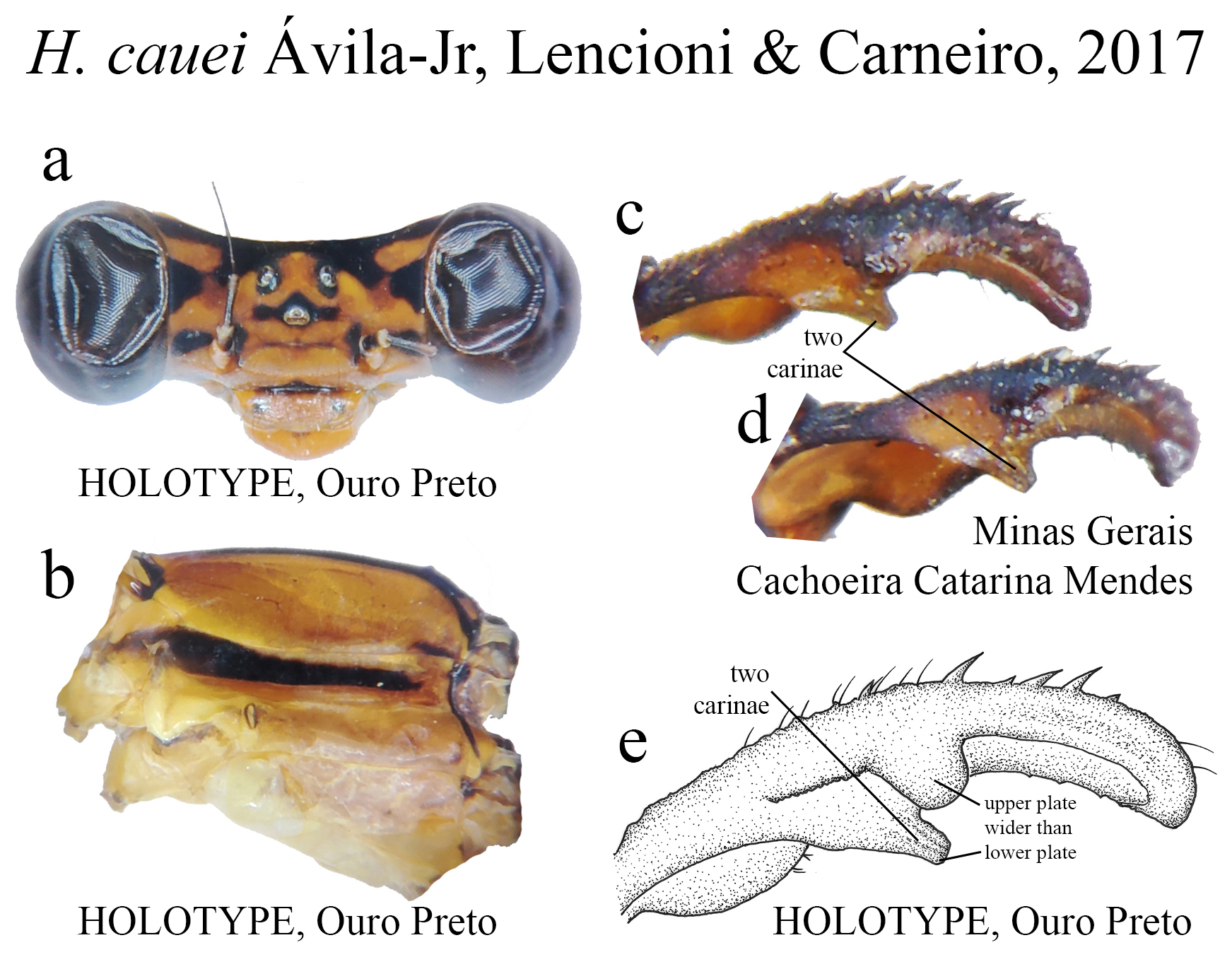
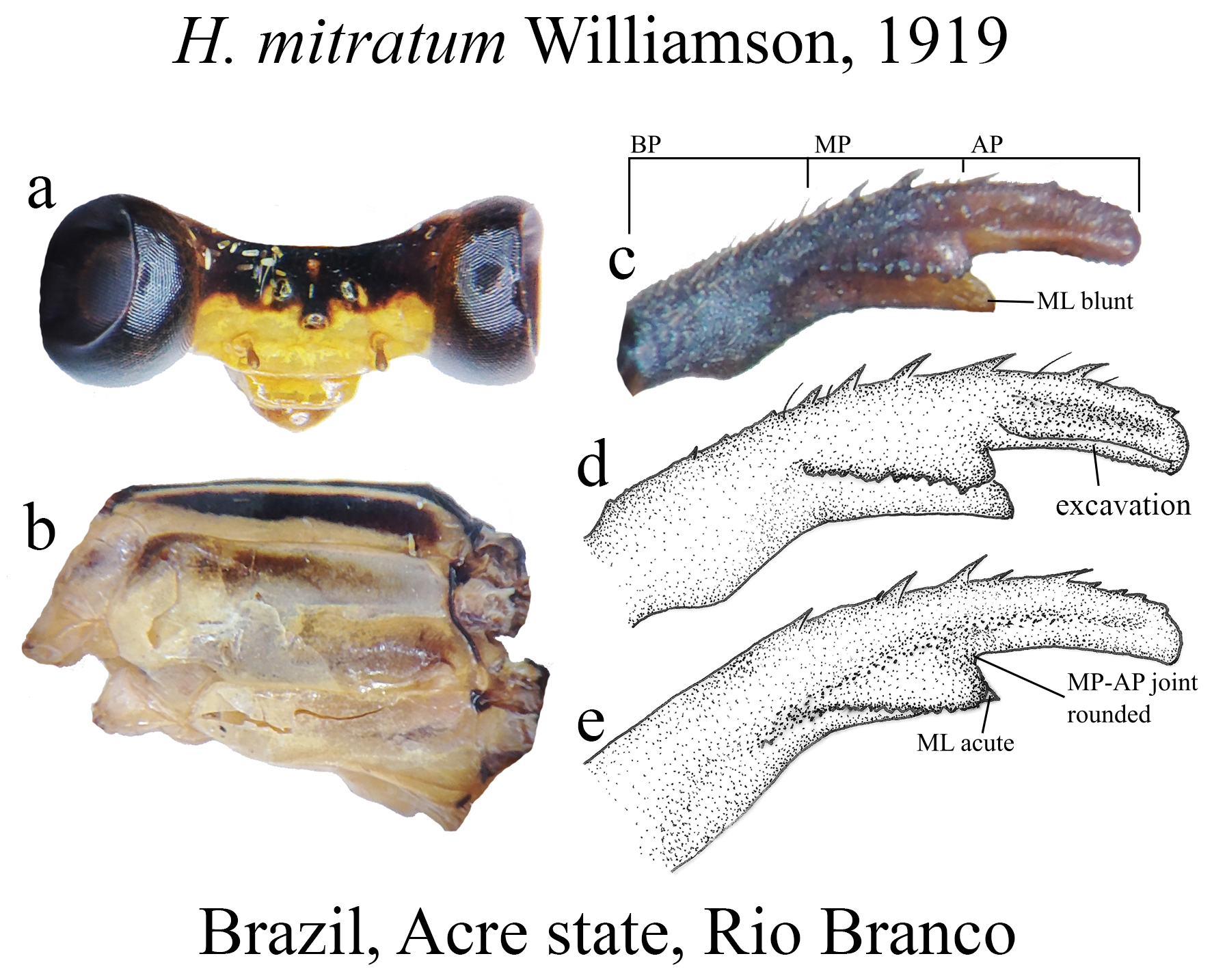
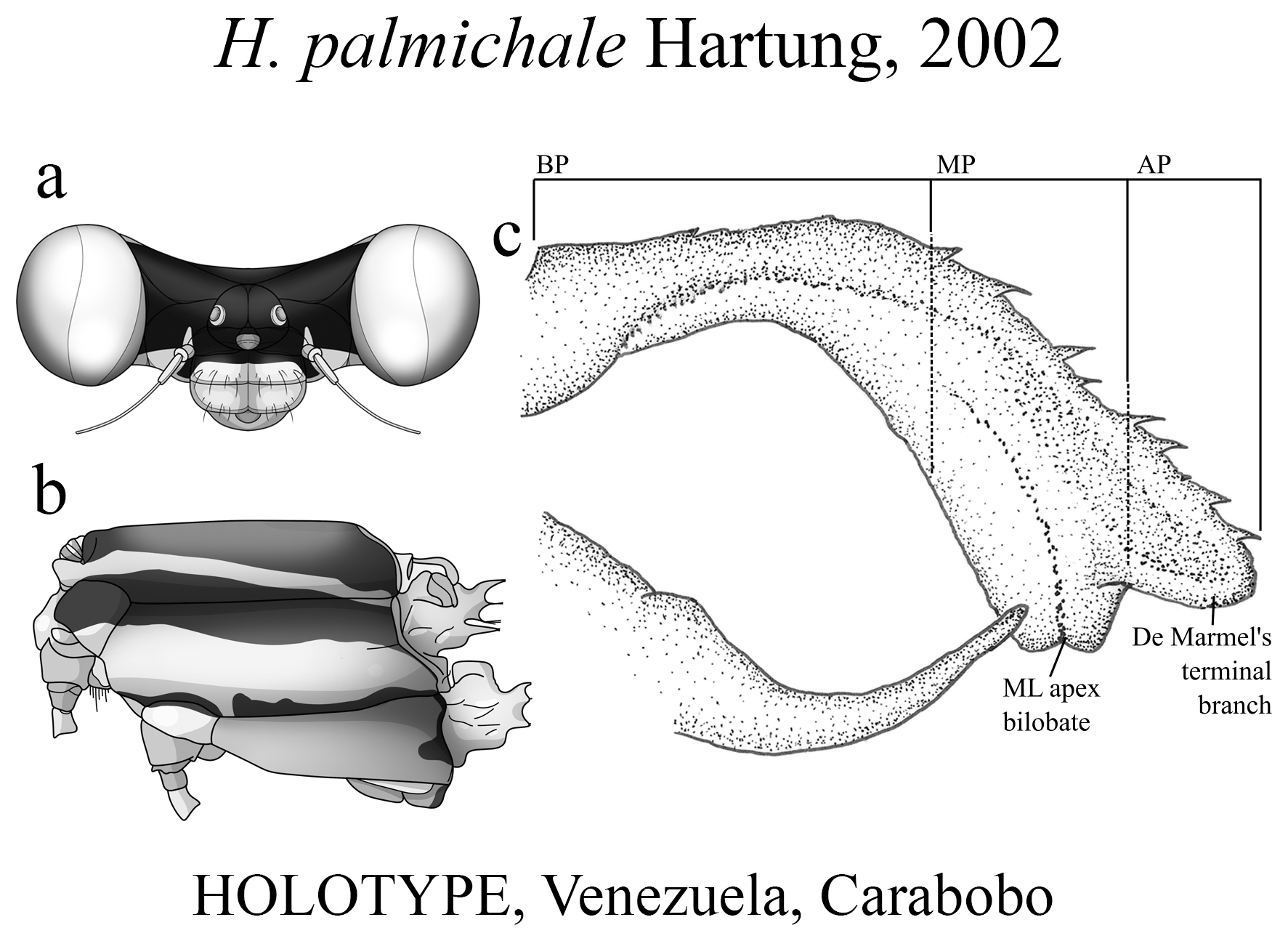
The taxonomic diversity of Heteragrion is evident when examining its representatives, that stands out among other members of the family ( Williamson 1919; Garrison et al. 2010; Lencioni 2013; Stand-Pérez et al. 2019). Species delimitation in this genus is mostly based on the morphology of the male anal appendages, as knowledge of female structures is still limited and requires further extensive study, although recent publications have made significant progress on this topic ( Bota-Sierra & Novelo-Gutiérrez 2017; Stand-Pérez et al. 2019; Vilela et al. 2019a). When comparing the two groups of species in Heteragrion (A and B), it is possible to observe some distinct differences in their appendages: 1) in Group A species, the cerci have a more truncate and forcipate aspect (“ en crochets ”, as stated by Selys 1862), with basal dilatations and broad plates (see Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 for comparisons between the groups); and 2) in Group B species, the cerci are more slender, spear-like and often with acute tips. However, there are some exceptions. For instance, H. aurantiacum ( Figs. 12f–i View FIGURE 12 ), a Group A species, has the basal portion of cercus poorly expanded, in contrast to other species from the same group, like H. brianmayi ( Fig. 18d View FIGURE 18 ) or H. cauei ( Fig. 21d View FIGURE 21 ). Similarly, H. archon ( Fig. 11c View FIGURE 11 ) and H. palmichale ( Fig. 53c View FIGURE 53 ), both Group B species, have broad and downward curved cercus, standing out not only from other Group B species, but from all congeners.
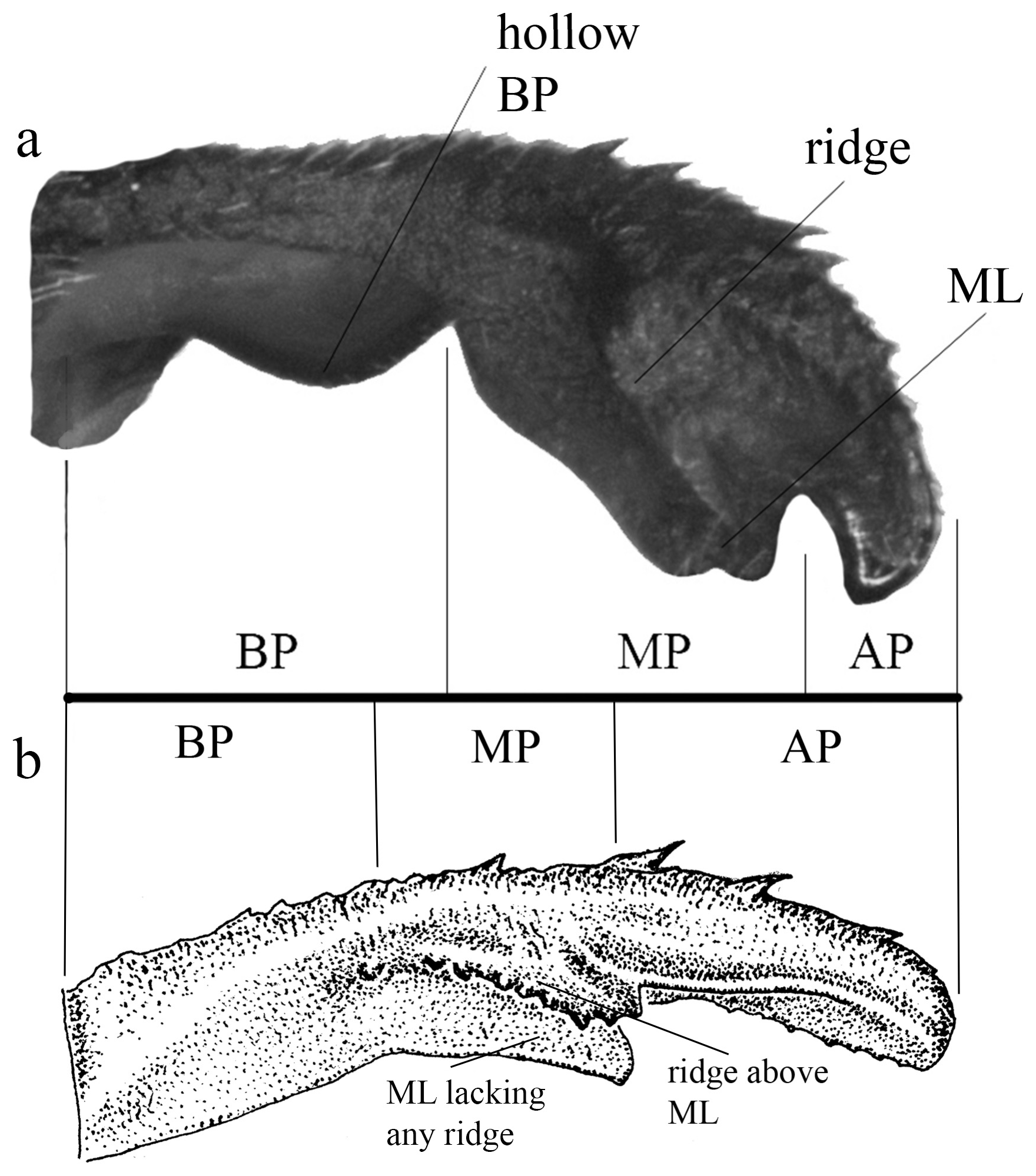
The cercus in Heteragrion (see also Lencioni 2013 for further images on different angles) can be divided into three main portions ( Figs. 1a–b View FIGURE 1 , 2a–h View FIGURE 2 , 3a–f View FIGURE 3 ):
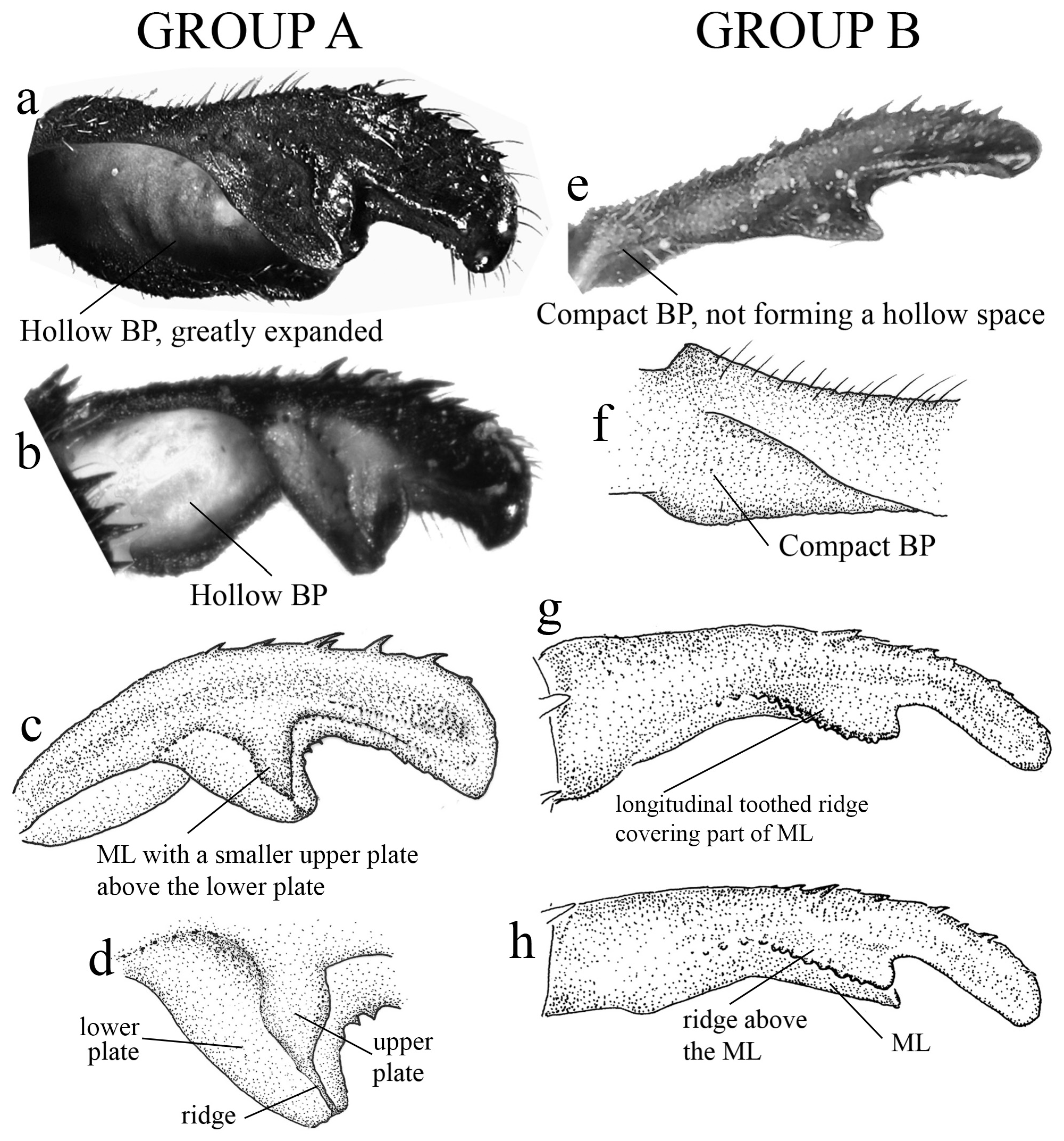
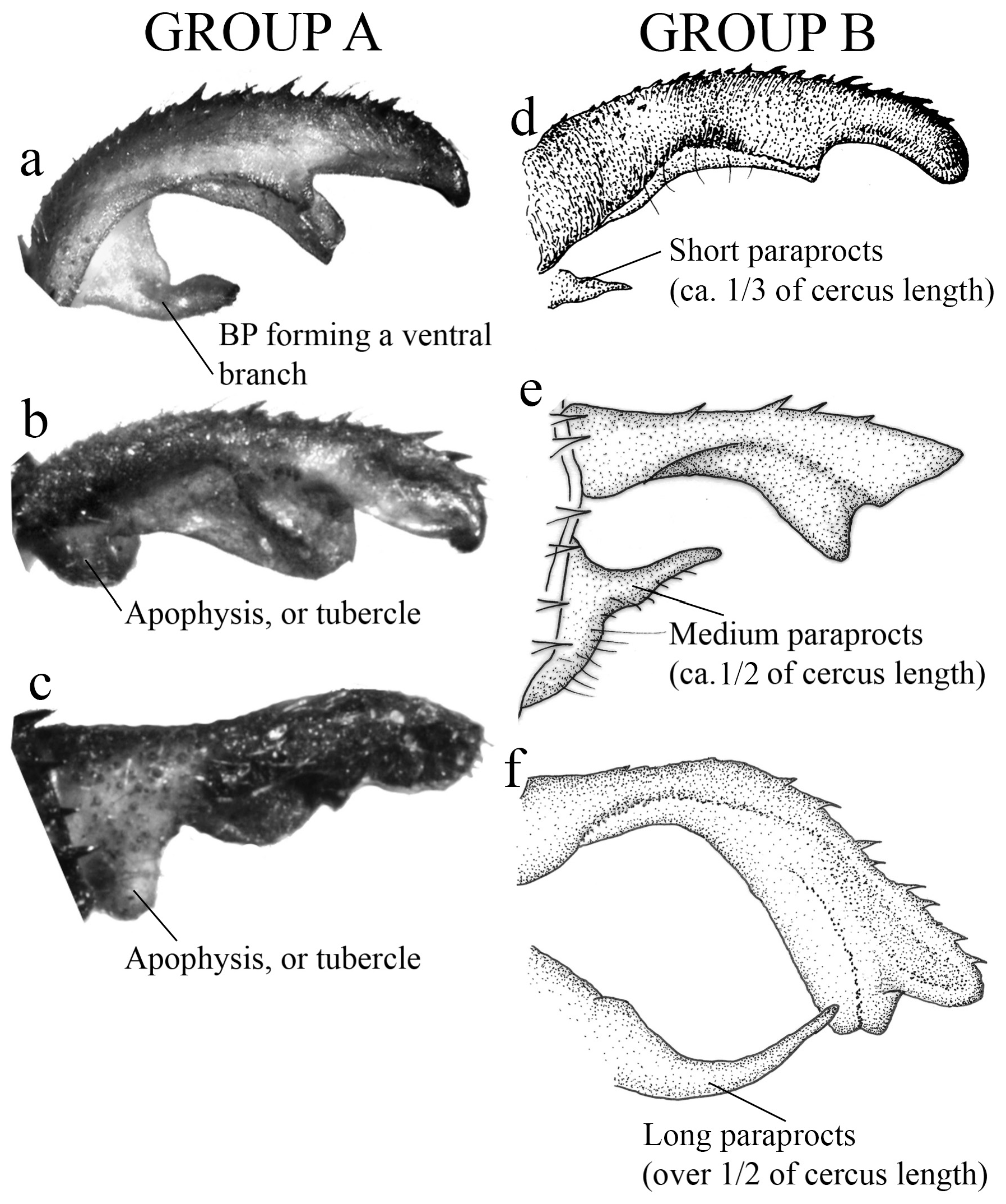
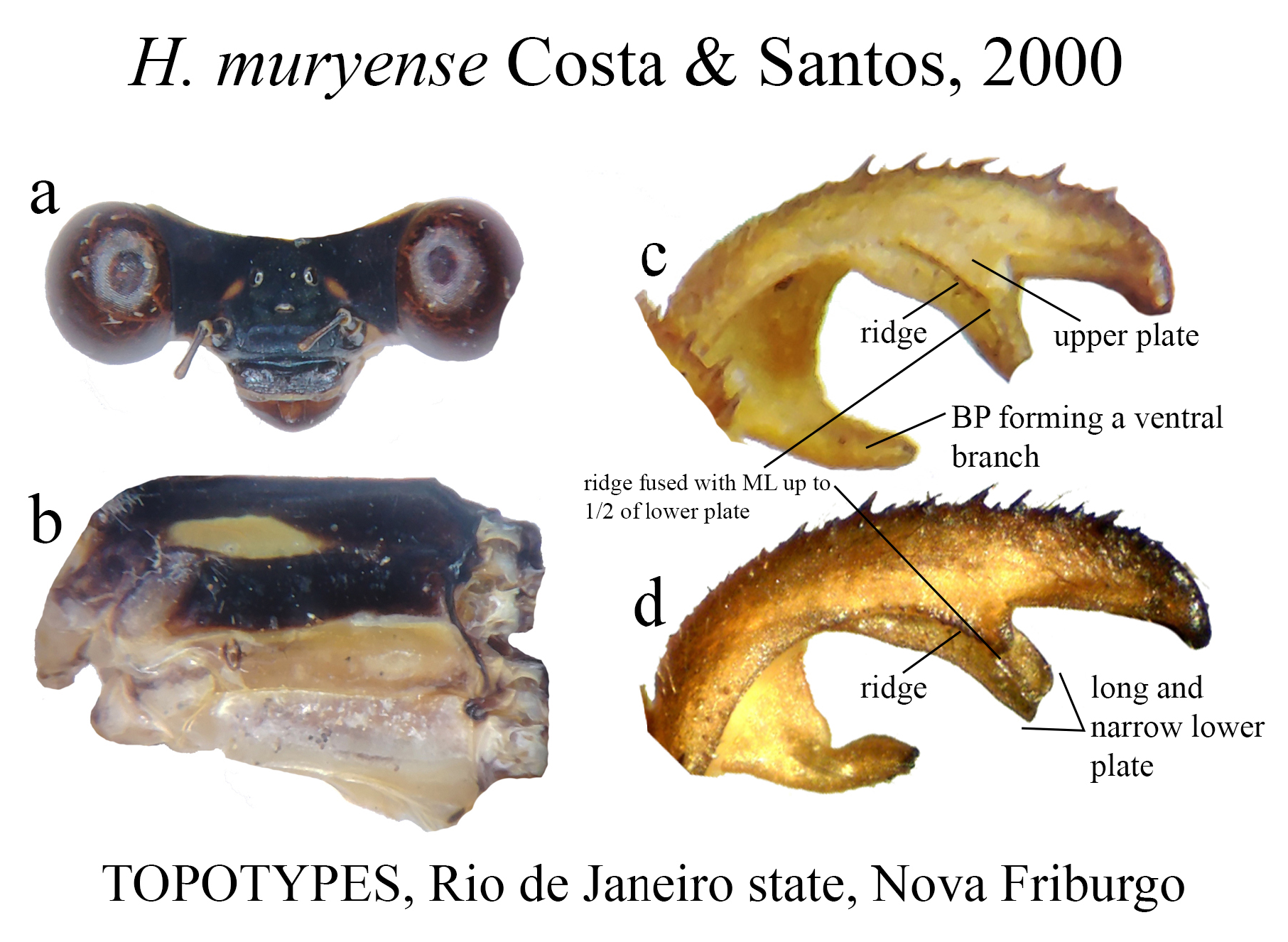
(i) the basal portion (BP), which is the part of the cercus attached to S10, displays a wide range of morphological variations among the different species. They can be compact ( Figs. 2e–f View FIGURE 2 ); bear many degrees of convexities ( Figs. 10f View FIGURE 10 , 14e, g View FIGURE 14 ) or hollow concavities ( Figs. 15c View FIGURE 15 , 25e View FIGURE 25 , 52e View FIGURE 52 ); tubercles or apophyses ( Figs. 4b–c View FIGURE 4 ); or unique ventral branches ( H. muryense , Figs. 51c–d View FIGURE 51 ). The structural features of the BP are far more diversified in shape and size on Group A species, whereas Group B species have usually compact BPs, lacking any expanded concavities in this area. On the other hand, BP convexities (as seen in Figs. 10f View FIGURE 10 , 14e, g View FIGURE 14 ) are a Group B feature. It is important to notice the difference between the convexities present in some Group B species and the ventral tubercle (or apophysis, Figs. 4b–c View FIGURE 4 ) present in some Group A species: whereas the convexities are expanded medially on the medial margin of the BP, the tubercles are extended downwards, being restricted to that ventral area.
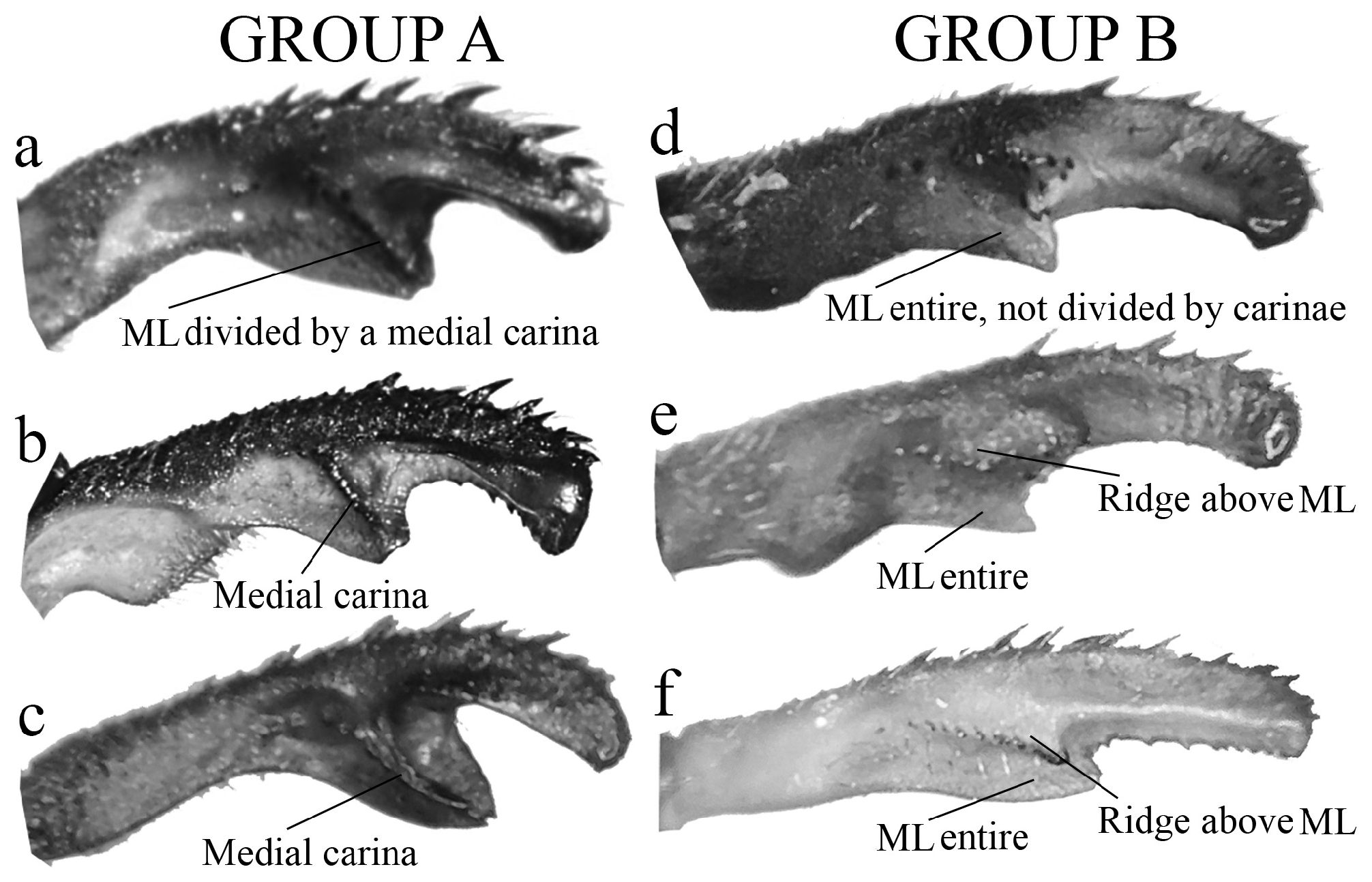
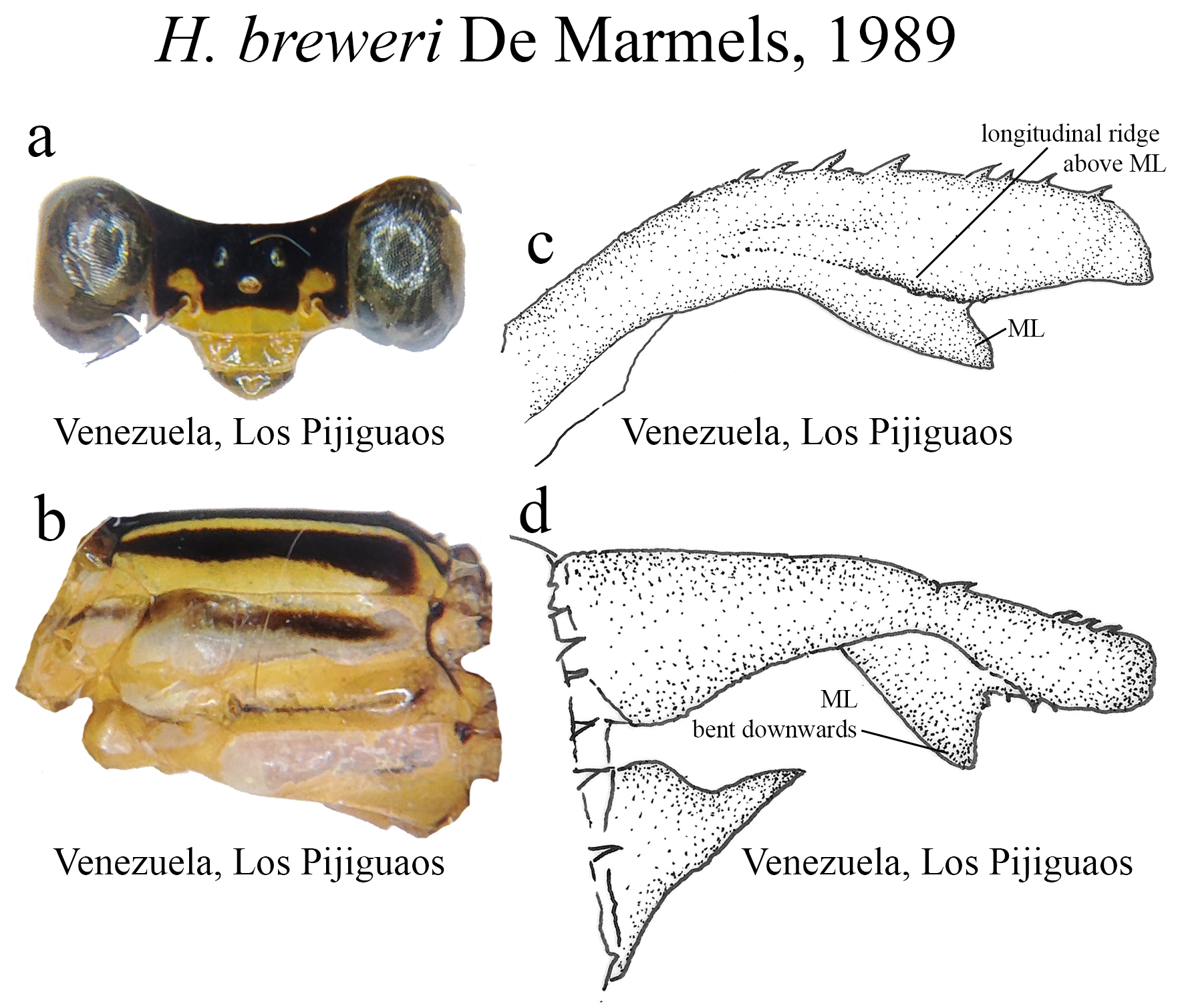
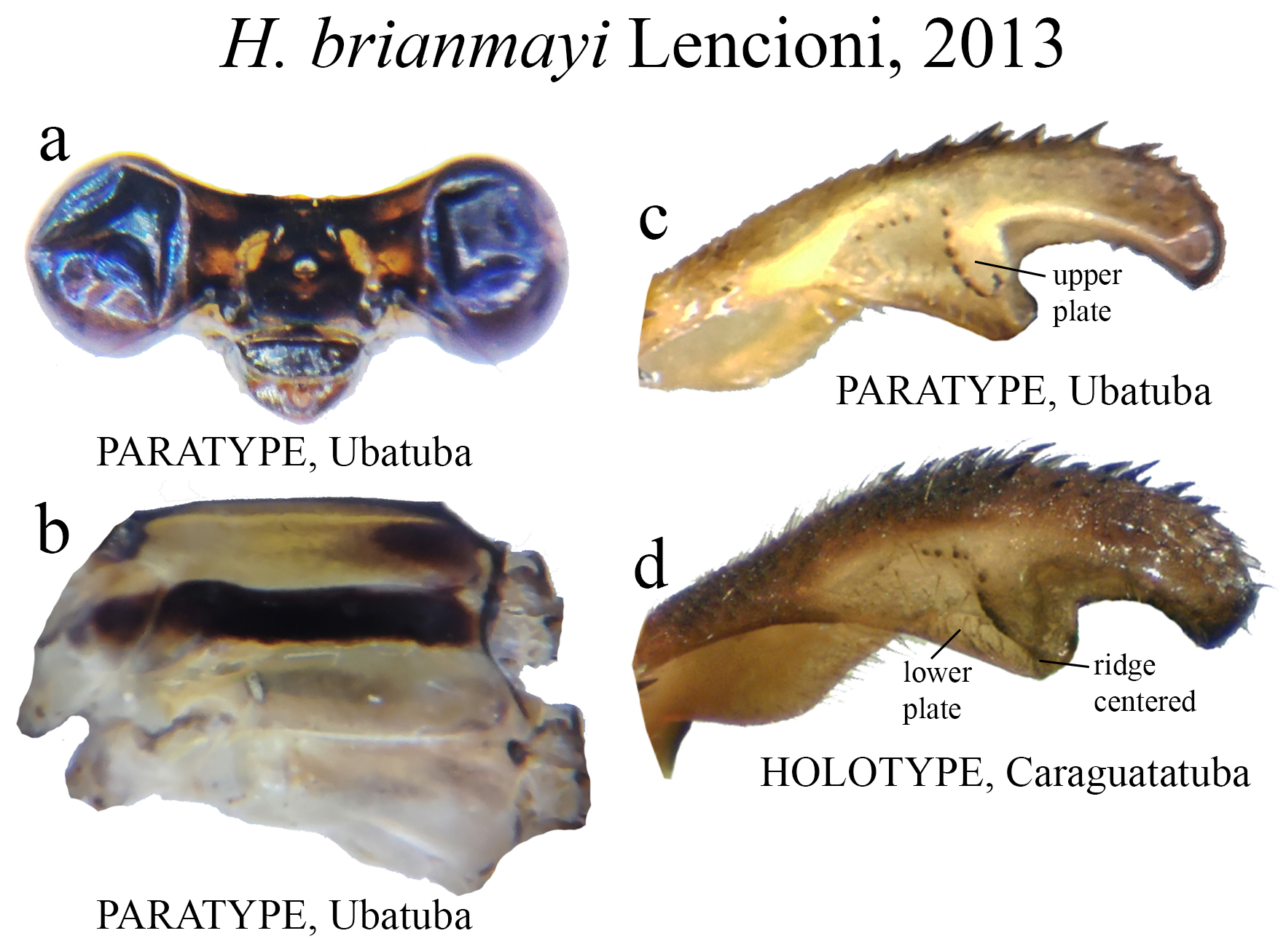
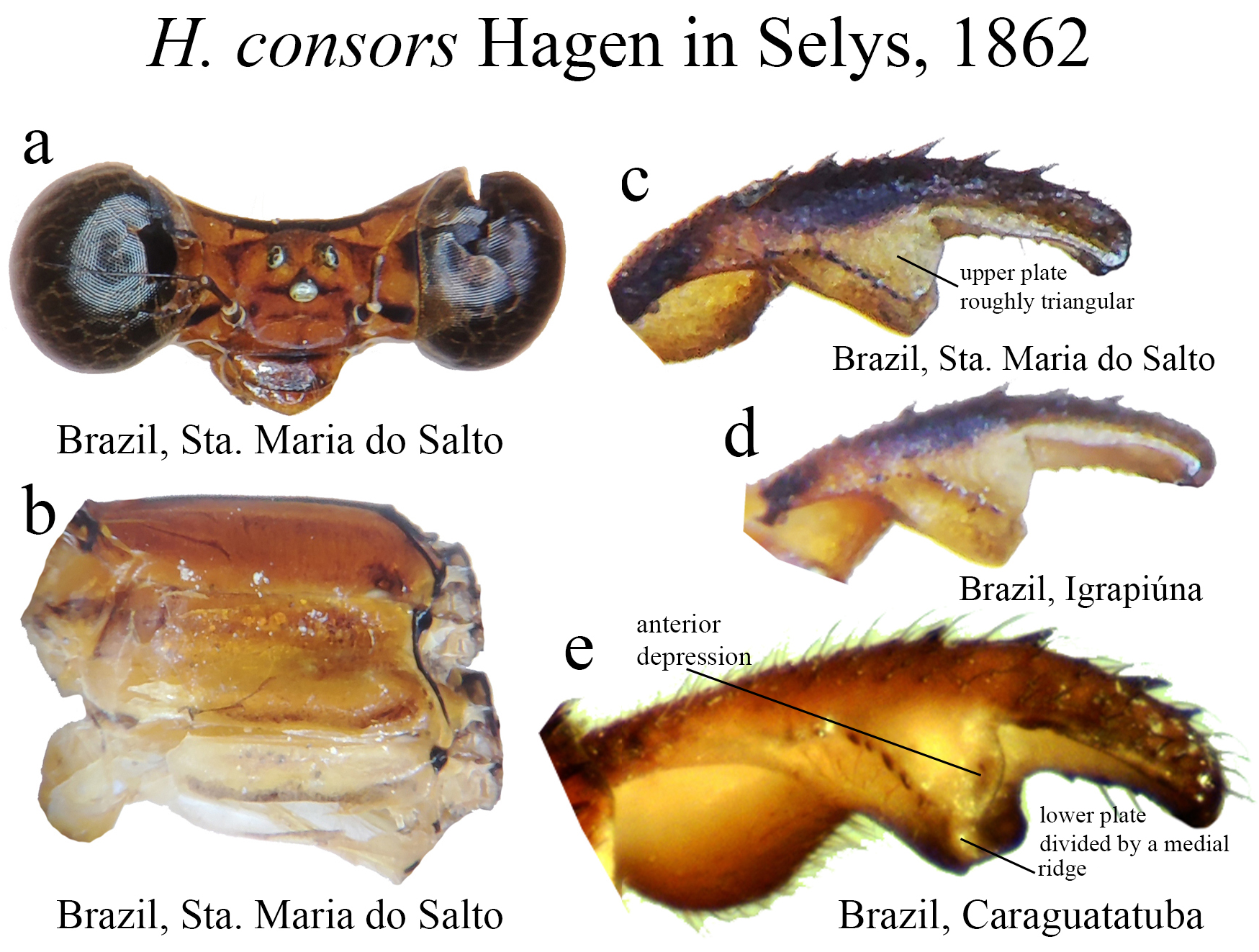
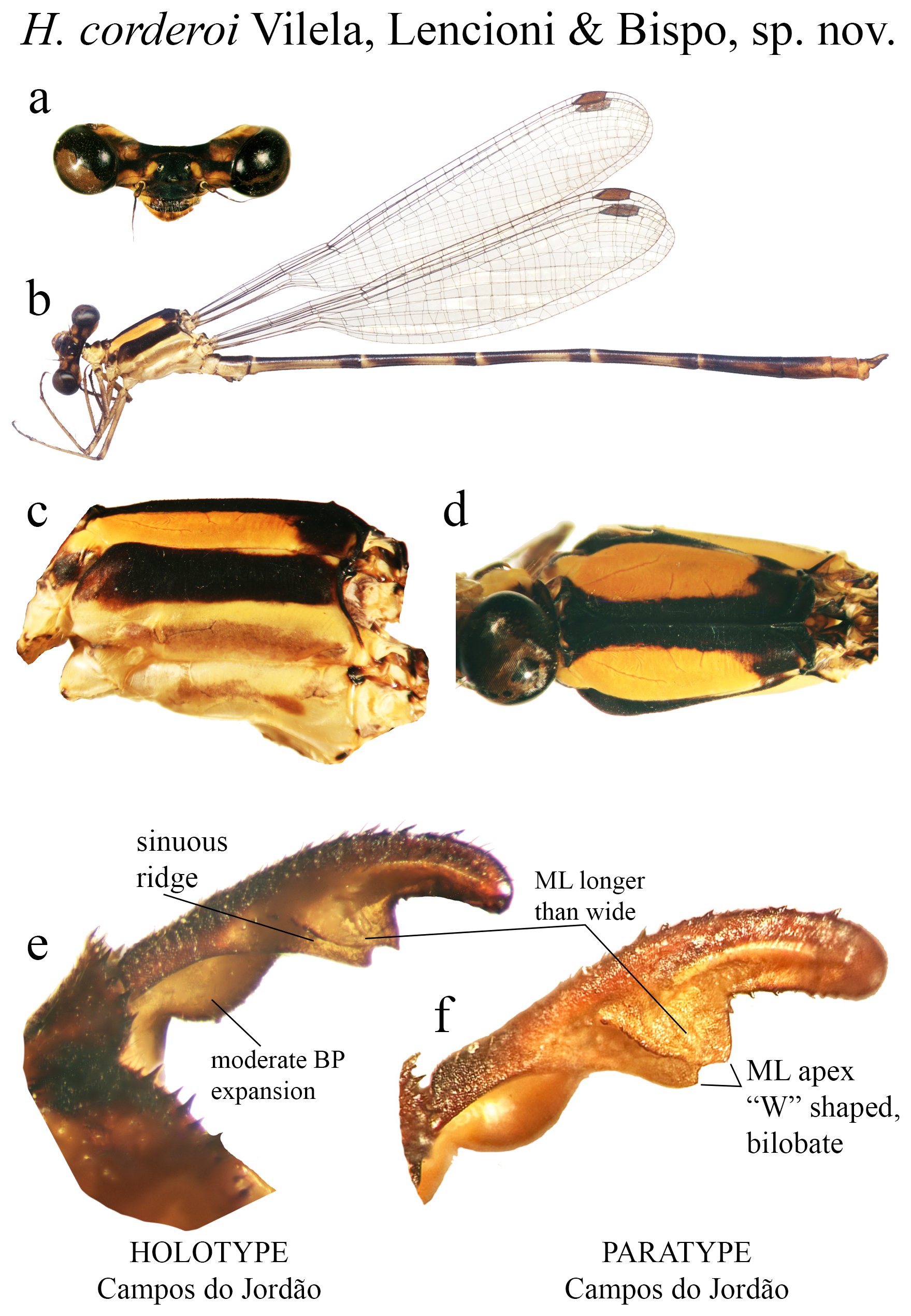
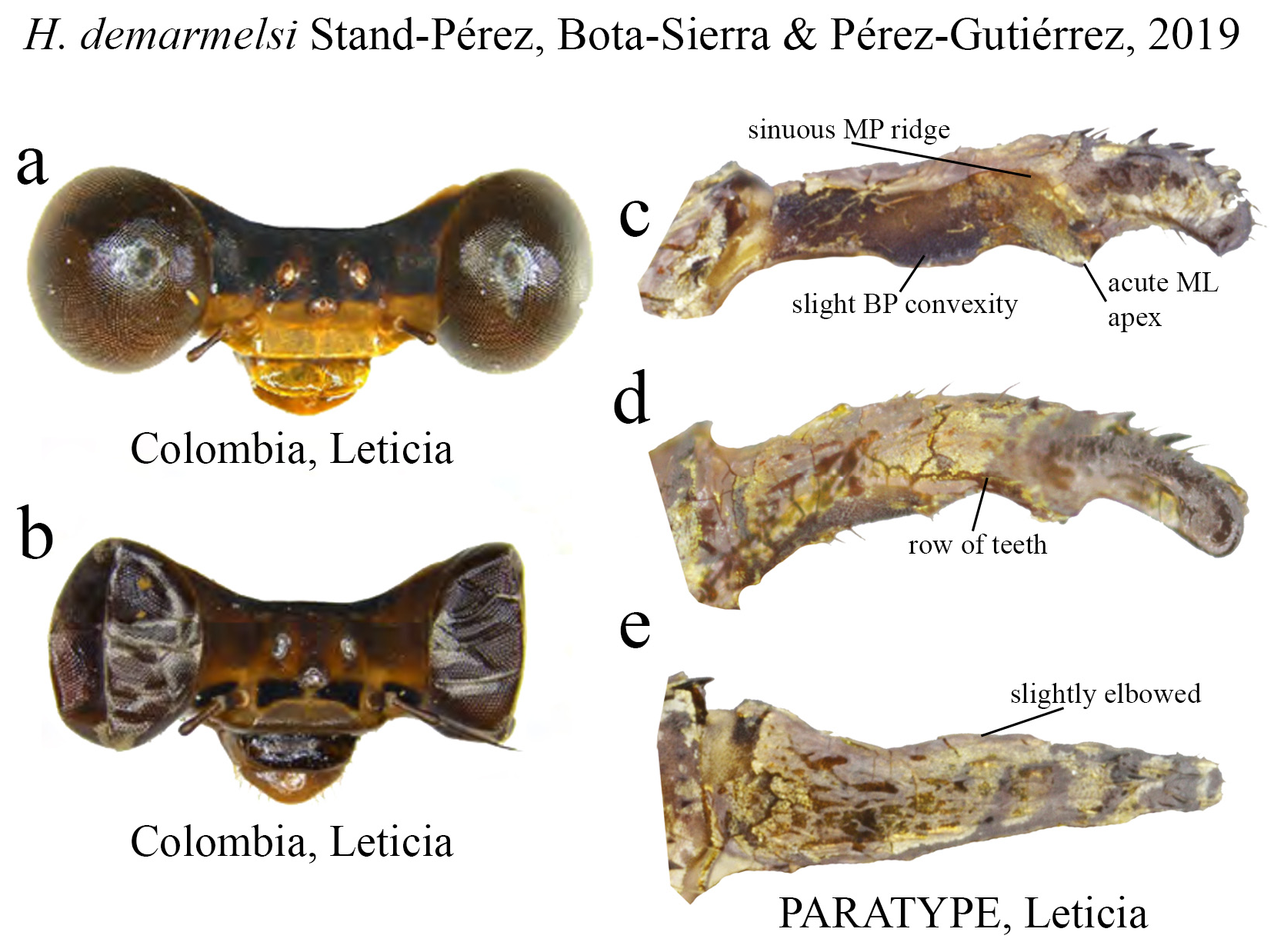
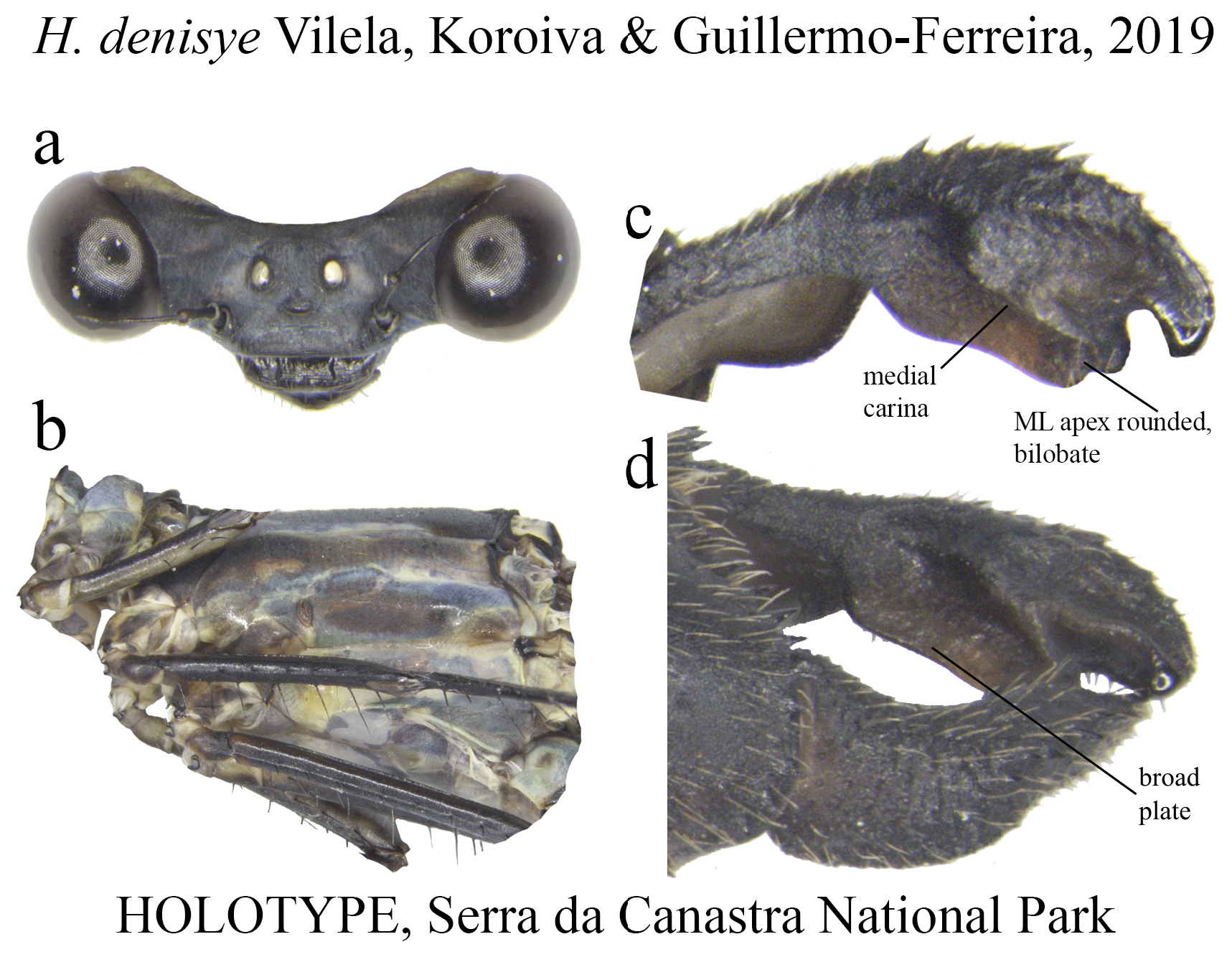
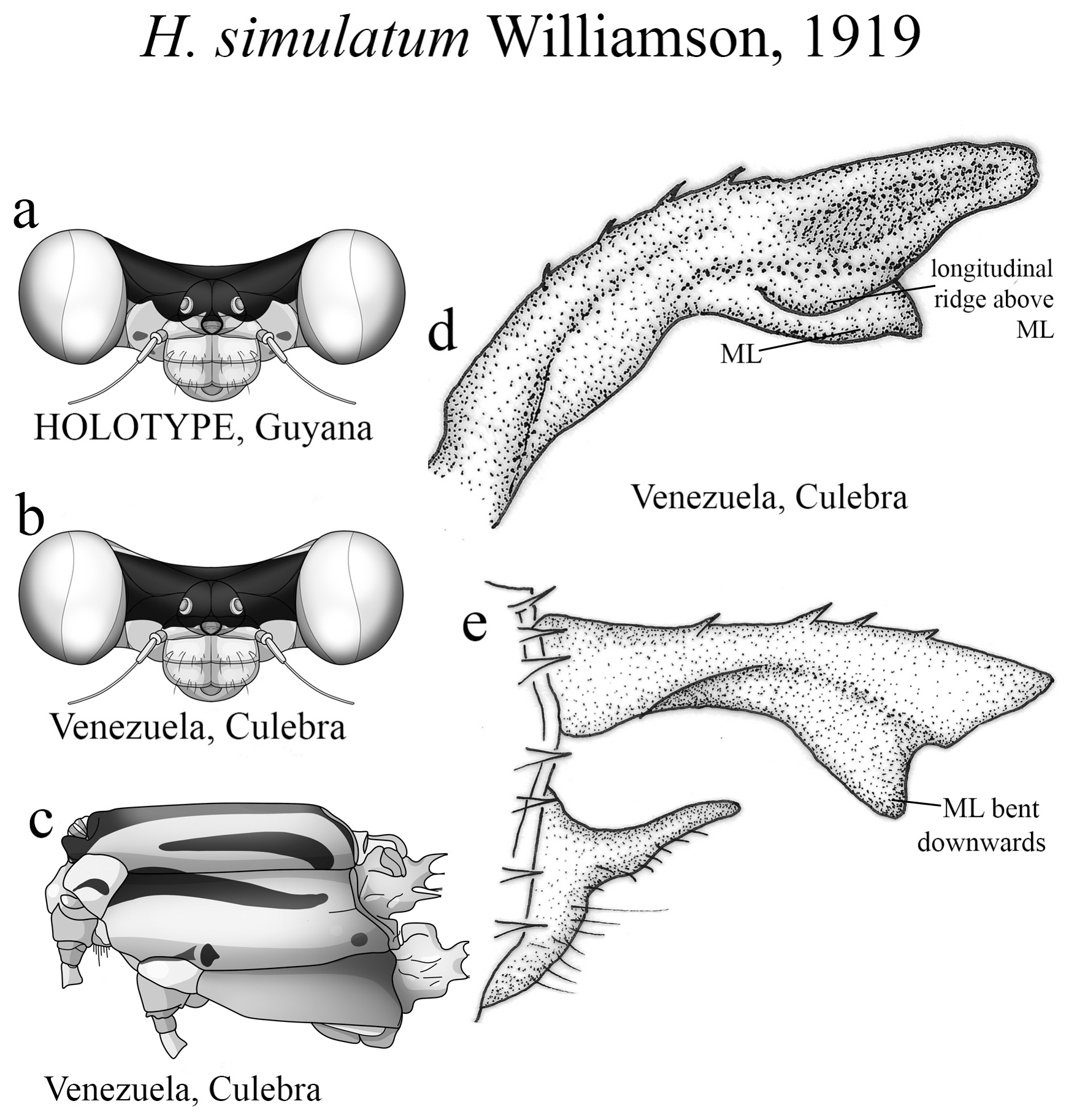
(ii) the medial portion (MP), whose extension is delimited by the size and development of the medial lobe (ML, Fig. 1a View FIGURE 1 ) and the extension of the toothed carina ( Fig. 1b View FIGURE 1 ), bears the most important character-delimiting species on the genus. The ML is a more complex structure in Group A than in Group B species. Some Group A species have the ML composed of a lower plate, an upper plate, and a carina that extends between them, in a “two-story” structure ( Figs. 2a–d View FIGURE 2 ). Other species of the same group have more simple ML structures, bearing a medial toothed carina (elevated carina in H. cooki , Fig. 26c View FIGURE 26 ), dividing the plate and reaching or not its apex ( Figs. 3a–c View FIGURE 3 ). The apex is also a structure that varies in shape and size, being squared ( Fig. 63c View FIGURE 63 , 65c View FIGURE 65 ), rounded ( Fig. 28c View FIGURE 28 , 45d View FIGURE 45 ), bilobate ( Fig. 30c View FIGURE 30 ) or undulate ( Figs. 37c–d View FIGURE 37 ). On species from Group B, the medial portion (MP) features a toothed ridge, located immediately above the ML ( Figs. 1b View FIGURE 1 , 2g –h View FIGURE 2 , 3d–f View FIGURE 3 ). In some species, the toothed ridge is robust, bearing strong teeth and covering part of the ML in dorsal and dorsolateral views ( Figs. 2g –h View FIGURE 2 ). In other species, the ridge bears only small teeth, not covering the ML in any view ( Figs. 9e View FIGURE 9 , 20e View FIGURE 20 , 32c–d View FIGURE 32 ). Or the ML is free of any dividing ridges, the plate entire or smooth ( Figs. 2e–h View FIGURE 2 ). In some cases, a row of small teeth on the ML do not form a ridge ( Fig. 62d View FIGURE 62 ). In contrast, all Group A species bear a strong ridge dividing the ML, in most cases at its middle.
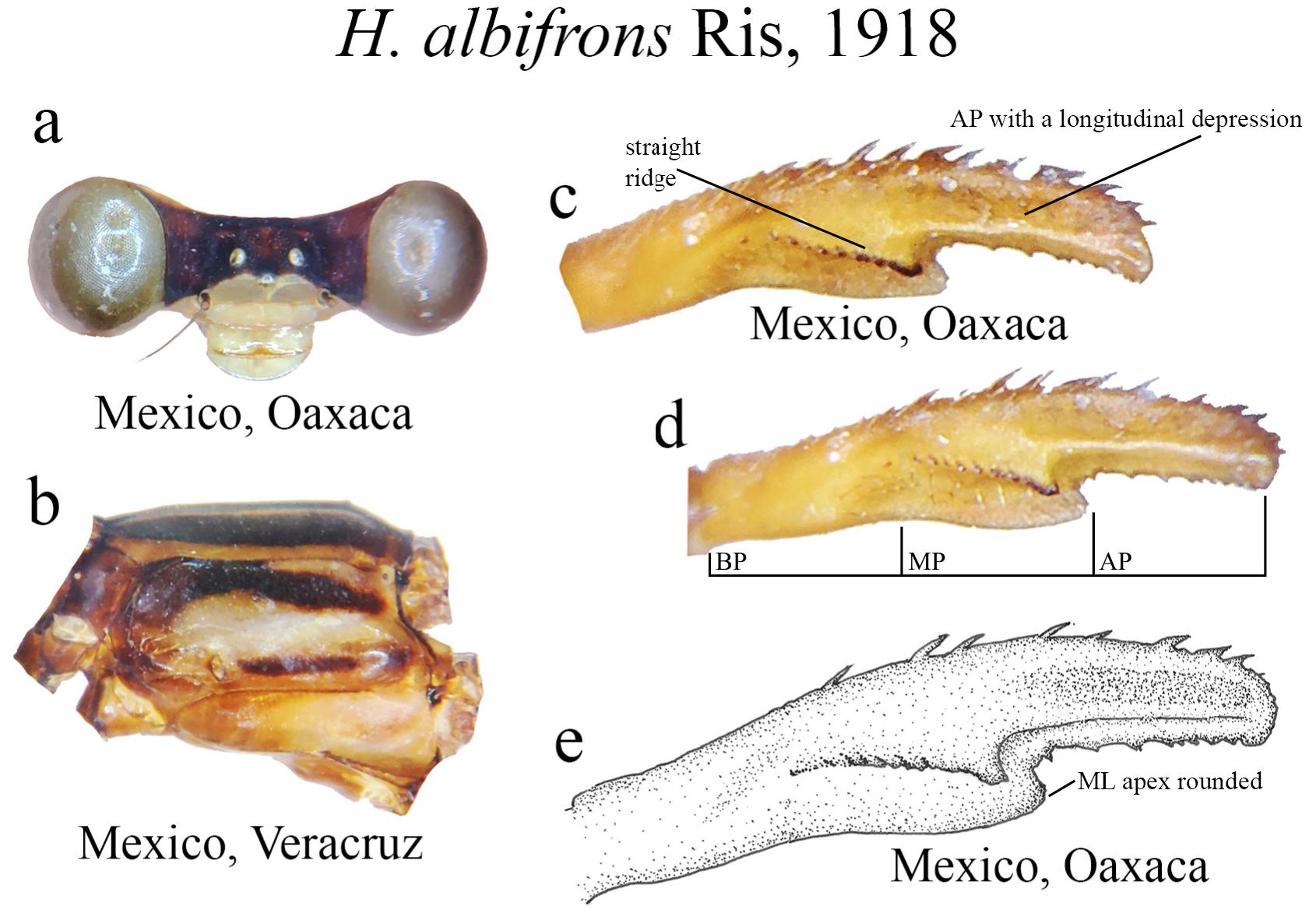
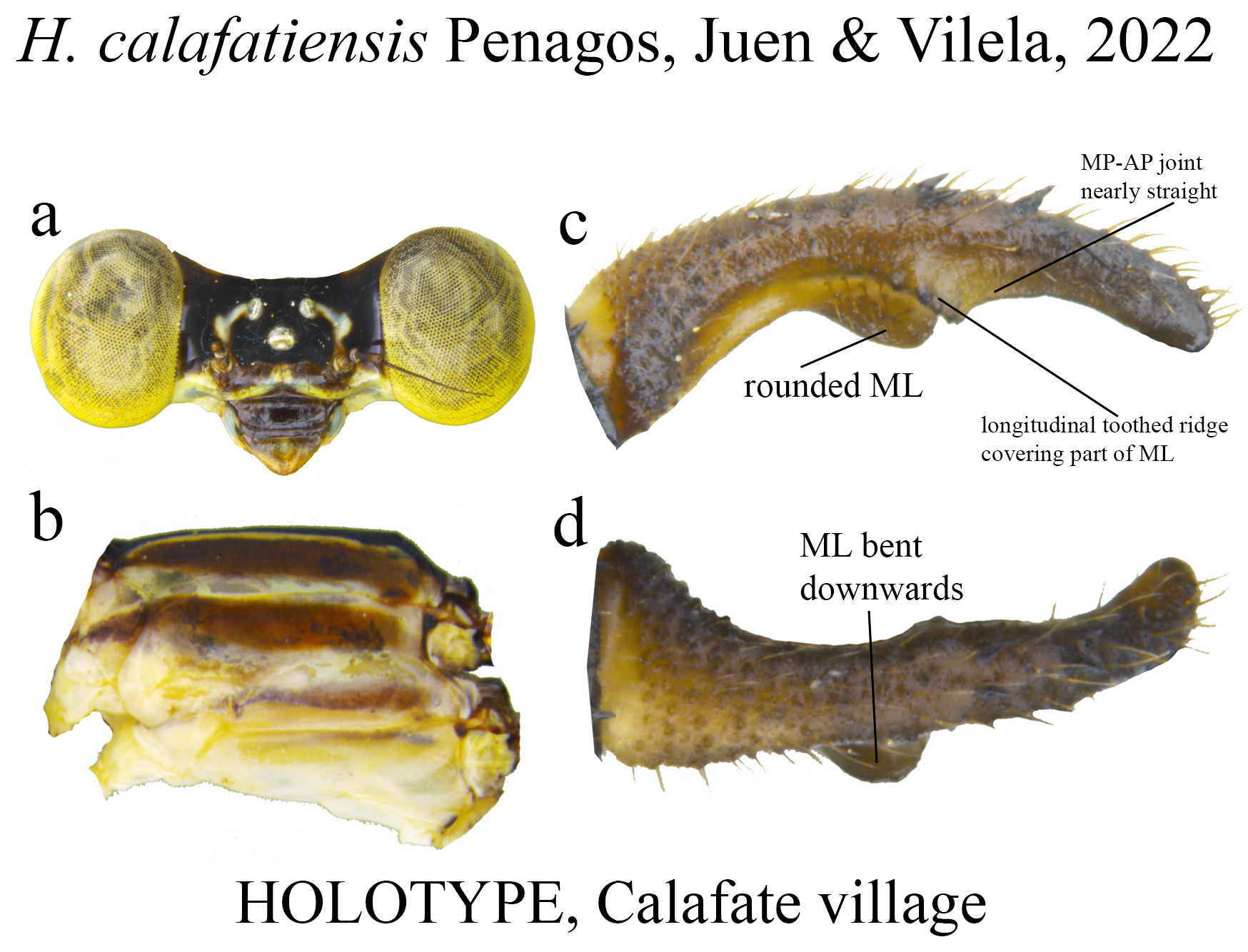
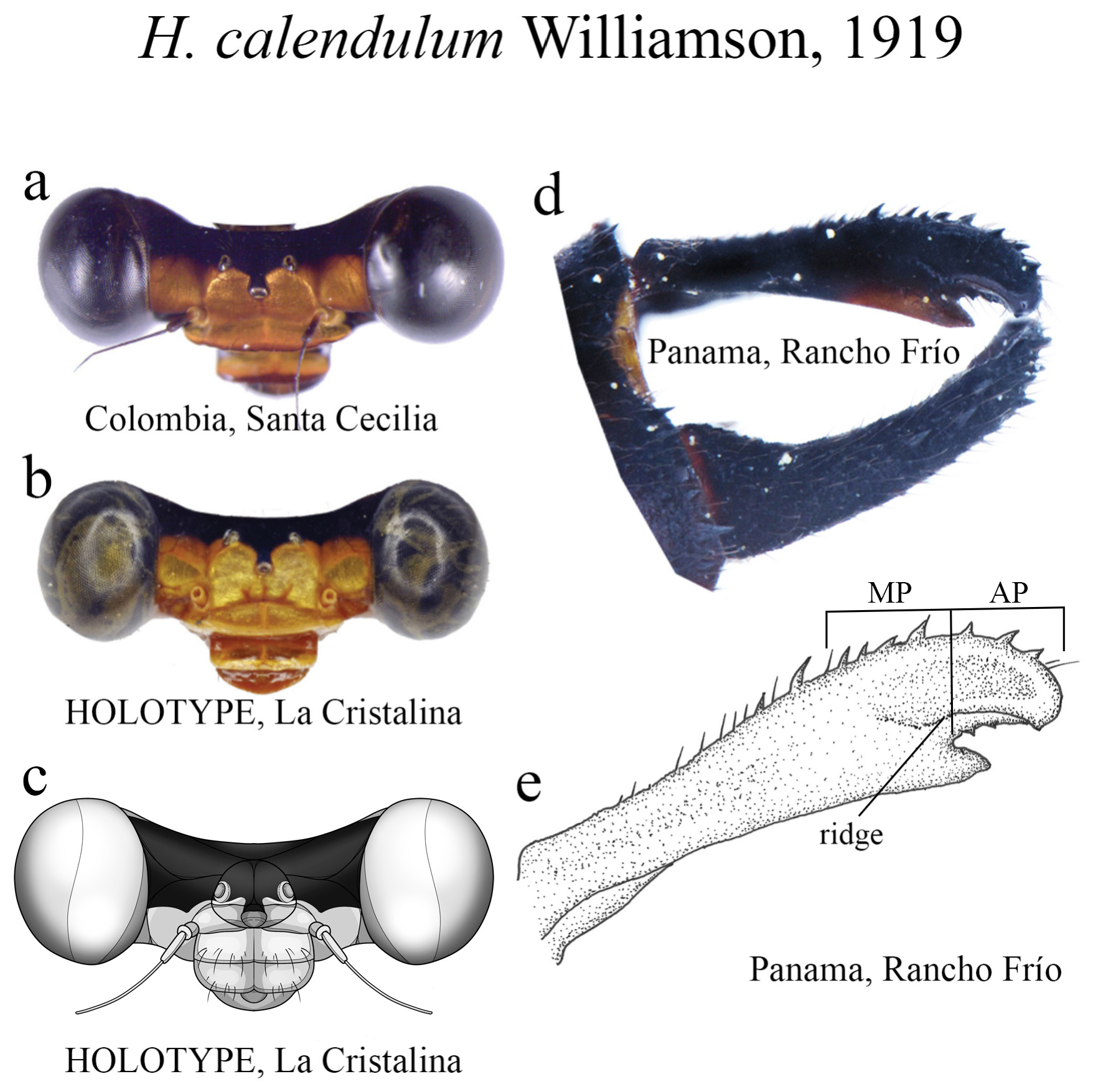
(iii) the apical portion (AP) is delimited from the MP by a junction that may be in different shapes: angled ( Fig. 14d–e View FIGURE 14 ), rounded ( Fig. 14g View FIGURE 14 ), or nearly straight ( Fig. 19c View FIGURE 19 ). Where this junction is not well delimited at the base of ML, as seen in H. calafatiensis ( Fig. 19 View FIGURE 19 ), the MP-AP border is delimited by the apical end of the toothed ridge (i.e., apical tooth, Fig. 19c View FIGURE 19 ). AP varies greatly in length, being long, as in H. albifrons ( Figs. 8c–d View FIGURE 8 ) and H. consors ( Fig. 25c View FIGURE 25 ), or very short, as in H. archon ( Fig. 11c View FIGURE 11 ), H. palmichale ( Fig. 53c View FIGURE 53 ) and H. calendulum ( Fig. 20e View FIGURE 20 ). In species with long AP portions, it is quite common for these structures to include a longitudinal depression ( Fig. 31c View FIGURE 31 ). In medial view, the AP does not bear any morphological character important in taxonomic distinction. Instead, it is invariably a slightly concave structure bearing no ridges or carinas; those are confined to the upper margin ( Figs. 14e View FIGURE 14 , 16e View FIGURE 16 ).
Intraspecific variation and species delimitation
Especially for species in which we could examine a large series of specimens, a wide range of variation in terms of color and morphology were observed ( Figs. 5 View FIGURE 5 , 12 View FIGURE 12 , 42 View FIGURE 42 , 60 View FIGURE 60 ). As discussed by Bota-Sierra & Novelo-Gutiérrez (2017), color and morphological variation are known and well documented for only a few species of the genus, and based on what we could observe among the examined material and literature, this variability has been recorded for only a few species due to inadequate sampling and, in some cases, poor preservation of deposited specimens.
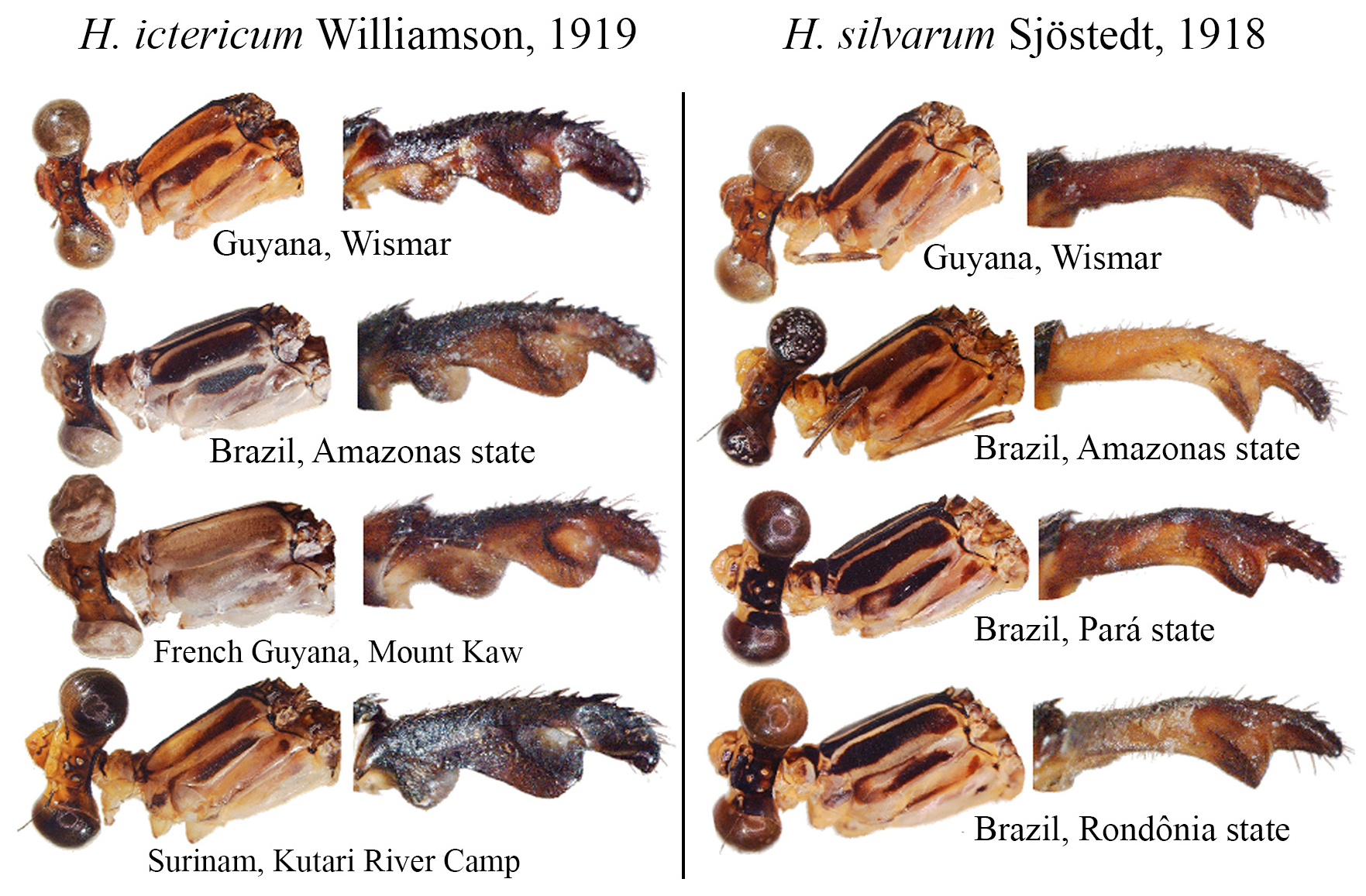
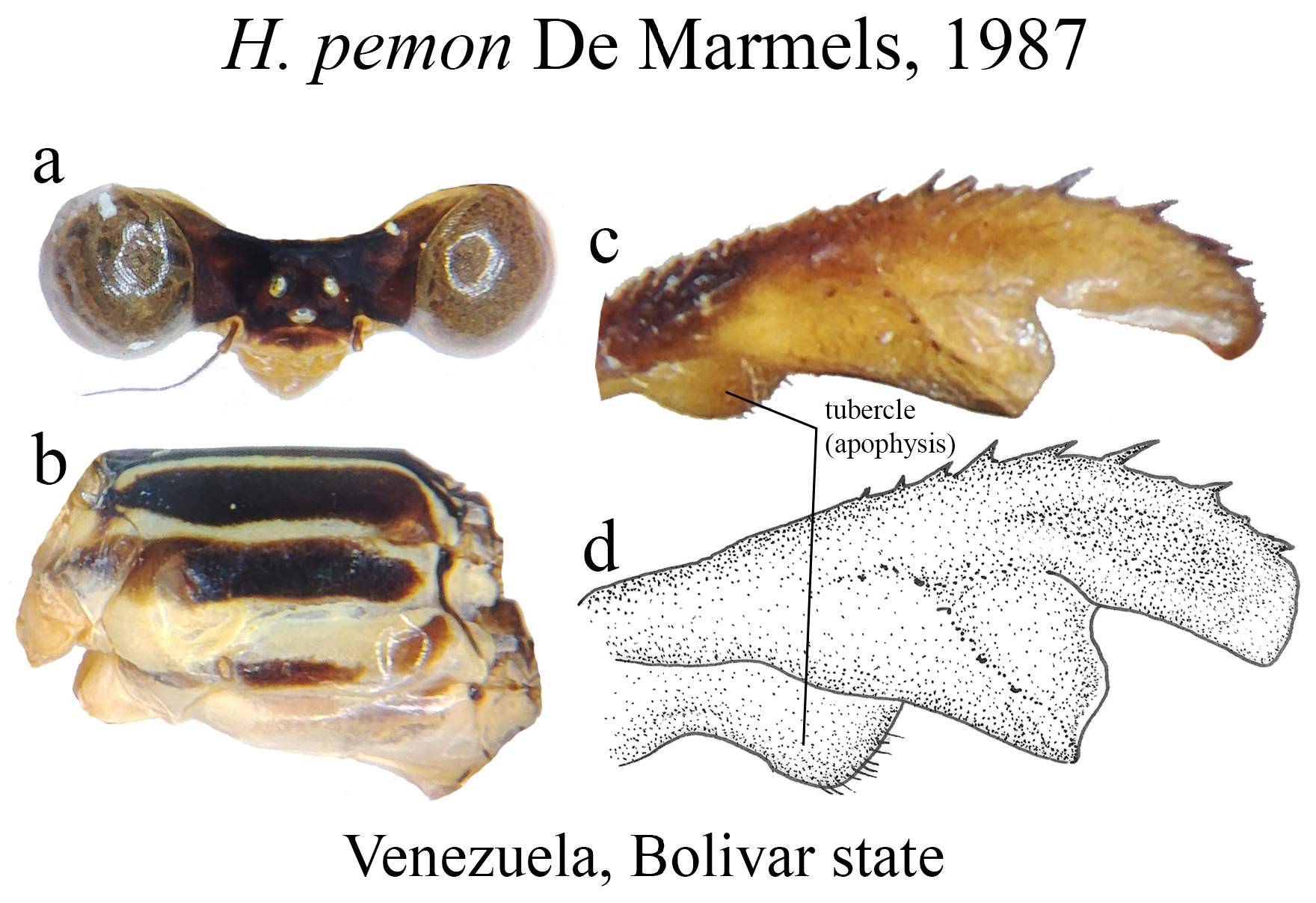
Some boundaries between species are difficult to delimit, mainly because many of them are represented by few individuals, making difficult the analysis of intra- and interspecific variability. For example, similar species such as H. ictericum and H. pemon ; H. palmichale and H. archon ; H. rogertaylori and H. thais are morphologically close, and new synonymies may be feasible pending studies on a molecular level or further morphological assessment with more specimens. Thus, we can assume that the intraspecific variation of nearly half of the Heteragrion species is unknown or poorly known, and this can lead us to misinterpret variations, causing species to be described as new that later can have their names synonymized.
In this study, we treat these species as valid and consider the combination of characters provided by the original authors to separate morphologically close taxa.
Several Heteragrion species (14, to be accurate) have their conservation status assessed as Data Deficient in the IUCN Red List, mainly due to the paucity of specimens known and recent specimens lacking (see species accounts). These species total nearly 23% of the known species, and this percentage may grow even more if we add recently described species (i.e., in the last 30 years) based on small series collected in one or two localities, such as H. muryense , H. gracile , H. mantiqueirae , H. freddiemercuryi , H. brianmayi , H. johndeaconi , H. cyane , H. thais , H. denisye , and H. roquei .
Distribution
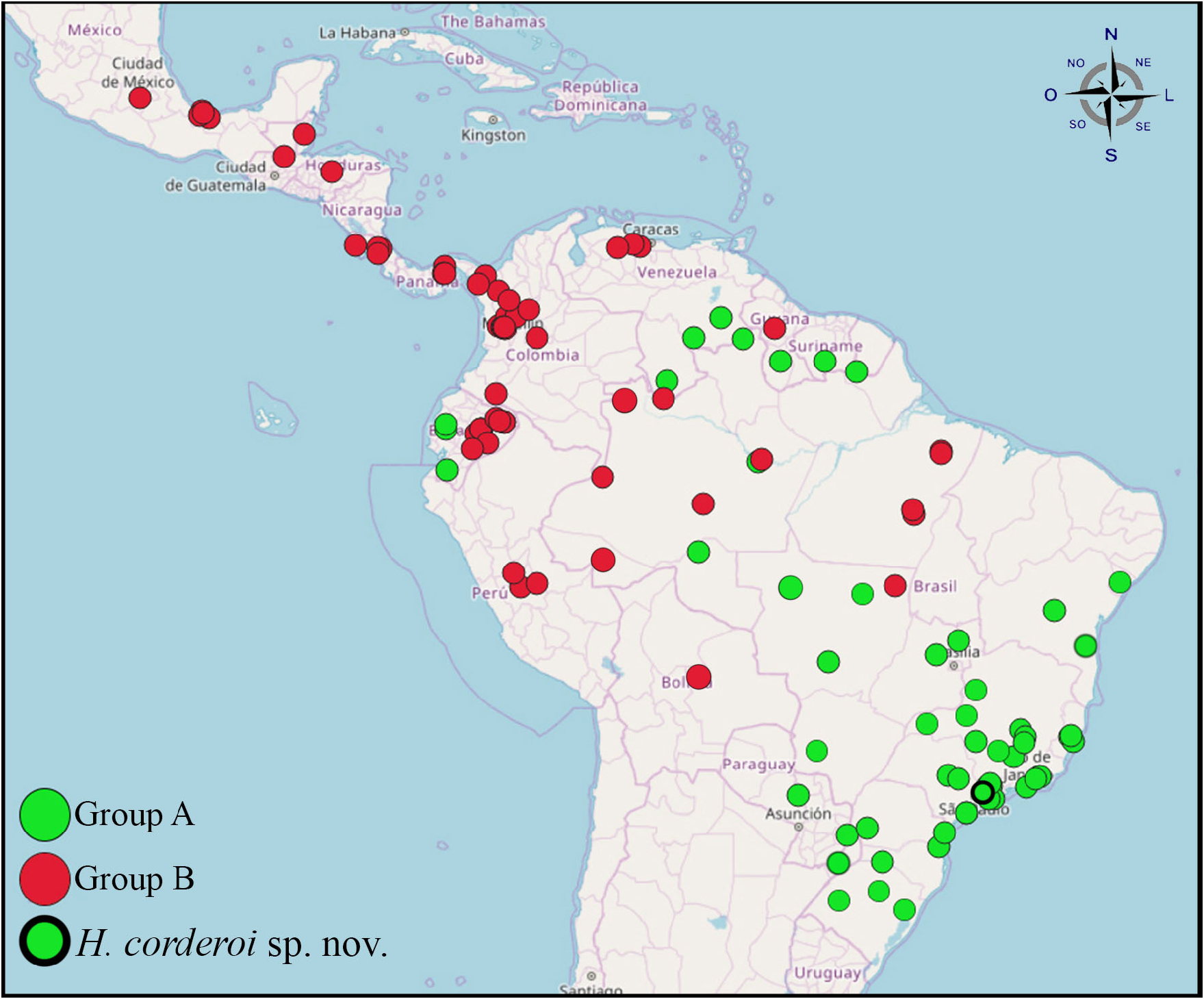
This genus is widespread in the Neotropical region, being found from Central Mexico to Southern Brazil and Northeastern Argentina ( Fig. 6 View FIGURE 6 ). As discussed in the previous section, the distributional data for most species is probably still inadequate, but even with this data deficit we can spot a difference in distribution between the two distinct groups of species.
Group B species ( Fig. 6 View FIGURE 6 , red dots) are mostly found in the Andean region, Central America, and Mexico, whereas Group A species ( Fig. 6 View FIGURE 6 , green dots) are found mostly in Brazil (from North to South), but also being recorded in Venezuela, Ecuador and in the southern Brazilian border with Argentina. There is a “common area”, where species from both groups can be found, that ranges from southern Venezuela to the Brazilian state of Mato Grosso, comprising, therefore most of the Brazilian and Venezuelan Amazon Basin.
Identification key
Key to male Heteragrion species ( H. dorsale View in CoL , H. ochraceum View in CoL and H. obsoletum View in CoL omitted due to lack of information on types, and taxonomic uncertainty)
Important note: This identification key relies heavily on the morphology of the cerci, including their ridges, carinas, and overall ML shape. Other features, such as body coloration, may also be used as secondary characteristics. Frequently the examined structures may be hidden, depending on the specimen, so we recommend relaxing and spreading the anal appendages to obtain a better view of the diagnostic characters.
1. Paraprocts poorly developed (vestigial); overall aspect of cercus oblong, swollen ( Figs. 2a–c View FIGURE 2 , 4a–c View FIGURE 4 ); in most species, BP forming a hollow space that varies in size and shape ( Figs. 2a–b View FIGURE 2 , 15c View FIGURE 15 , 18c View FIGURE 18 ); ML with a developed ridge or carina ( Figs. 3a–c View FIGURE 3 ), frequently dividing ML into two plates and forming an additional upper plate ( Figs. 2c–d View FIGURE 2 , 18c–d View FIGURE 18 , 25c–e View FIGURE 25 )................................................................................................. Group A key (couplet 2)
1’. Paraprocts developed, being short (ca. 1/3 of cercus length, Fig. 4d View FIGURE 4 ), medium (ca. 2/3 of cercus length, Fig. 4e View FIGURE 4 ) or long sized (over 1/2 of cercus length, Fig. 4f View FIGURE 4 ); BP compact, not forming a hollow space ( Figs. 2e–h View FIGURE 2 , 7e–f View FIGURE 7 ); ML entire, not divided by a ridge or carina ( Figs. 3d–f View FIGURE 3 , 8c–e View FIGURE 8 ), only with small teeth in a few species ( Figs. 55c–e View FIGURE 55 ); a longitudinal toothed ridge above ML ( Figs. 2g –h View FIGURE 2 , 3e–f View FIGURE 3 , 8c–e View FIGURE 8 )............................................................. Group B key (couplet 2)
Key to Group A species
2 (1). In the medial portion of cercus (MP, see Fig. 1a View FIGURE 1 ), medial lobe (ML) consisting of a single plate with a medial carina, reaching or not ML apex (e.g., Figs. 3a–c View FIGURE 3 , 12f–j View FIGURE 12 , 38c View FIGURE 38 ).................................................... 3
2’. In the MP, ML consisting of two plates (upper and lower), sustained by a medial ridge (e.g., Figs. 2c–d View FIGURE 2 , 18c–d View FIGURE 18 , 21e View FIGURE 21 ).... .............................................................................................. 19
3 (2). BP consisted of a hairy tubercle or apophysis, not forming a hollow ventral concavity (e.g., Figs. 4b–c View FIGURE 4 , 65c–d View FIGURE 65 )....... 4
3’. BP lacking a tubercle, its ventral portion poorly dilated or forming a hollow, dilated ventral concavity, varying in expansion ventrally and posteriorly (e.g., Figs. 2a–b View FIGURE 2 , 15c View FIGURE 15 , 18c View FIGURE 18 )...................................................... 6
4 (3). ML narrow, with squared apex ( Fig. 65c View FIGURE 65 ), ridge slightly curved, small, bearing strong teeth ( Fig. 65c–d View FIGURE 65 )................................................................................................. H. triangulare View in CoL
4’. ML broad, with roughly squared apex, ridge bearing small teeth (e.g., Figs. 54c–d View FIGURE 54 )............................. 5
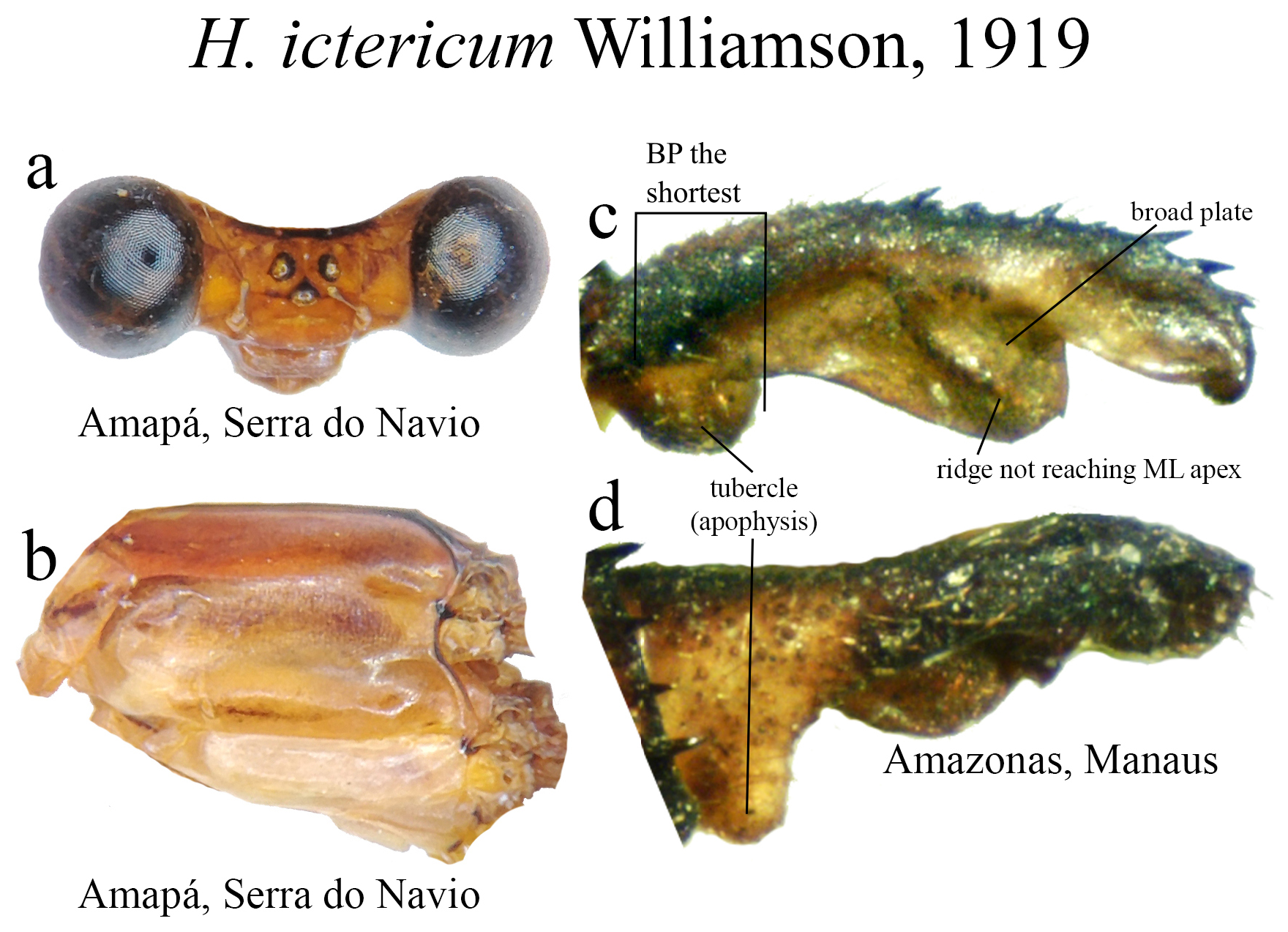
5 (4’). Head lacking a basal line of the frontoclypeal suture; restricted to Northern Brazil ( Figs. 38c–d View FIGURE 38 ).......... H. ictericum View in CoL
5’. Head with a basal line on the frontoclypeal suture; restricted to the Venezuelan Tepuis ( Figs. 54c–d View FIGURE 54 )........ H. pemon View in CoL
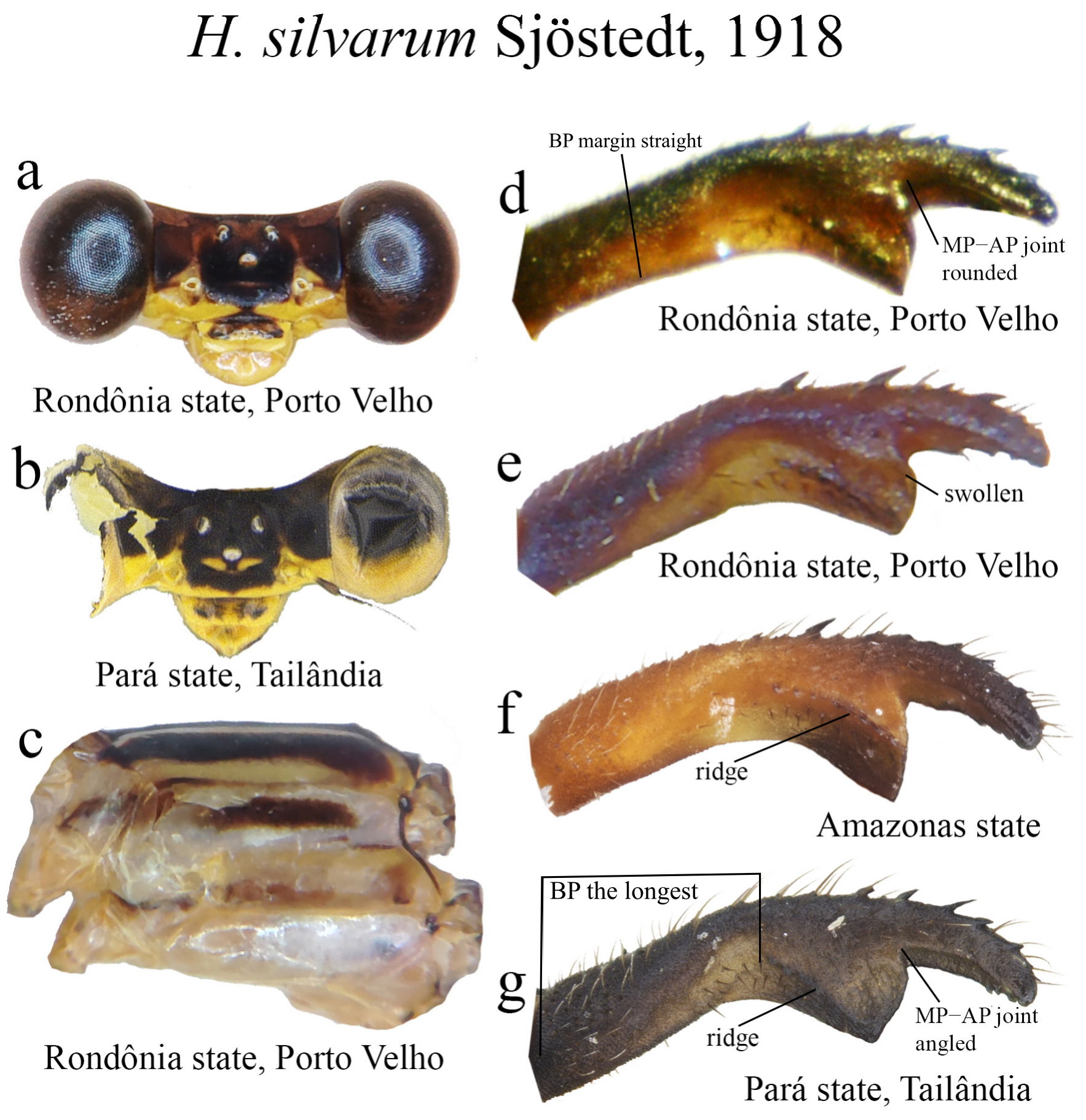
6 (3’). BP the longest cercus portion, little dilated ventrally; MP and AP subequal in length (e.g., Figs. 60d–f View FIGURE 60 )............. 7
6’. BP not the longest cercus portion, moderately to dilated ventral concavity; MP and AP not subequal in length (e.g., Fig. 12g View FIGURE 12 , 28c View FIGURE 28 , 30c View FIGURE 30 ).................................................................................... 9
7 (6). BP margin straight ( Figs. 26c–d View FIGURE 26 , 60d View FIGURE 60 ); ML broad, roughly squared; ML apex not bilobate, or if so, bearing only a small cleft ( Figs. 26c View FIGURE 26 , 60d–g View FIGURE 60 )............................................................................. 8
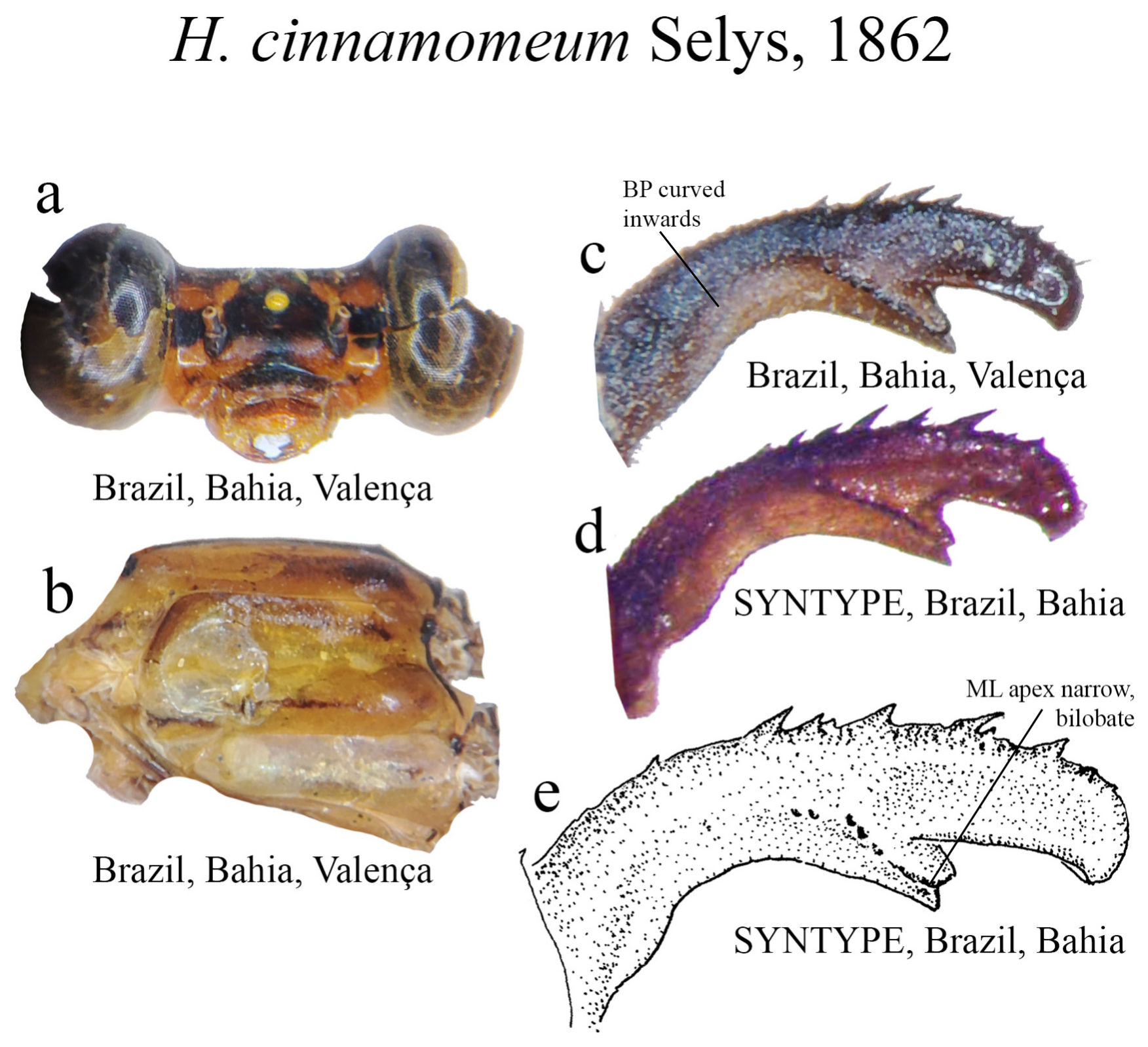
7’. BP slightly curved inwards ( Fig. 24c View FIGURE 24 ); ML narrow, ridge bearing strong teeth ( Fig. 24e View FIGURE 24 ); ML apex bilobate, W shaped ( Figs. 24c–e View FIGURE 24 )....................................................................... H. cinnamomeum View in CoL
8 (7). ML ridge short, nearly straight, reaching or not ML apex ( Fig. 60f View FIGURE 60 ); ML apex blunt, lacking cleft......... H. silvarum View in CoL
8’. ML ridge long, bearing an elevated ridge ( Fig. 26c View FIGURE 26 ); ML apex with a small cleft ( Fig. 26d View FIGURE 26 )................. H. cooki View in CoL
9 (6’). Overall body coloration consisting of shades of blue and green, with black background (e.g., Figs. 28a–b View FIGURE 28 , 30b View FIGURE 30 , 36b View FIGURE 36 ).... .............................................................................................. 10
9’. Overall body coloration consisting of shades of yellow and orange, with black background (e.g., Figs. 12a–e View FIGURE 12 , 27a View FIGURE 27 , 46ab View FIGURE 46 )............................................................................................ 12
10 (9). ML ridge not reaching its apex ( Fig. 28c View FIGURE 28 ); ML apex rounded, not bilobate ( Figs. 28c–d View FIGURE 28 )................... H. cyane
10’. ML ridge almost reaching its apex ( Figs. 30c View FIGURE 30 , 36 View FIGURE 36 ); ML apex not rounded, bilobate ( Figs. 30d View FIGURE 30 , 36 c–d View FIGURE 36 )............. 11
11 (10’). Lobes of ML apex rounded ( Fig. 30c View FIGURE 30 )......................................................... H. denisye
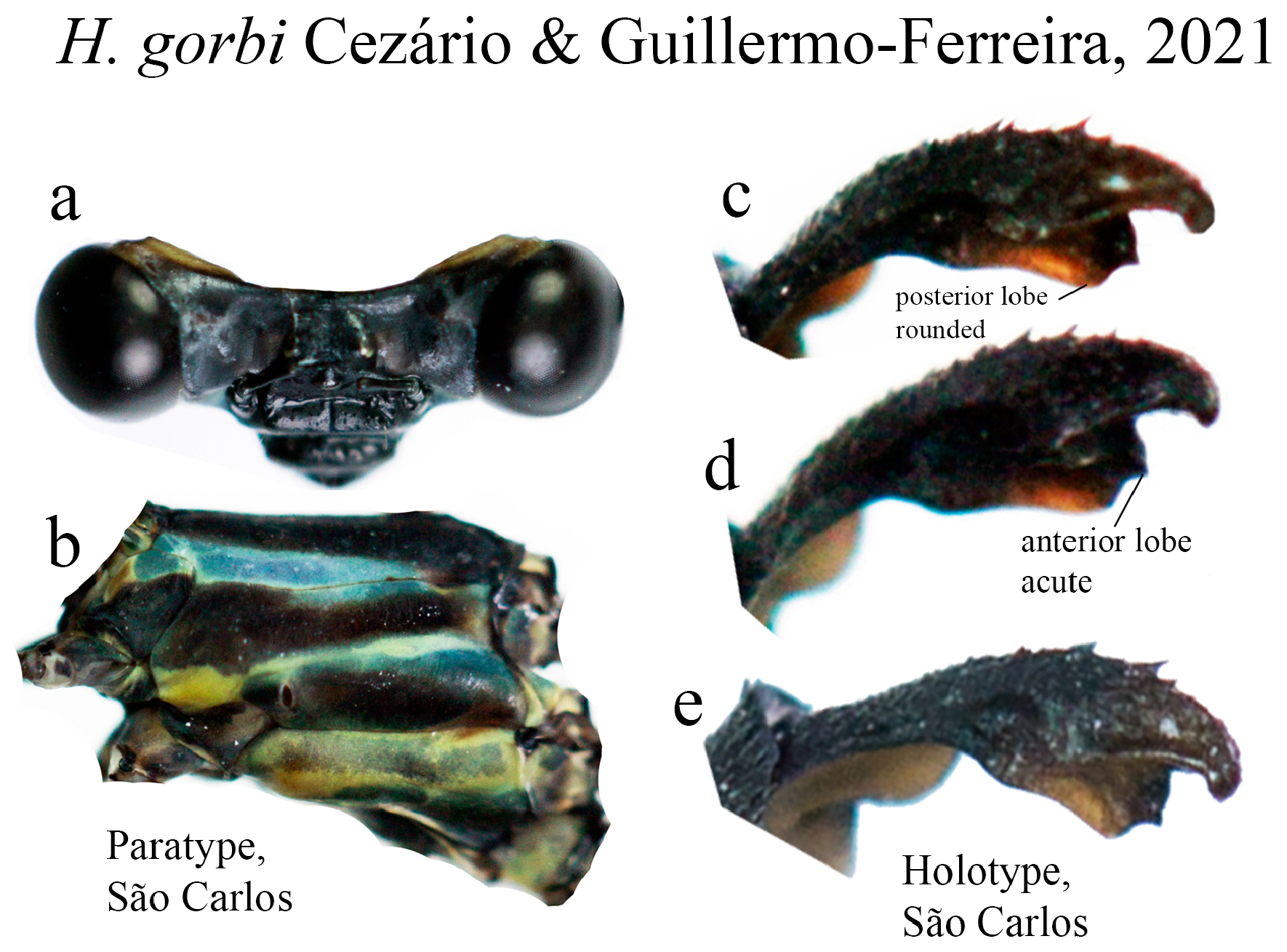
11’. Anterior lobe of ML acute, posterior lobe rounded ( Figs. 36c–d View FIGURE 36 )...................................... H. gorbi
12 (9’). ML apex cleft or bilobate (e.g., Figs. 12f–j View FIGURE 12 , 27d View FIGURE 27 , 46d View FIGURE 46 ).................................................... 13
12’. ML apex entire or undulated, not cleft or bilobate (e.g., Figs. 15c View FIGURE 15 , 37c–d View FIGURE 37 , 45c–d View FIGURE 45 ).............................. 15
13 (12). ML ridge nearly straight, long, reaching its apex, lacking an additional ridge and hollow space, bearing several small teeth ( Figs. 12f View FIGURE 12 , 27e–f View FIGURE 27 )................................................................................ 14
13’. ML ridge short, bearing strong teeth ( Fig. 46c View FIGURE 46 ), with an additional ridge forming a hollow space posteriorly ( Figs. 46e–f View FIGURE 46 )..................................................................................... H. luizfelipei View in CoL
14 (13). ML longer than wide, its apex bilobate, ‘W’ shaped ( Figs. 27d View FIGURE 27 ).............................. H. corderoi sp. nov.
14’. ML wider than long, apex not bilobate varying in width, but ending in a usually narrow cleft apex ( Figs. 12f–j View FIGURE 12 )........................................................................................... H. aurantiacum View in CoL
15 (12’). BP not greatly expanded or dilated ventrally (e.g., Fig. 15c View FIGURE 15 )............................................... 16
15’. BP expanded ventrally, forming a hollow concavity (e.g., Figs. 48d View FIGURE 48 , 49c View FIGURE 49 ).................................... 17
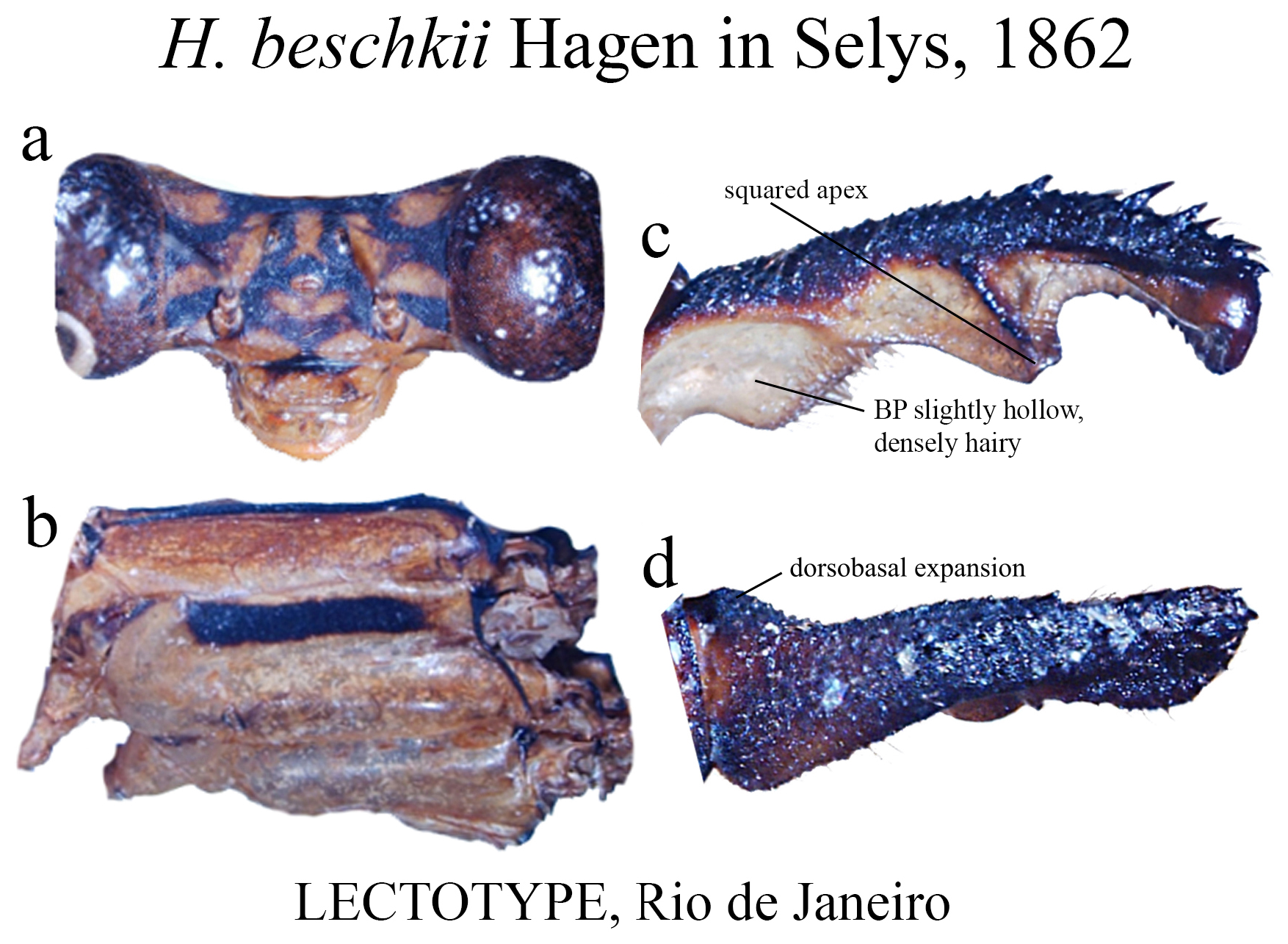
16 (15). BP densely hairy ( Fig. 15c View FIGURE 15 ); ML ridge bearing strong teeth, reaching its apex ( Fig. 15c View FIGURE 15 ); in lateral view, BP with a dorsobasal expansion ( Fig. 15d View FIGURE 15 ); ML apex square ( Fig. 15c View FIGURE 15 )....................................... H. beschkii View in CoL
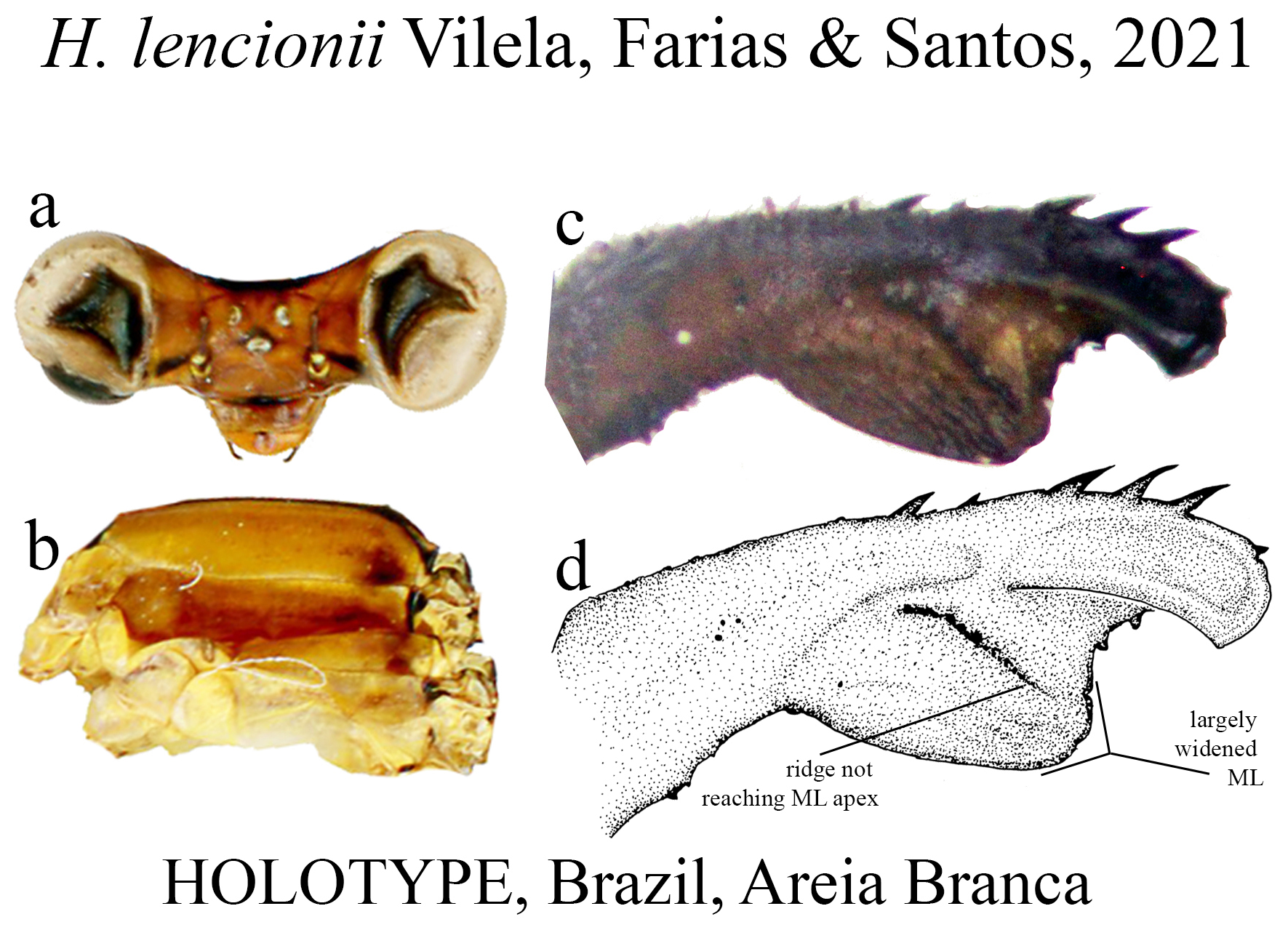
16’. BP not densely hairy; ML formed by a broad plate ( Figs. 45c–d View FIGURE 45 ); ML ridge short, not reaching its apex ( Fig. 45d View FIGURE 45 ); in lateral view, BP lacking dorsobasal expansion; ML apex rounded ( Fig. 45d View FIGURE 45 )............................... H. lencionii
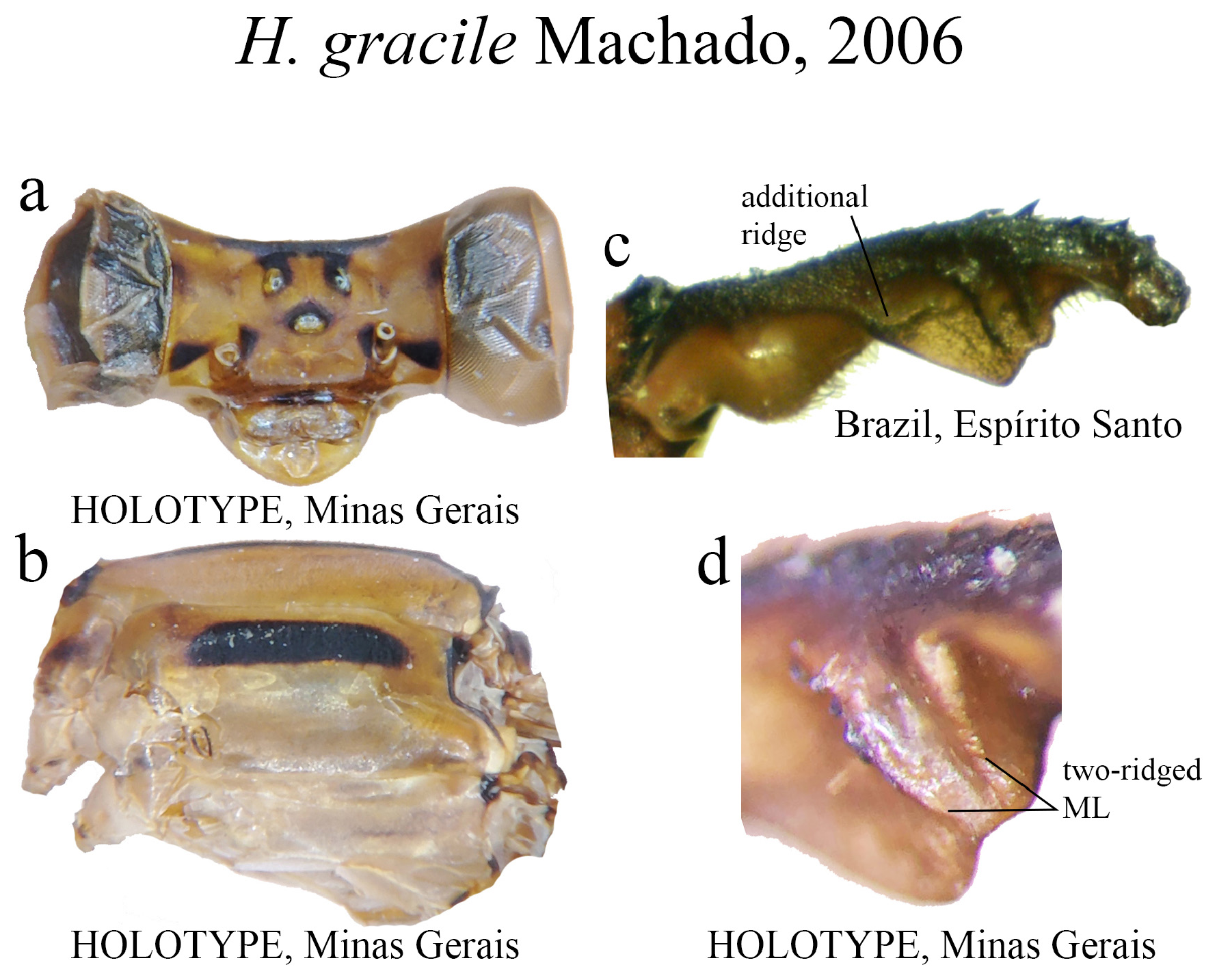
17 (15’). ML with two parallel ridges on its medial space ( Fig. 37d View FIGURE 37 ), plus an additional basal ridge ( Fig. 37c View FIGURE 37 ); ML apex undulate ( Figs. 37c–d View FIGURE 37 ).............................................................................. H. gracile View in CoL
17’. ML with a single ridge on medial space ( Figs. 48c–d View FIGURE 48 , 49c View FIGURE 49 ), lacking an additional basal ridge; ML apex not undulate ( Figs. 48c View FIGURE 48 , 49c View FIGURE 49 )....................................................................................... 18
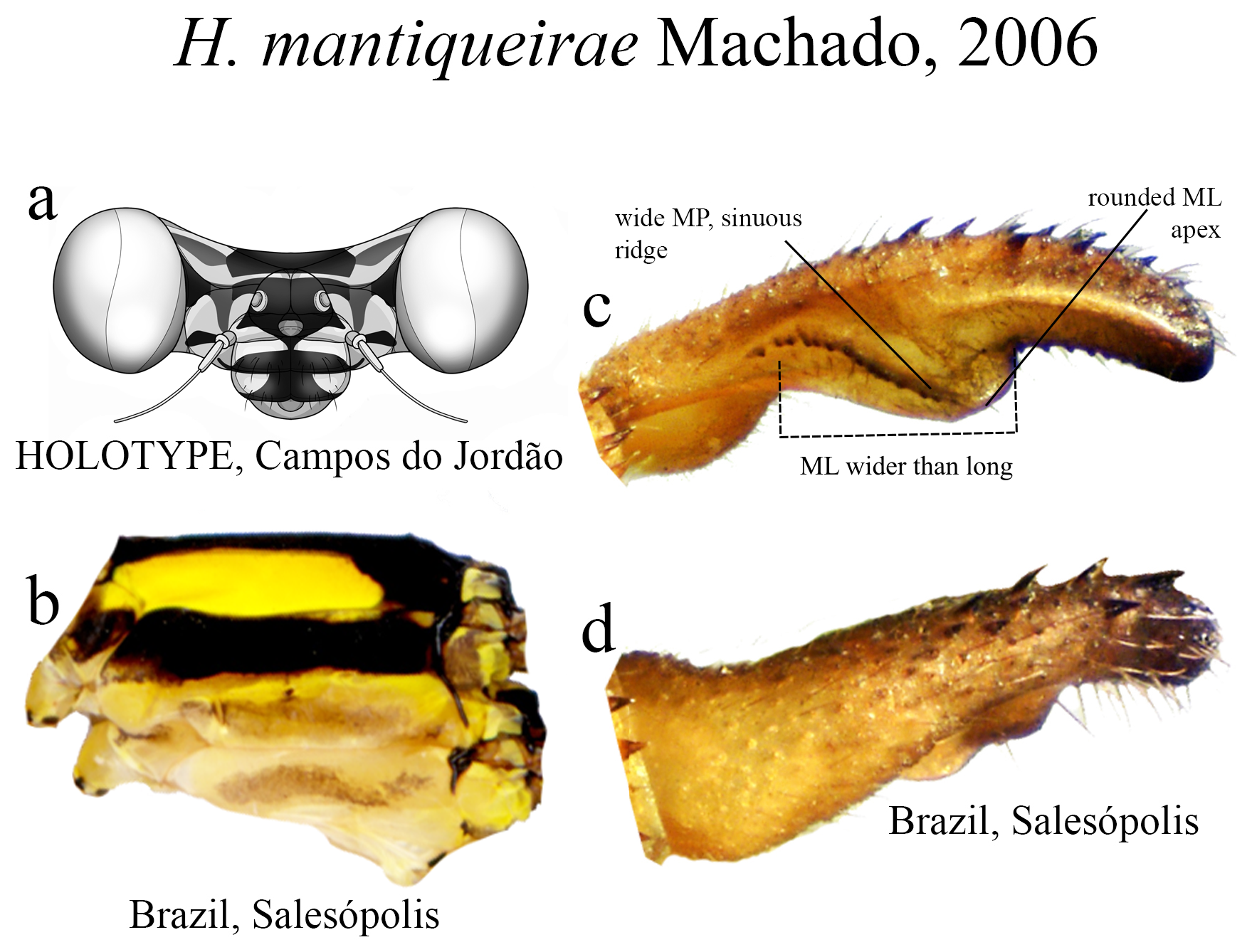
18 (17’). ML ridge long, sinuous, bearing strong teeth, reaching ML apex ( Fig. 49c View FIGURE 49 ); AP margin lacking any basal swelling ( Fig. 49c View FIGURE 49 )................................................................................ H. mantiqueirae View in CoL
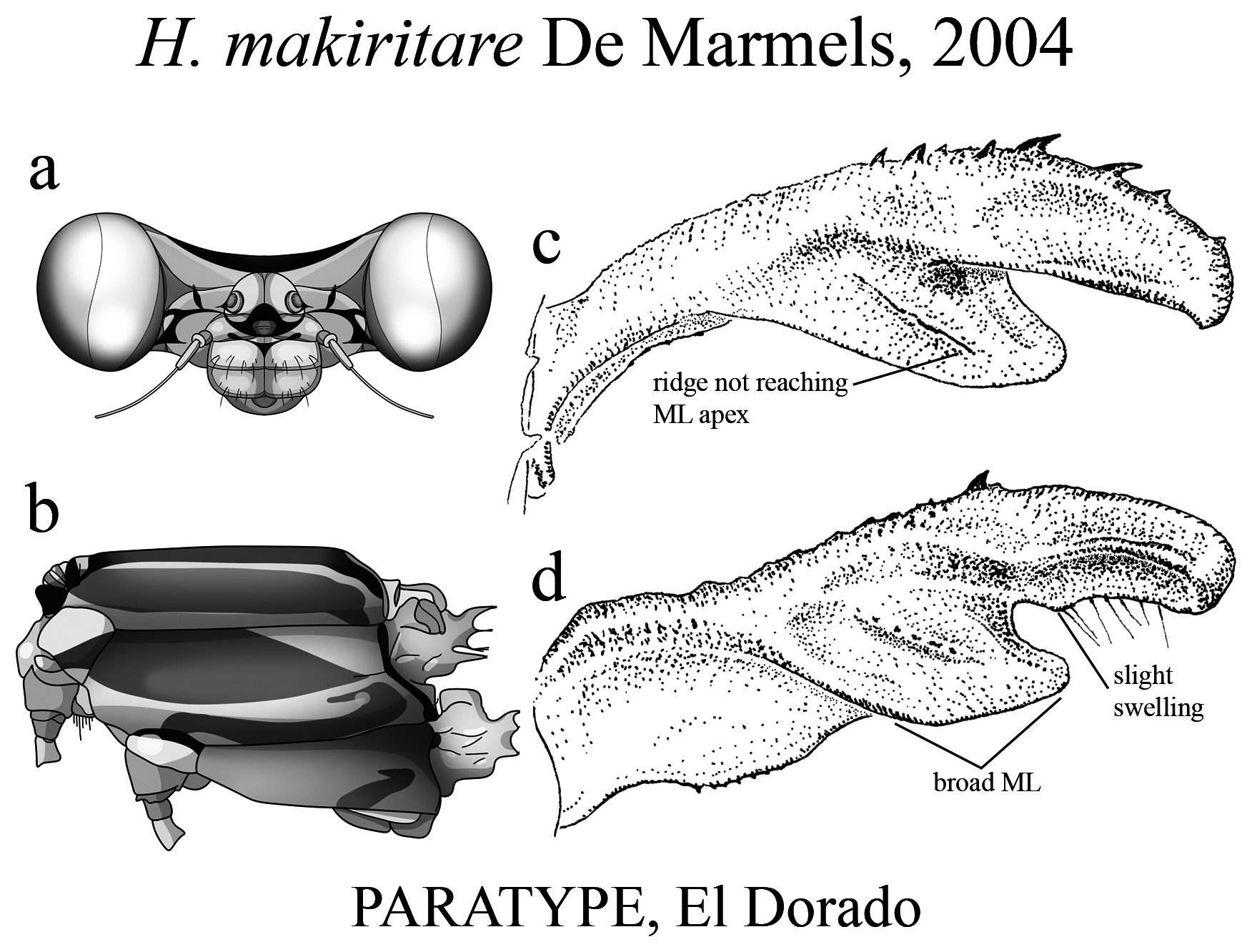
18’. ML ridge short, bearing small teeth, not reaching ML apex ( Fig. 48c View FIGURE 48 ); AP margin bearing a small basal swelling ( Fig. 48d View FIGURE 48 )................................................................................. H. makiritare View in CoL
19 (2’). BP uniquely with a long, narrow ventral branch ( Figs. 51c–d View FIGURE 51 )..................................... H. muryense View in CoL
19’. BP lacking a long, narrow ventral branch, instead forming a hollow, dilated ventral concavity, varying in expansion (e.g., Figs. 2a–b View FIGURE 2 , 18c View FIGURE 18 )................................................................................. 20
20 (19’). Upper plate of MP subequal in size to lower plate (e.g., Figs. 21e View FIGURE 21 , 25c–e View FIGURE 25 , 56c–d View FIGURE 56 ).............................. 21
20’. Upper plate of MP smaller than lower plate (e.g., Fig. 18c–d View FIGURE 18 , 43c–e View FIGURE 43 , 52e View FIGURE 52 ).................................... 23
21 (20). Upper plate rounded, lower plate roughly squared ( Figs. 21e View FIGURE 21 , 56c View FIGURE 56 )......................................... 22
21’. Upper plate and lower plate with similar shape ( Figs. 25c–e View FIGURE 25 )....................................... H. consors View in CoL
22 (21). Upper plate broader than lower plate; lower plate bearing two carinae and squared apex ( Fig. 21e View FIGURE 21 )........... H. cauei
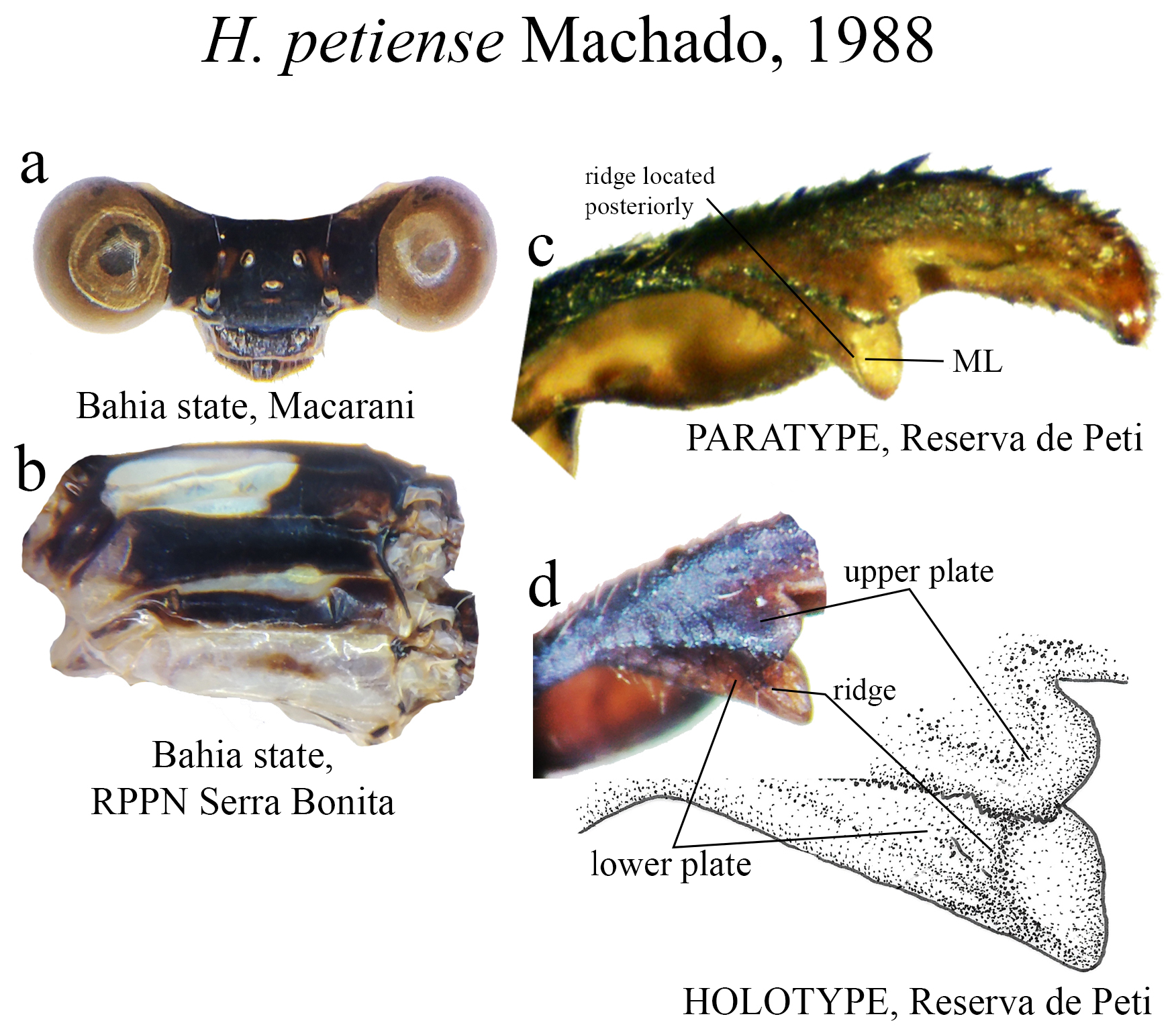
22’. Upper plate broader than lower plate; lower plate lacking two carinae, bearing acute apex ( Fig. 56c View FIGURE 56 )....... H. petiense View in CoL
23 (20’). BP hollow, greatly expanded ventrally, uniquely surpassing MP level ( Figs. 52e–h View FIGURE 52 )..................... H. ovatum View in CoL
23’. BP hollow, but ventral expansion not surpassing MP level (remainder of Group A species)...................... 24
24 (23’). Upper plate narrow, longer than wide (e.g., Figs. 18c–d View FIGURE 18 , 43c–e View FIGURE 43 )............................................ 25
24’. Upper plate in a different shape (small, with rounded edges, as in Figs. 44c View FIGURE 44 , 35c–d View FIGURE 35 ; short, wider than long, as in Fig. 64d View FIGURE 64 ; or inverted triangle, with angled corners, as in Fig. 57e View FIGURE 57 ).................................................. 26
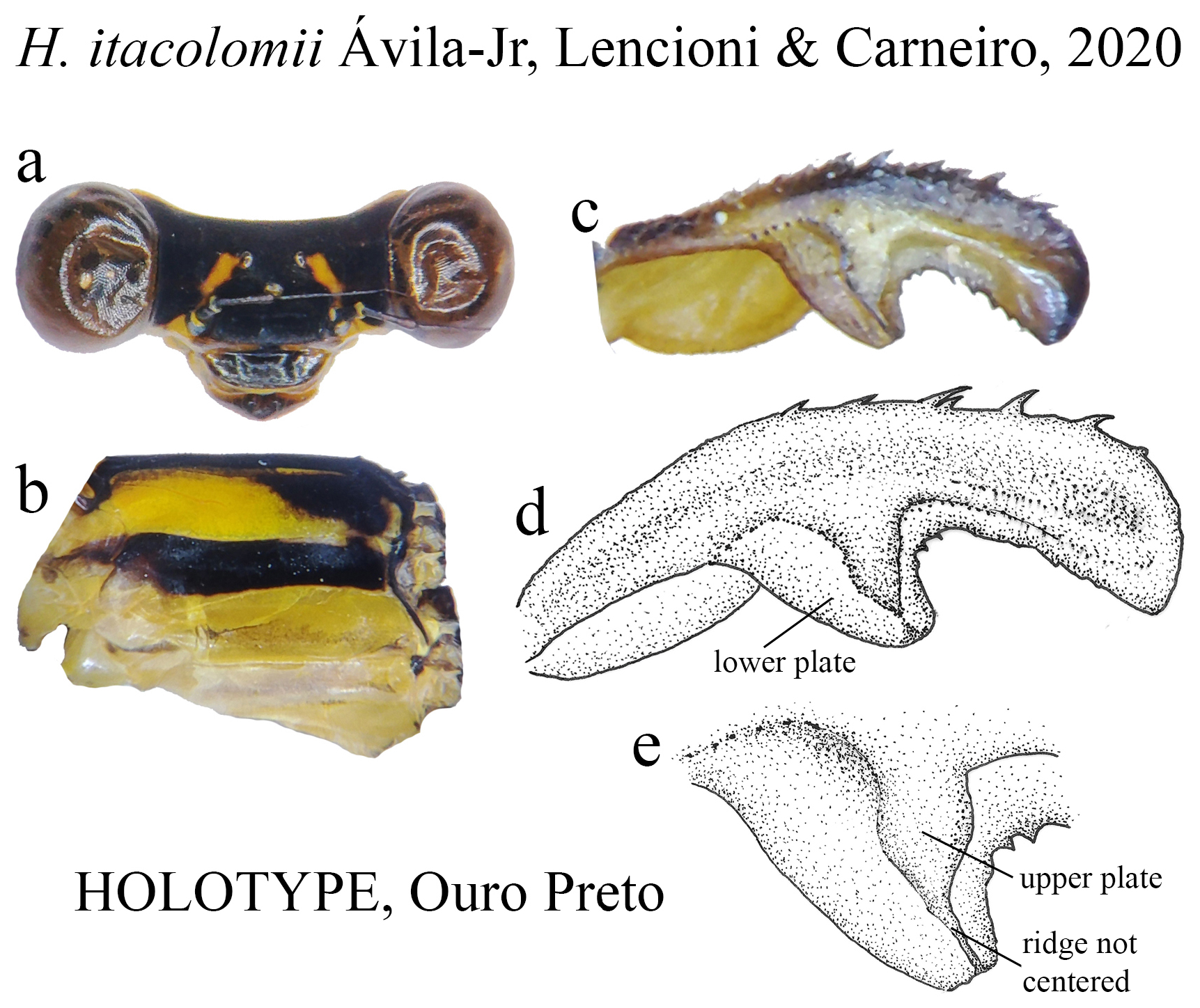
25 (24). BP forming a greatly ventral expanded concavity ( Fig. 43c View FIGURE 43 ); ML ridge not centered ( Figs. 43d–e View FIGURE 43 )........ H. itacolomii
25’. BP ventral concavity not greatly expanded ( Figs. 18c–d View FIGURE 18 ); ML ridge centered ( Fig. 18d View FIGURE 18 )................ H. brianmayi
26 (24’). Upper plate with rounded edges (e.g., Figs. 44c View FIGURE 44 , 35c–d View FIGURE 35 ).................................................. 27
26’. Upper plate with angled edges (e.g., Figs. 64d View FIGURE 64 , 57e View FIGURE 57 )..................................................... 28
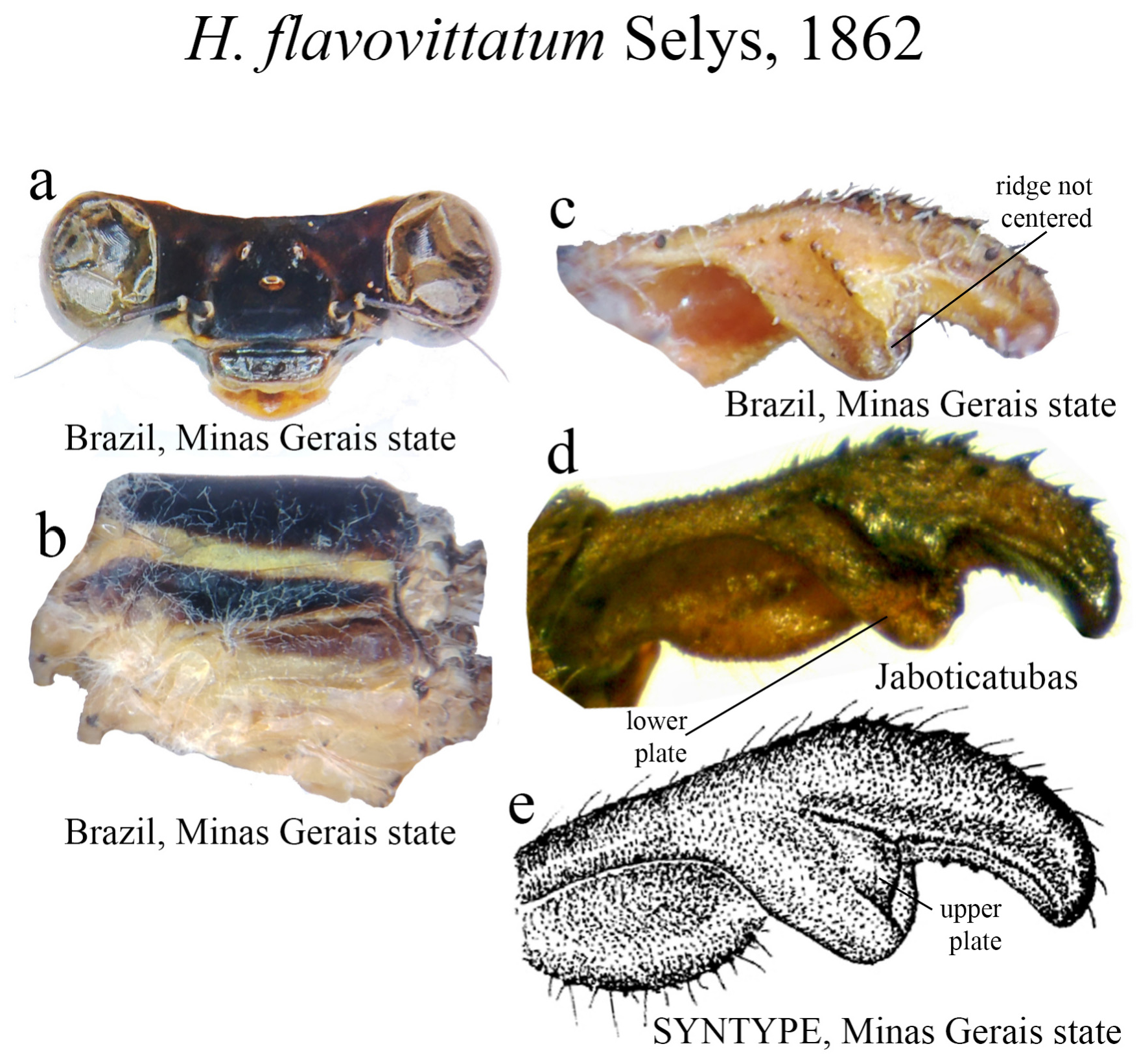
27 (26). ML apex rounded ( Figs. 34c–e View FIGURE 34 ).......................................................... H. flavovittatum View in CoL
27’. ML apex roughly square ( Figs. 35c View FIGURE 35 , 44c View FIGURE 44 ).............................................................. 29
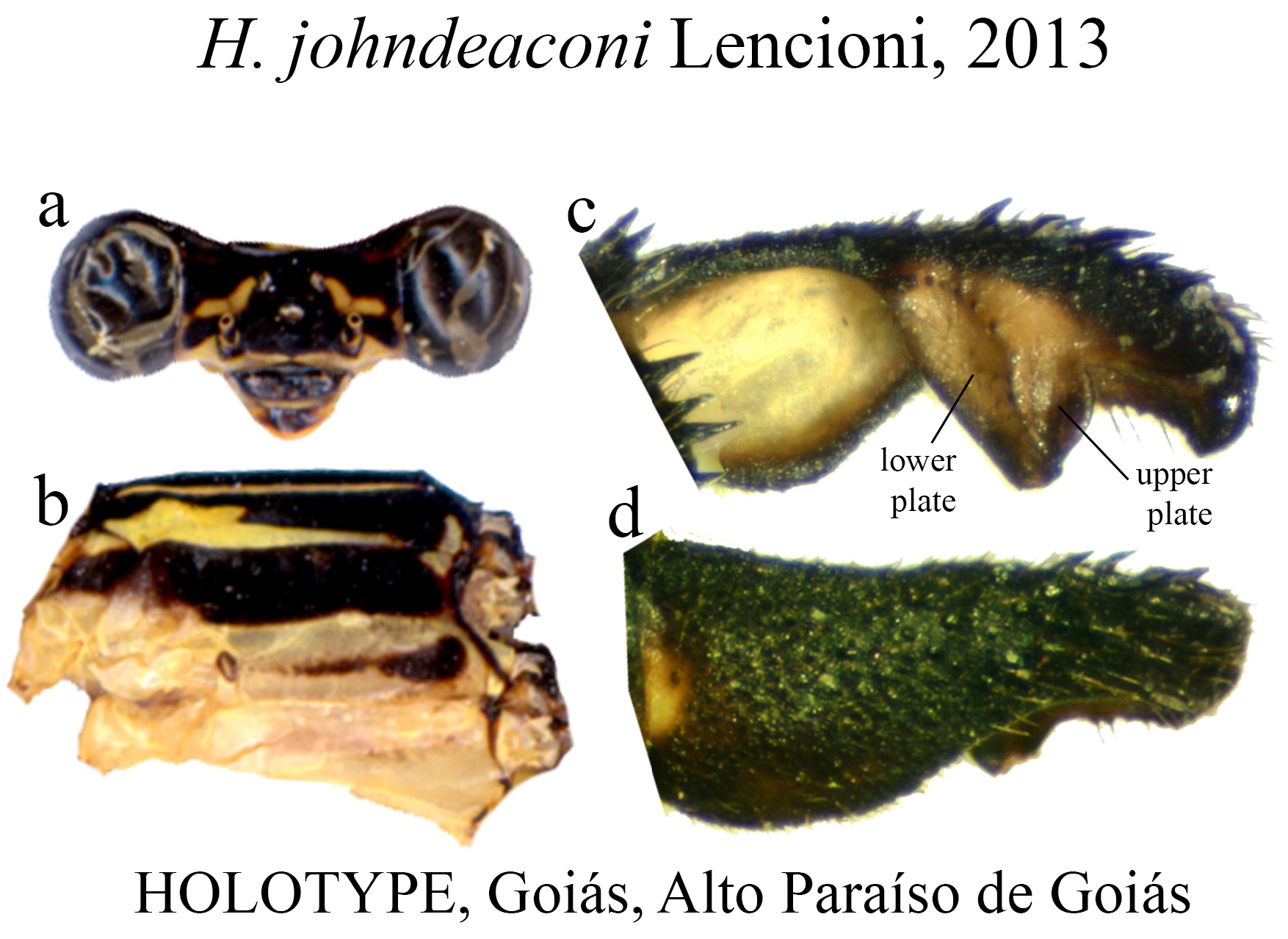
28 (26’). Upper plate bearing a posterior row of small teeth, not centered ( Fig. 44c View FIGURE 44 ); broadly developed BP ventral concavity; ridge fused with ML at the apex ( Fig. 44d View FIGURE 44 )...................................................... H. johndeaconi
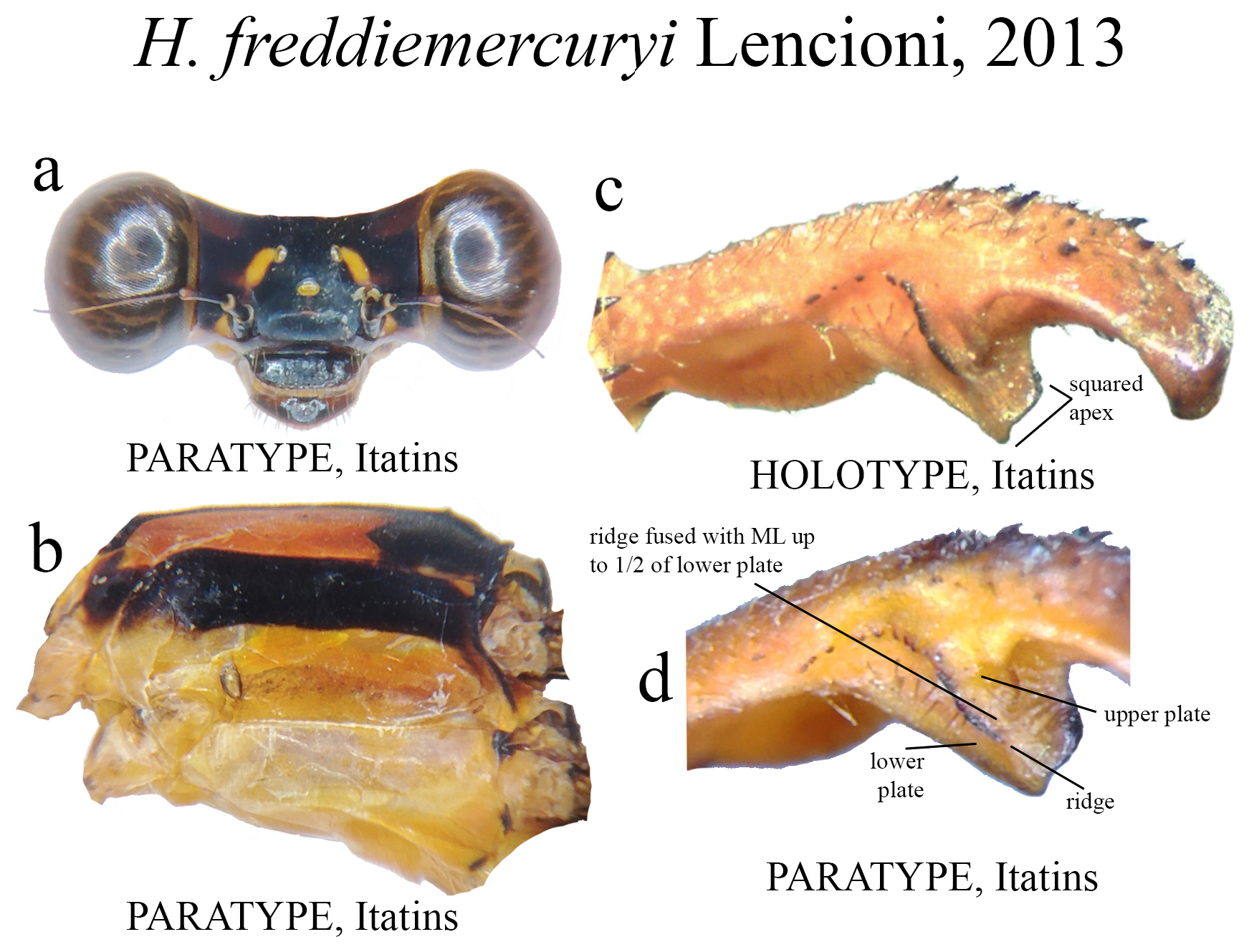
28’. Upper plate bearing a posterior row of strong teeth, centered ( Fig. 35c View FIGURE 35 ); moderately developed BP concavity; ridge fused with ML up to 1/2 of lower plate ( Fig. 35d View FIGURE 35 ).............................................. H. freddiemercuryi
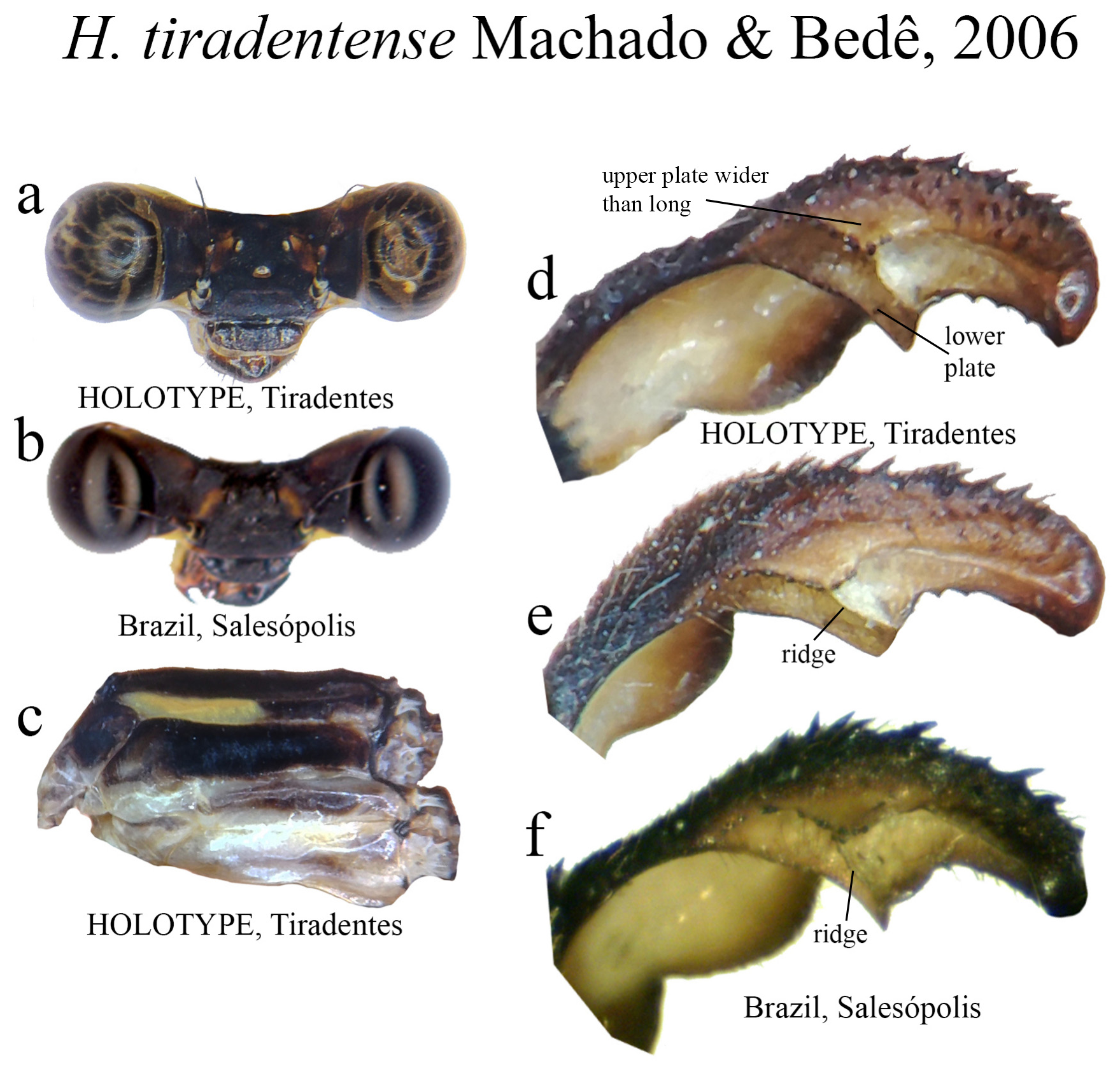
29 (27’). Upper plate wider than long, roughly triangular, bearing small teeth on its border ( Figs. 64d–f View FIGURE 64 ); ML apex acute ( Fig. 64f View FIGURE 64 ) H. tiradentense View in CoL
29’. Upper plate longer than wide, shaped like an inverted triangle (e.g., Figs. 57e View FIGURE 57 , 58c View FIGURE 58 , 63c View FIGURE 63 ); ML apex squared (e.g., Fig. 63c View FIGURE 63 ) or bilobate (e.g., Fig. 57e View FIGURE 57 )......................................................................... 30
30 (29’). ML apex not bilobate, roughly squared ( Figs. 58c View FIGURE 58 , 63c–d View FIGURE 63 )................................................ 31
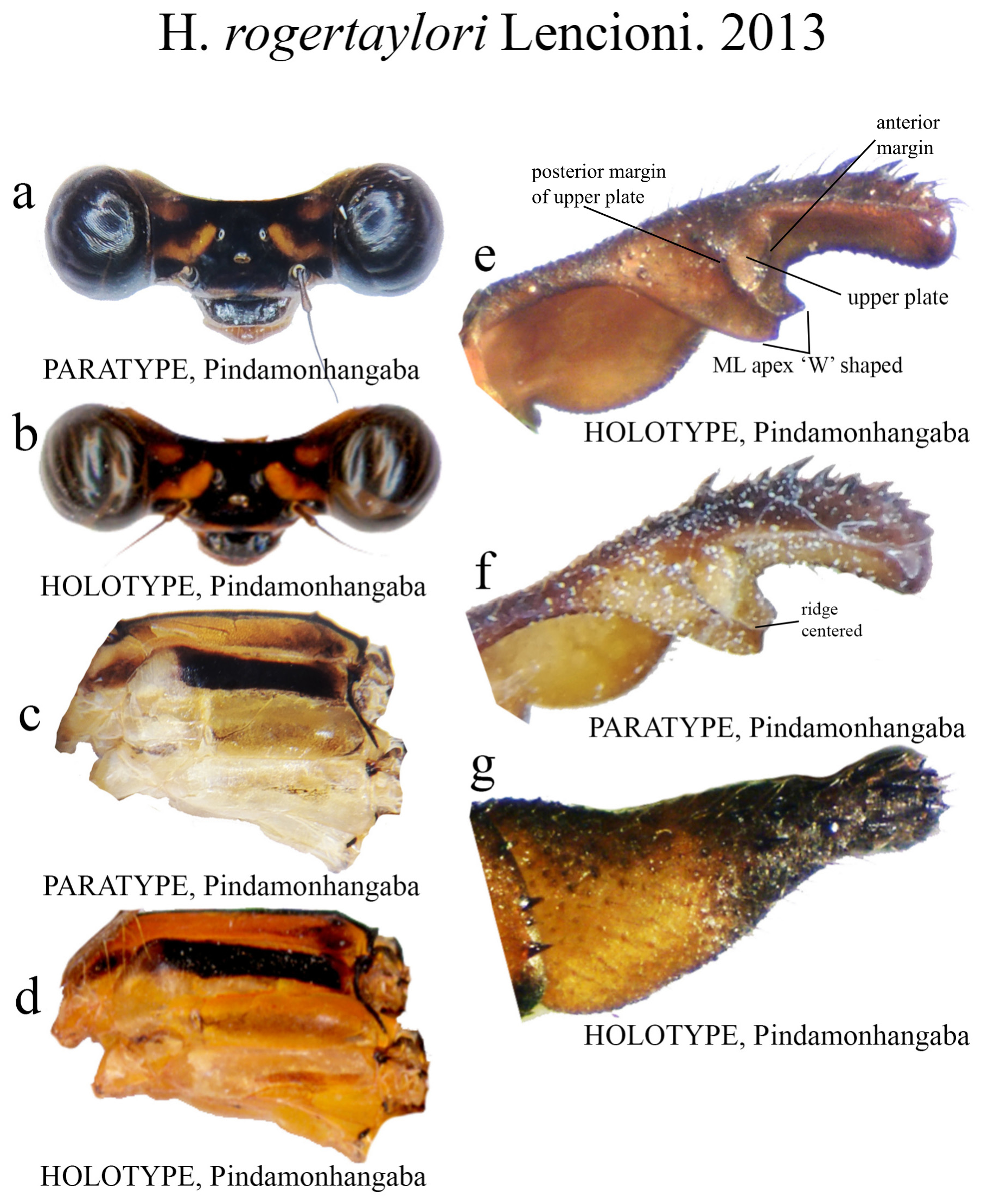
30’. ML apex bilobate, ‘W’ shaped ( Fig. 57e View FIGURE 57 ).................................................... H. rogertaylori
31 (30). Posterior margin of upper plate short, straight, bearing small teeth, anterior margin subequal in size ( Fig. 63c View FIGURE 63 ); upper plate markedly concave at its middle ( Fig. 63d View FIGURE 63 ); ML apex acutely squared ( Figs. 63c–d View FIGURE 63 )........................ H. thais
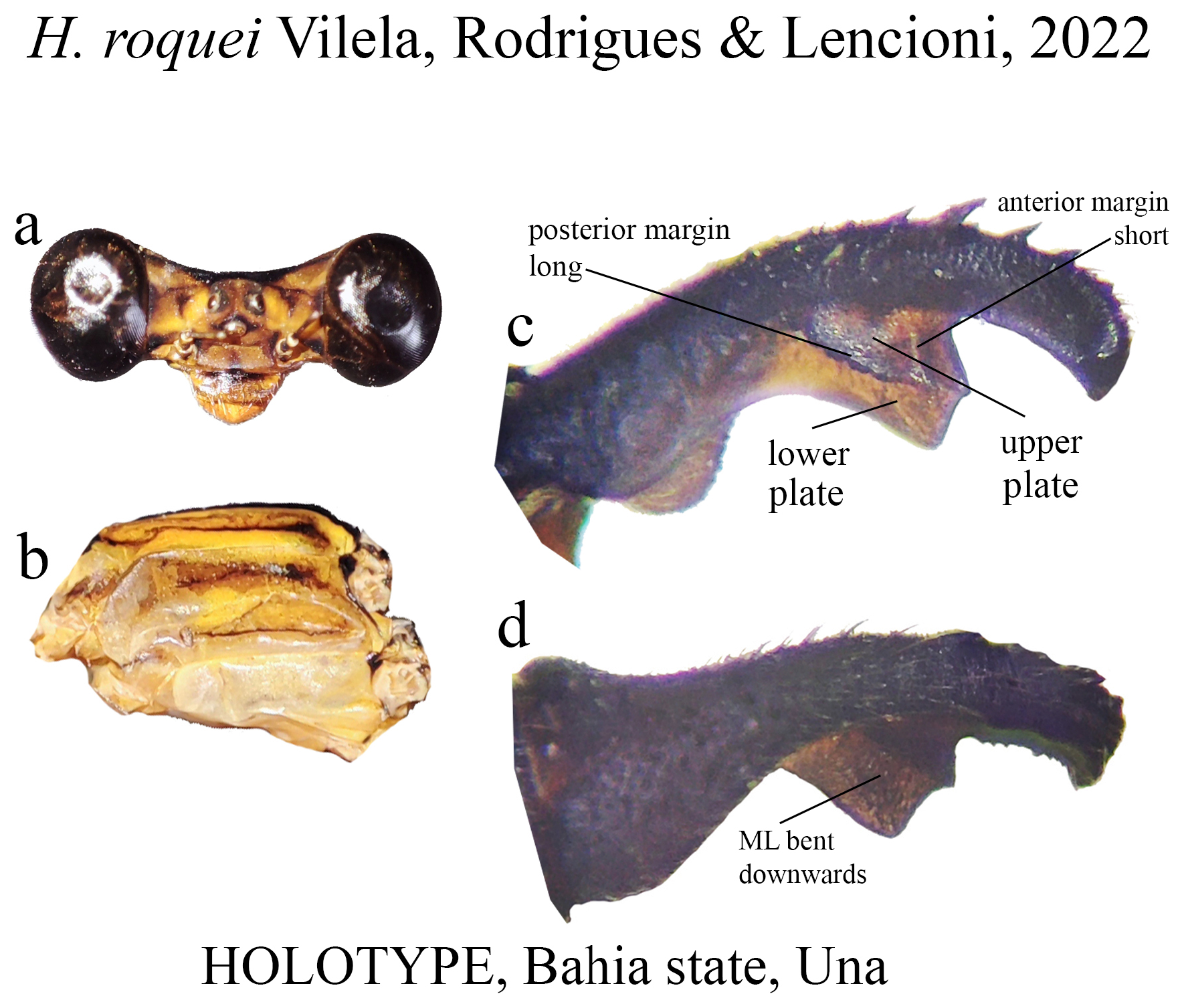
31’. Posterior margin of upper plate long, bearing a row of strong teeth, anterior margin short ( Fig. 58c View FIGURE 58 ); upper plate slightly concave at its middle; ML apex roughly squared ( Fig. 58c View FIGURE 58 )......................................... H. roquei
Key to Group B species
2 (1’). Paraprocts very long, surpassing 1/2 of cercus, almost reaching its apex (e.g., Figs. 4f View FIGURE 4 . 11c–d View FIGURE 11 ).................... 4
2’. Paraprocts from short to medium size, not surpassing 1/2 of cercus.......................................... 3
3. Paraprocts medium sized, almost reaching 1/2 of cercus length (e.g., Figs. 4e View FIGURE 4 , 17d View FIGURE 17 , 39e View FIGURE 39 )......................... 5
3’. Paraprocts short, thin, not surpassing 1/3 of cercus length (e.g., Figs. 4d View FIGURE 4 , 31b View FIGURE 31 , 59c View FIGURE 59 , 62d–e View FIGURE 62 , 67d View FIGURE 67 )................... 7
4 (2). In lateral view, cercus arched at midlength ( Fig. 53c View FIGURE 53 ); abdomen 52 mm ............................ H. palmichale View in CoL
4’. In lateral view, overall cercus shape less arched ( Fig. 11c View FIGURE 11 ) than H. palmichale View in CoL ( Fig. 53c View FIGURE 53 ); abdomen 49 mm ... H. archon View in CoL
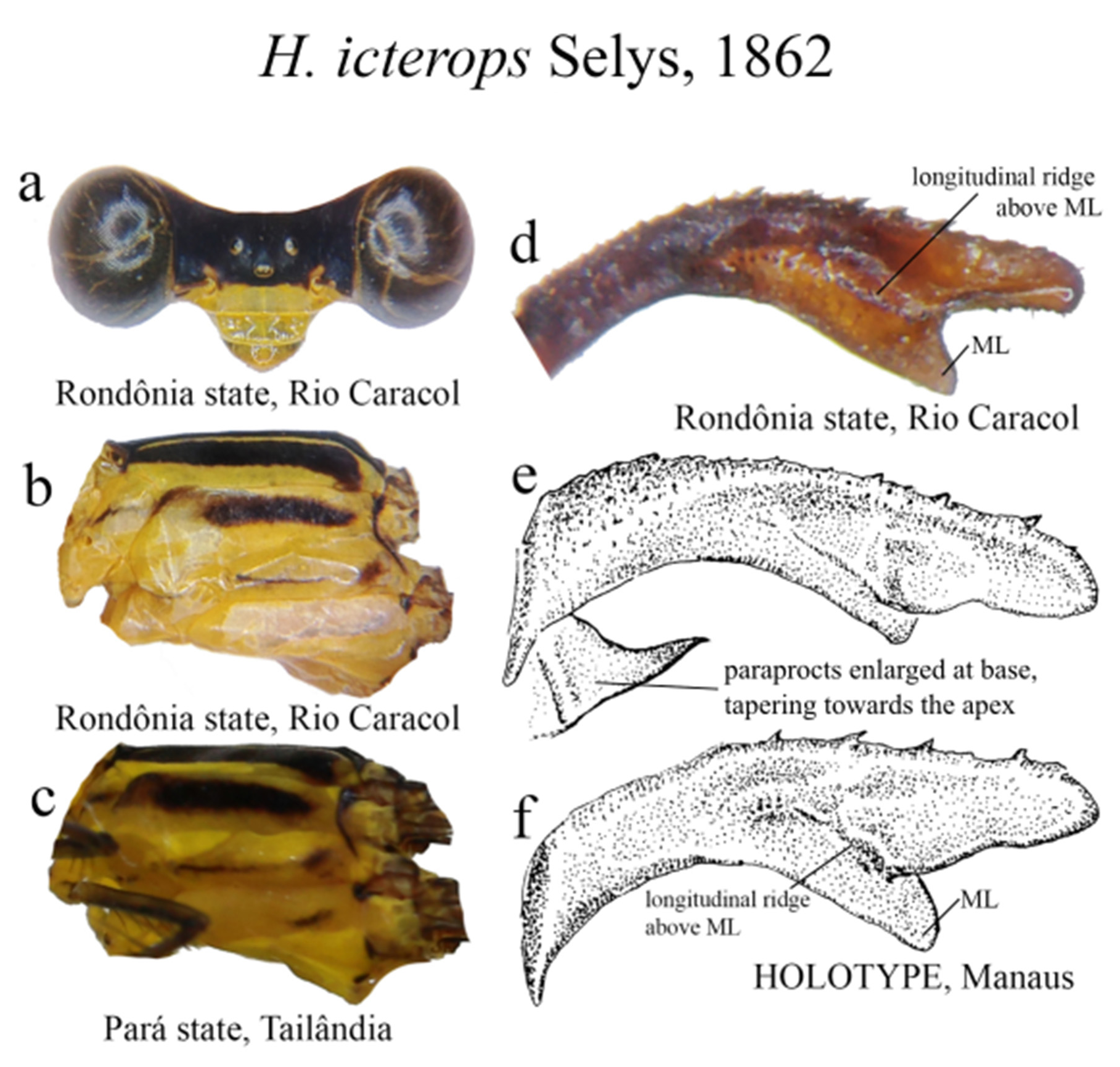
5 (3’). Ridge above ML long, sinuous consisting of small teeth, and a marked carina ( Figs. 39d–f View FIGURE 39 ); in lateral view, ML bent downwards ( Fig. 39f View FIGURE 39 ); AP the shortest ( Fig. 39d View FIGURE 39 ); S9–10 almost entirely black or dark brown............. H. icterops View in CoL
5’. Morphology as in H. icterops View in CoL , but larger yellow areas on head, thorax and abdomen............................ 6
6 (5’). Small yellow spots above each antenna ( Fig. 17a View FIGURE 17 ); S9–10 dark brown/yellow.......................... H. breweri View in CoL
6’. Small spots above antennae absent ( Fig. 61a View FIGURE 61 ); S9–10 paler...................................... H. simulatum View in CoL
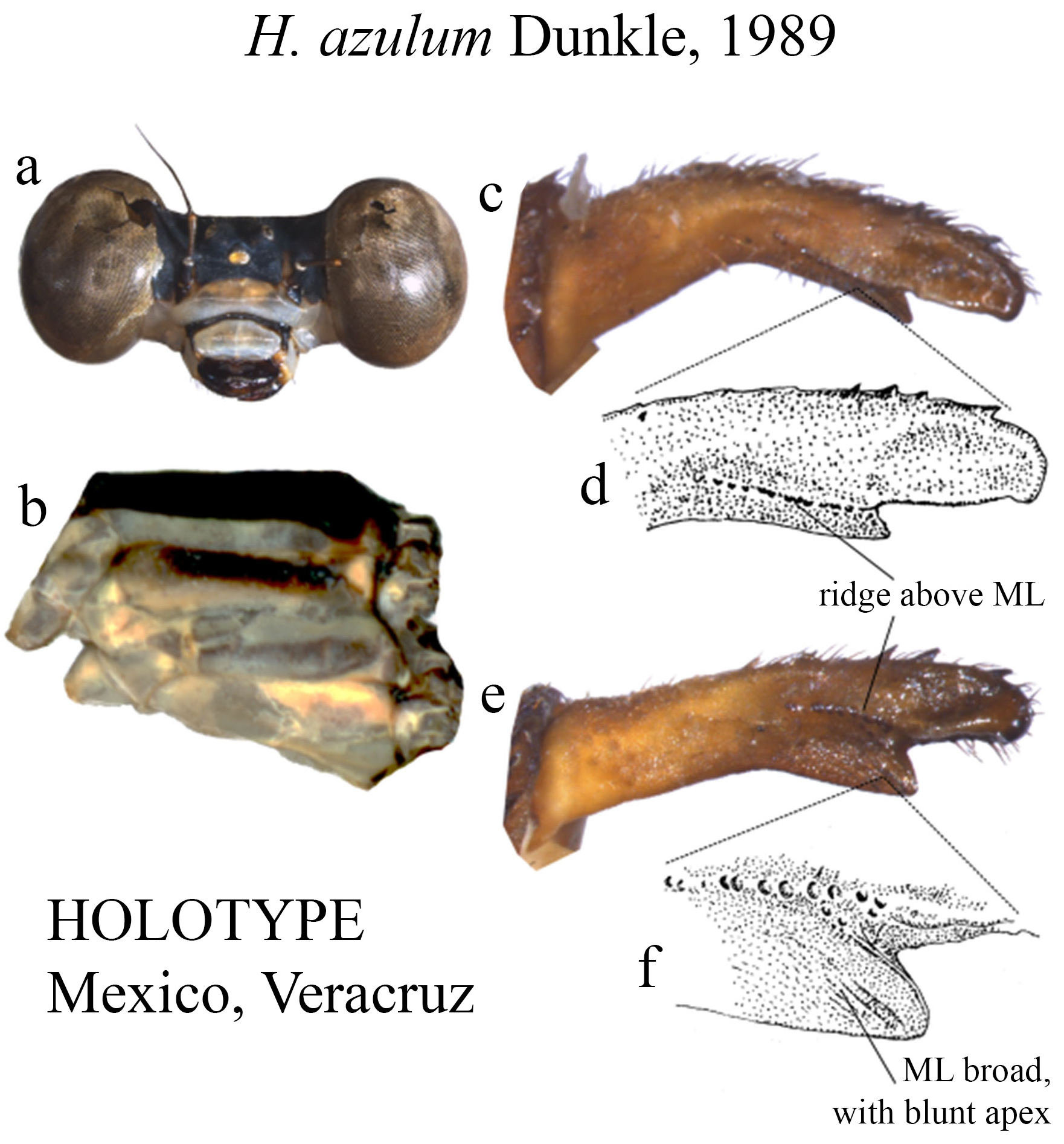
7 (3’). Overall body coloration consisting of shades of blue and gray, with black background ( Figs. 13a–b View FIGURE 13 ), S8–10 yellow ( Figs. 13c–e View FIGURE 13 ).................................................................................. H. azulum View in CoL
7’. Overall body coloration consisting of shades of yellow and orange, with black background, or largely red areas such as head and abdomen................................................................................ 8
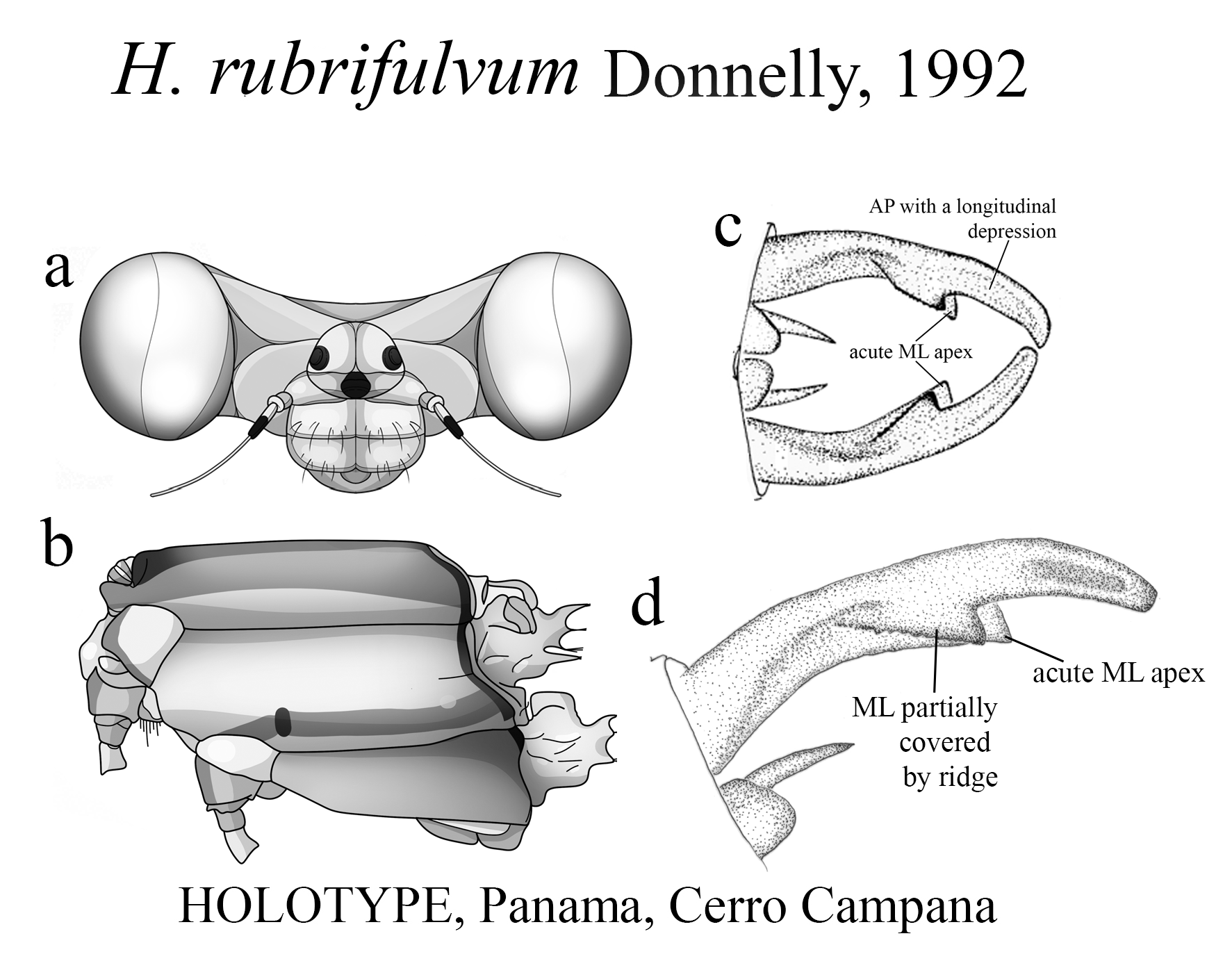
8. Largely red abdomen (e.g., H. erythrogastrum View in CoL and H. albifrons View in CoL ) or head (e.g., H. rubrifulvum View in CoL ).................... 9
8’. Abdomen and head not red......................................................................... 11
9 (8). Abdomen largely red, head largely black................................................. H. erythrogastrum View in CoL
9’. Some other combination of body coloration........................................................... 10
10 (9’). Head and mouthparts largely pale; S3–S7 black and red, S8–10 largely red........................... H. albifrons View in CoL
10’. Head red, remainder of body pale yellow.................................................. H. rubrifulvum View in CoL
11 (8’). Longitudinal toothed ridge not partially covering ML in dorsal and dorsolateral views (e.g., Figs. 9 c–e View FIGURE 9 , 55c–e View FIGURE 55 ; 62c View FIGURE 62 ).... .............................................................................................. 12
11’. Longitudinal toothed ridge partially covering ML in dorsal and dorsolateral views (e.g., Figs. 2g –h View FIGURE 2 , 7e–f View FIGURE 7 , 10f View FIGURE 10 , 14f–g View FIGURE 14 )... .............................................................................................. 19
12 (11). ML bearing small teeth on its plate (e.g., Figs. 55c–e View FIGURE 55 ; 62c View FIGURE 62 )............................................... 13
12’. ML bearing no teeth or carina on its plate (e.g., Figs. 8c–e View FIGURE 8 , 10g View FIGURE 10 , 14d–e View FIGURE 14 )..................................... 14
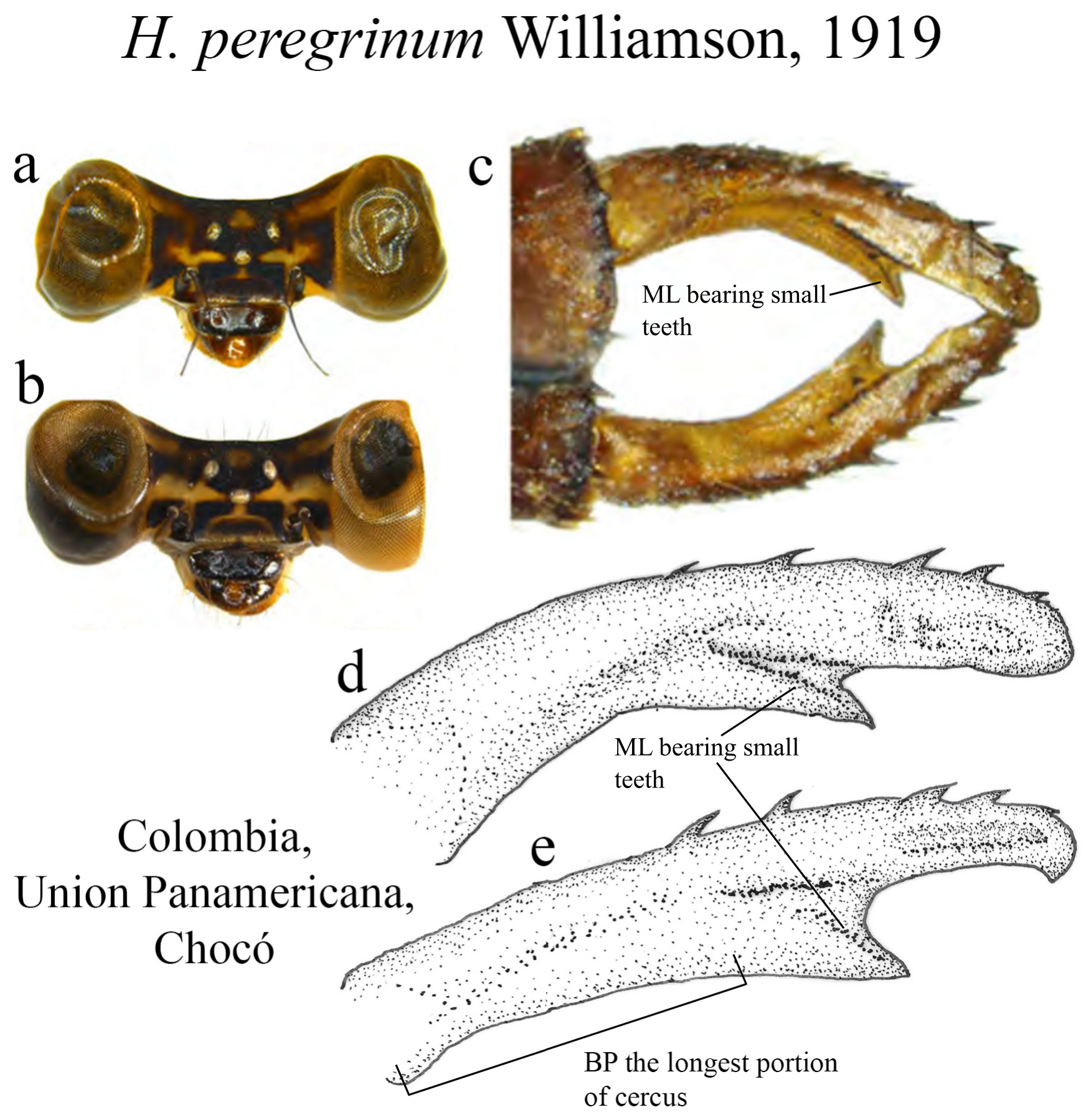
13 (12). BP the longest portion, with nearly straight margin ( Fig. 55e View FIGURE 55 ); MP subequal in length to AP ( Fig. 55e View FIGURE 55 ); ridge above ML long, bearing small teeth in a straight line, not reaching its apex ( Figs. 55c–e View FIGURE 55 ); ML apex acute ( Figs. 55c–e View FIGURE 55 ).............................................................................................. H. peregrinum View in CoL
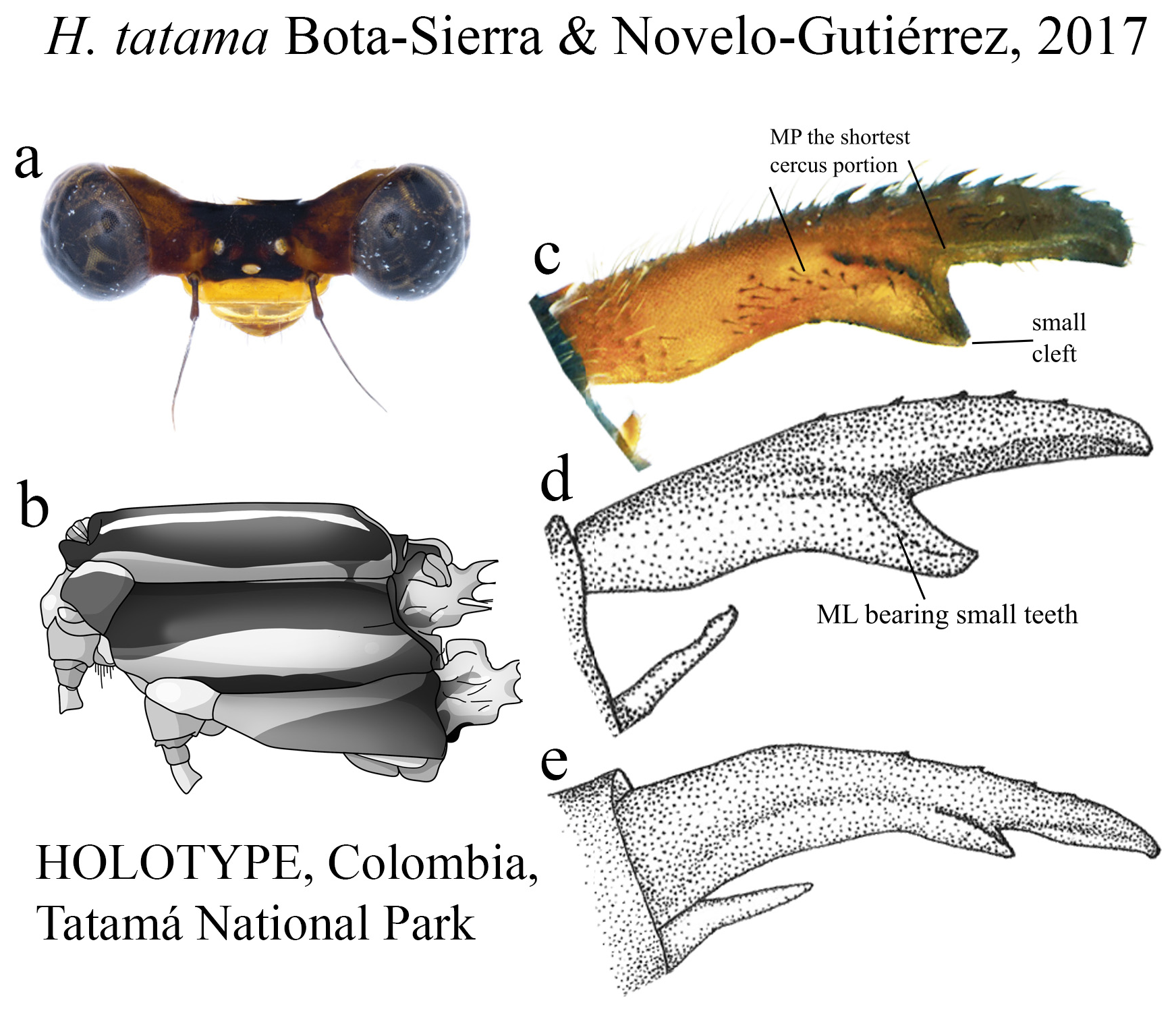
13’. BP subequal in length to AP, slightly convex margin ( Fig. 62c View FIGURE 62 ); MP the shortest ( Fig. 62c View FIGURE 62 ); ridge above ML bearing strong teeth on a nearly straight line, reaching its apex; ML apex with a small cleft ( Figs. 62c–e View FIGURE 62 )................. H. tatama
14 (12’). BP the longest cercus section in length (e.g., Figs. 9c–e View FIGURE 9 , 20e View FIGURE 20 , 67e View FIGURE 67 ).......................................... 15
14’. BP not the longest cercus section in length (e.g., Fig. 7e View FIGURE 7 , 23c View FIGURE 23 ; 31c View FIGURE 31 )......................................... 18
15 (14). Ridge above ML bearing strong teeth in a single row ( Figs. 20d–e View FIGURE 20 ); AP small, subequal to MP ( Fig. 20e View FIGURE 20 )................................................................................................. H. calendulum View in CoL
15’. Ridge above ML consisting of small denticles; AP not as above............................................ 16
16 (15’). Ridge above ML consisted of very small denticles in a single row ( Figs. 9e View FIGURE 9 , 16e View FIGURE 16 ).............................. 17
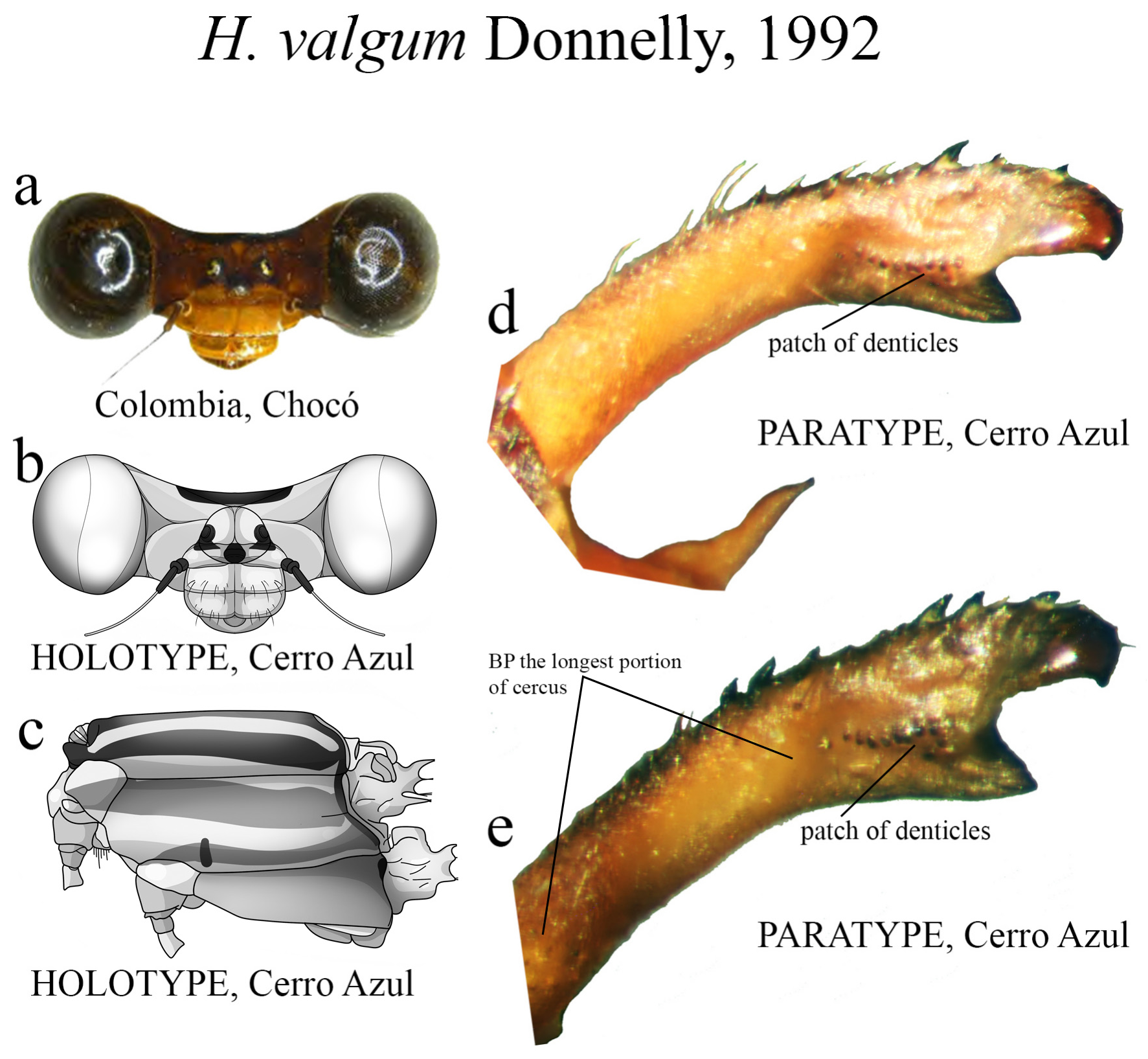
16’. Ridge above ML consisting of a patch of small denticles, not in a single row ( Figs. 67d–e View FIGURE 67 )............... H. valgum View in CoL
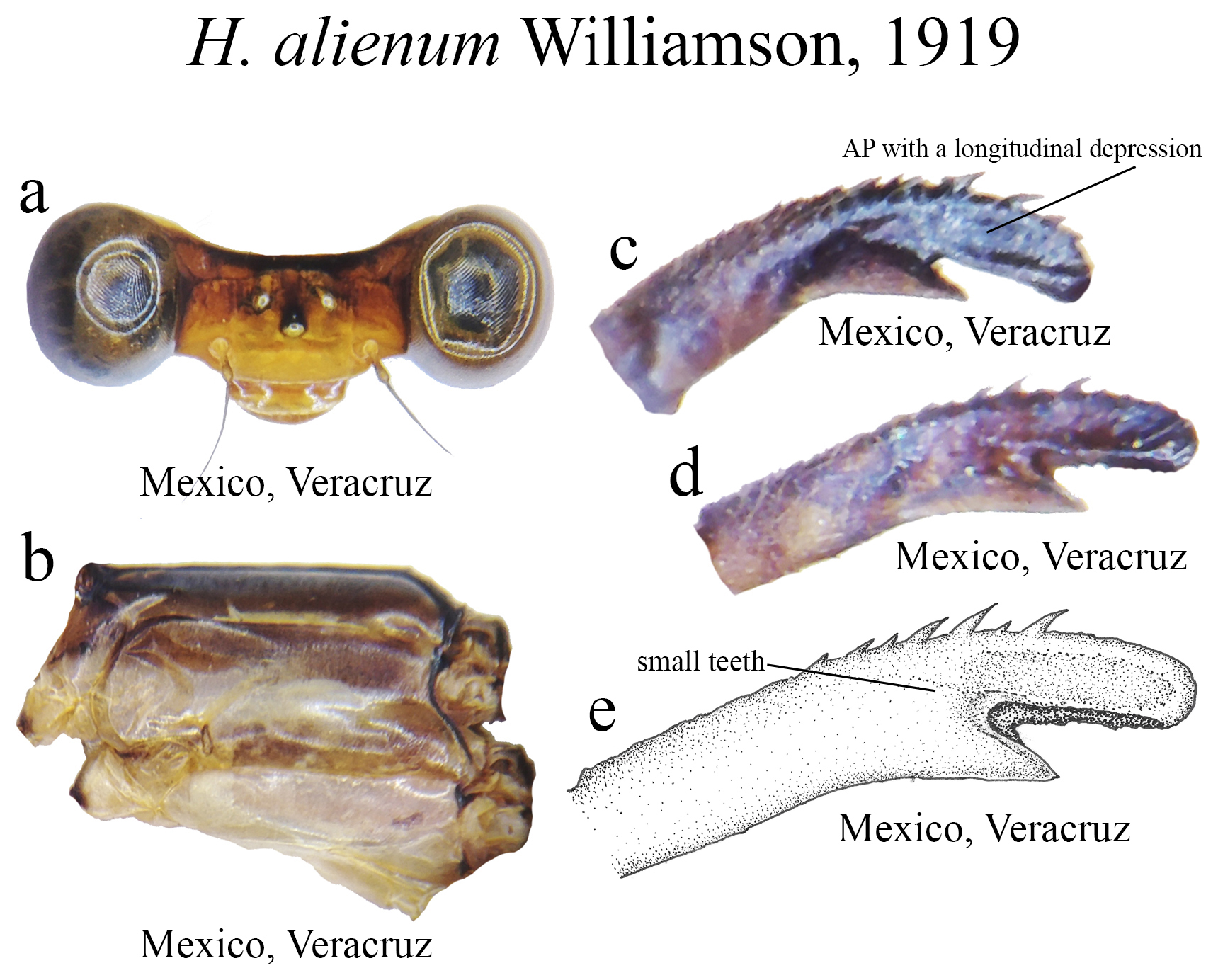
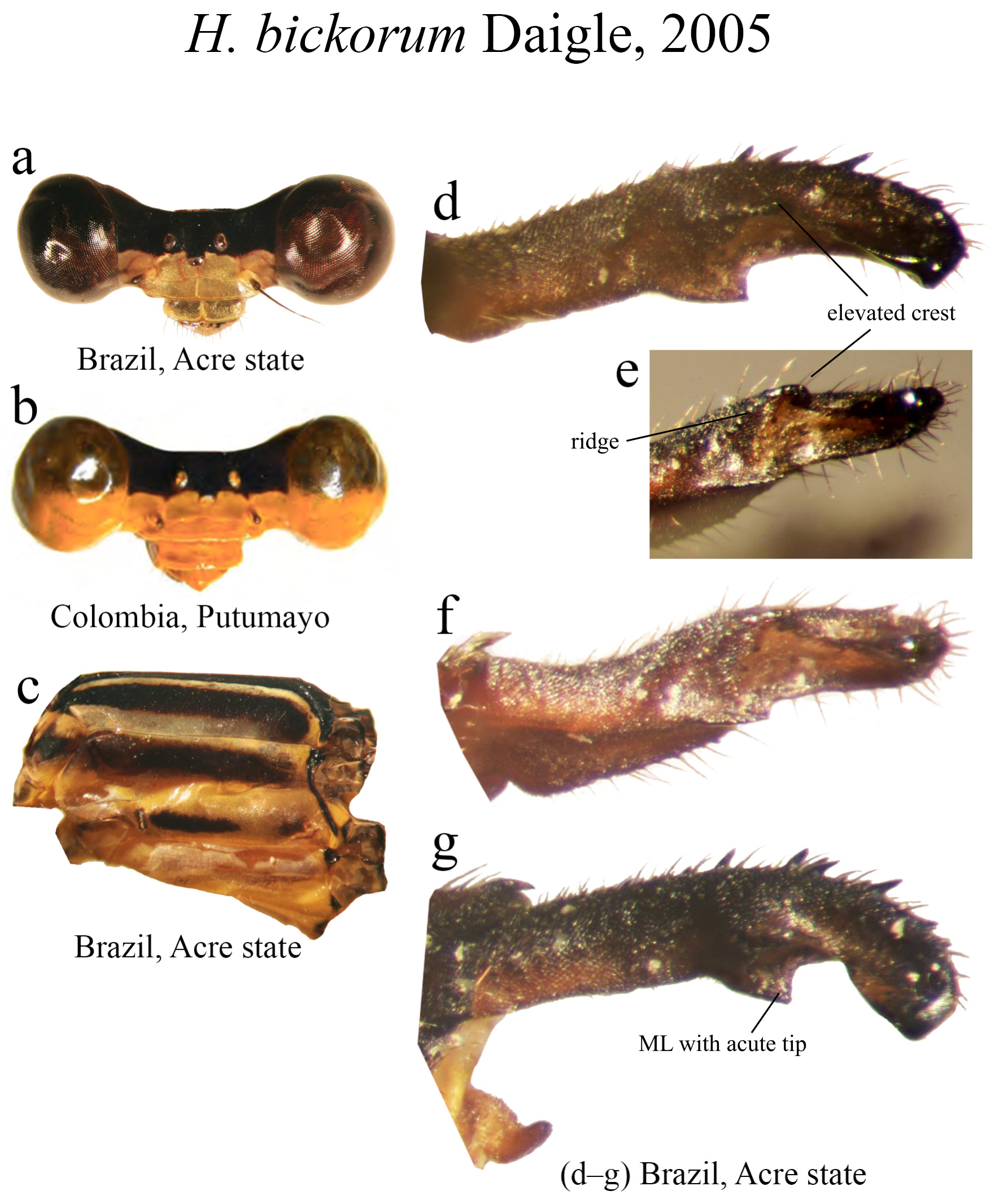
17 (16). MP lacking an elevated crest on its medial margin ( Figs. 9c–e View FIGURE 9 )..................................... H. alienum View in CoL
17’. MP bearing an elevated crest on its medial margin ( Figs. 16d–e View FIGURE 16 )................................... H. bickorum View in CoL
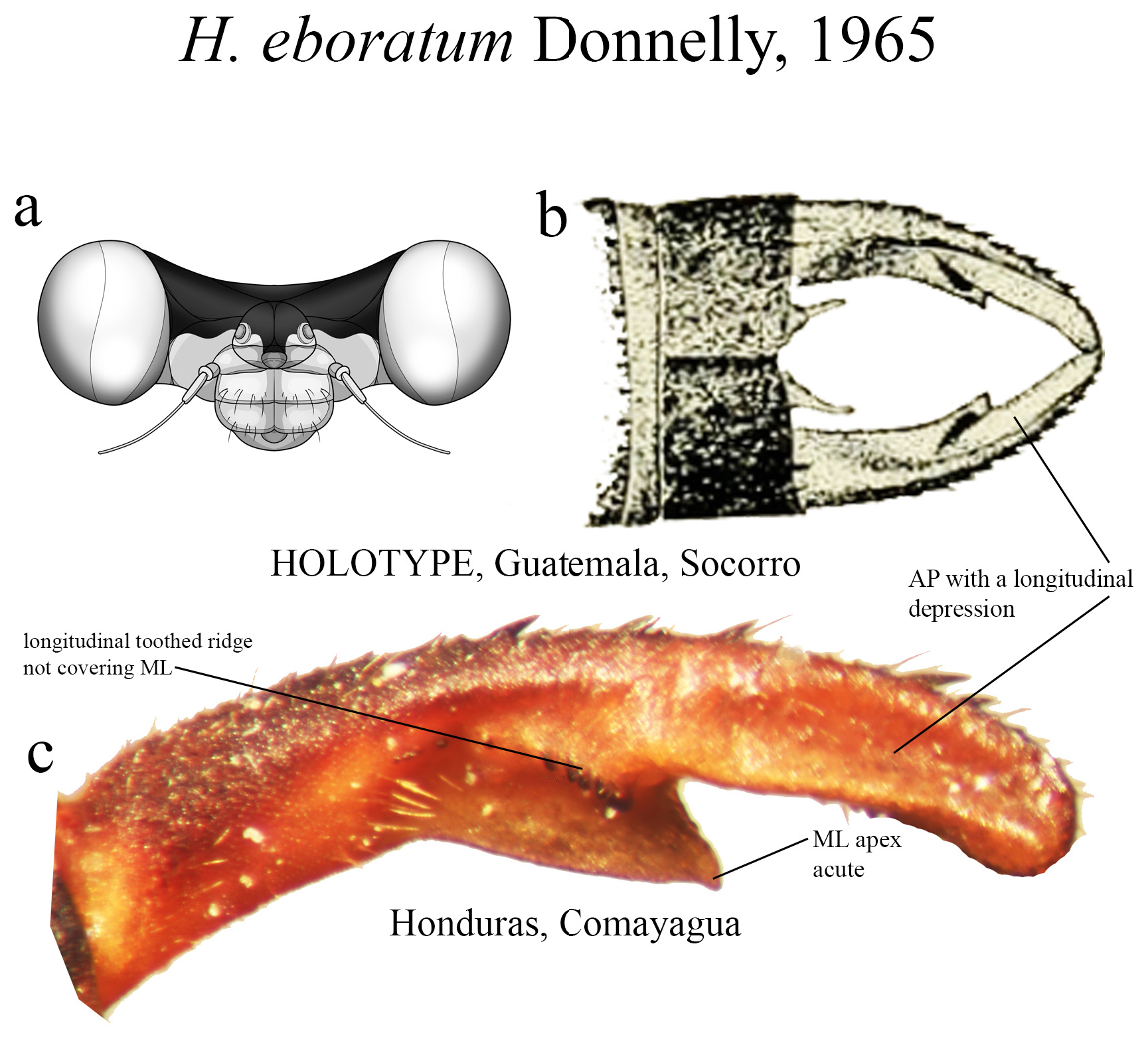
18 (14’). Ridge above ML bearing strong teeth on a single row ( Figs. 31b–c View FIGURE 31 ); ML apex acute ( Figs. 31b–c View FIGURE 31 )........ H. eboratum View in CoL
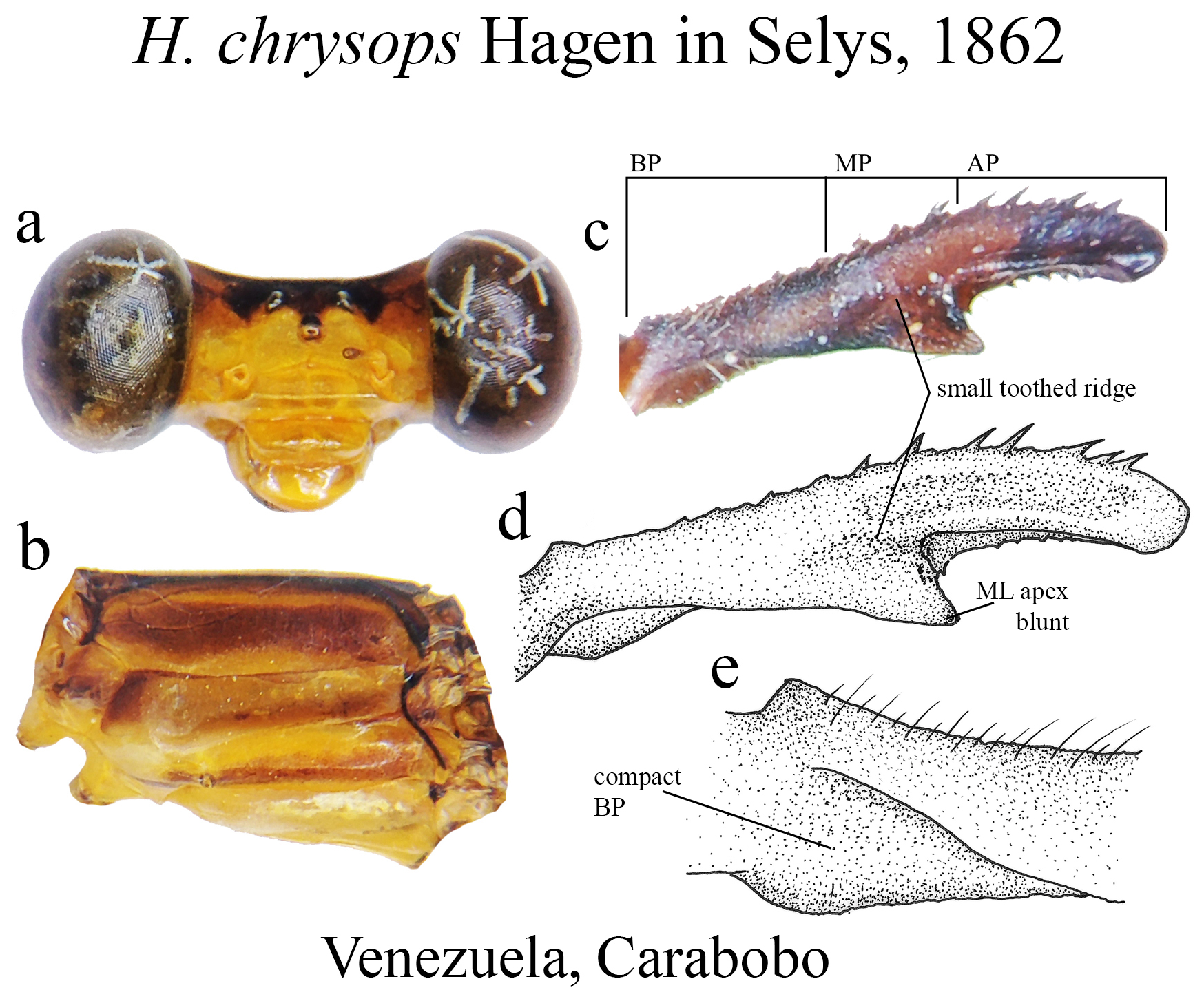
18’. Ridge above ML bearing very small denticles ( Figs. 23c–d View FIGURE 23 ); ML apex blunt ( Figs. 23c–d View FIGURE 23 )............... H. chrysops View in CoL
19 (11’). BP with a nearly straight margin, bearing no swelling or convexity (e.g., Figs. 7e–f View FIGURE 7 , 19c View FIGURE 19 , 47c–e View FIGURE 47 ).................. 20
19’ BP with some degree of convexity, varying from small to largely swollen (e.g., Figs. 14d–g View FIGURE 14 , 22c–e View FIGURE 22 , 33c–d View FIGURE 33 )......... 23
20 (19). MP the shortest portion of cercus in length (e.g., Figs. 7e View FIGURE 7 , 19c View FIGURE 19 , 47c–e View FIGURE 47 )...................................... 21
20’. Portions of cercus subequal in size or AP the longest (e.g., Figs. 8c–e View FIGURE 8 , 66c–e View FIGURE 66 )................................. 22
21 (20). Ridge above ML upcurved, bearing strong teeth not spaced ( Fig. 19c View FIGURE 19 ); MP–AP junction forming a nearly straight line with AP ( Fig. 19c View FIGURE 19 ); ML apex rounded ( Fig. 19c View FIGURE 19 )................................................. H. calafatiensis
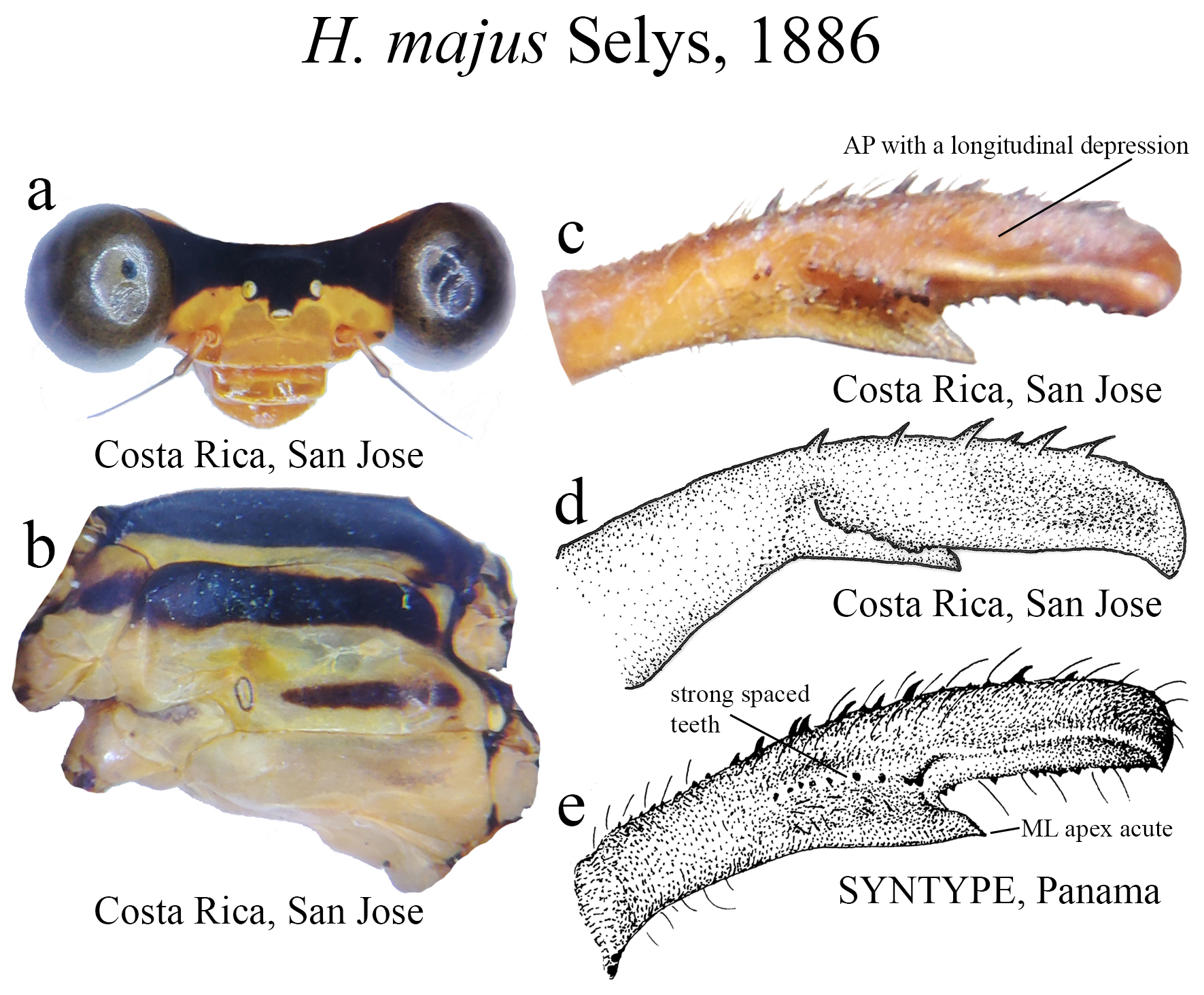
21’. Ridge above ML formed by spaced and very strong teeth ( Figs. 47c–e View FIGURE 47 ); MP–AP junction forming an angled corner; ML apex acute ( Figs. 47c–e View FIGURE 47 )...................................................................... H. majus View in CoL
22 (20’). Portions of cercus all subequal in size ( Fig. 8d View FIGURE 8 ); ridge above ML long, straight, its apex forming a narrow tip ( Figs. 8c–e View FIGURE 8 ); ML apex rounded ( Fig. 8e View FIGURE 8 ); mouthparts and frons largely pale, not yellow ( Fig. 8a View FIGURE 8 ).................... H. albifrons View in CoL
22’. AP the longest cercus portion, slightly longer than BP ( Fig. 66e View FIGURE 66 ); ridge above ML short, consisting of strong teeth ( Figs. 66c–e View FIGURE 66 ); ML apex acute ( Figs. 66c–e View FIGURE 66 ); mouthparts and frons yellow............................... H. tricellulare View in CoL
23 (19’). BP margin greatly swollen or expanded (e.g., Figs. 14d–e View FIGURE 14 , 22c–d View FIGURE 22 , 33c–d View FIGURE 33 ).................................... 24
23’. BP margin only slightly convex (e.g., Figs. 10d–g View FIGURE 10 , 16d–e View FIGURE 16 )................................................ 25
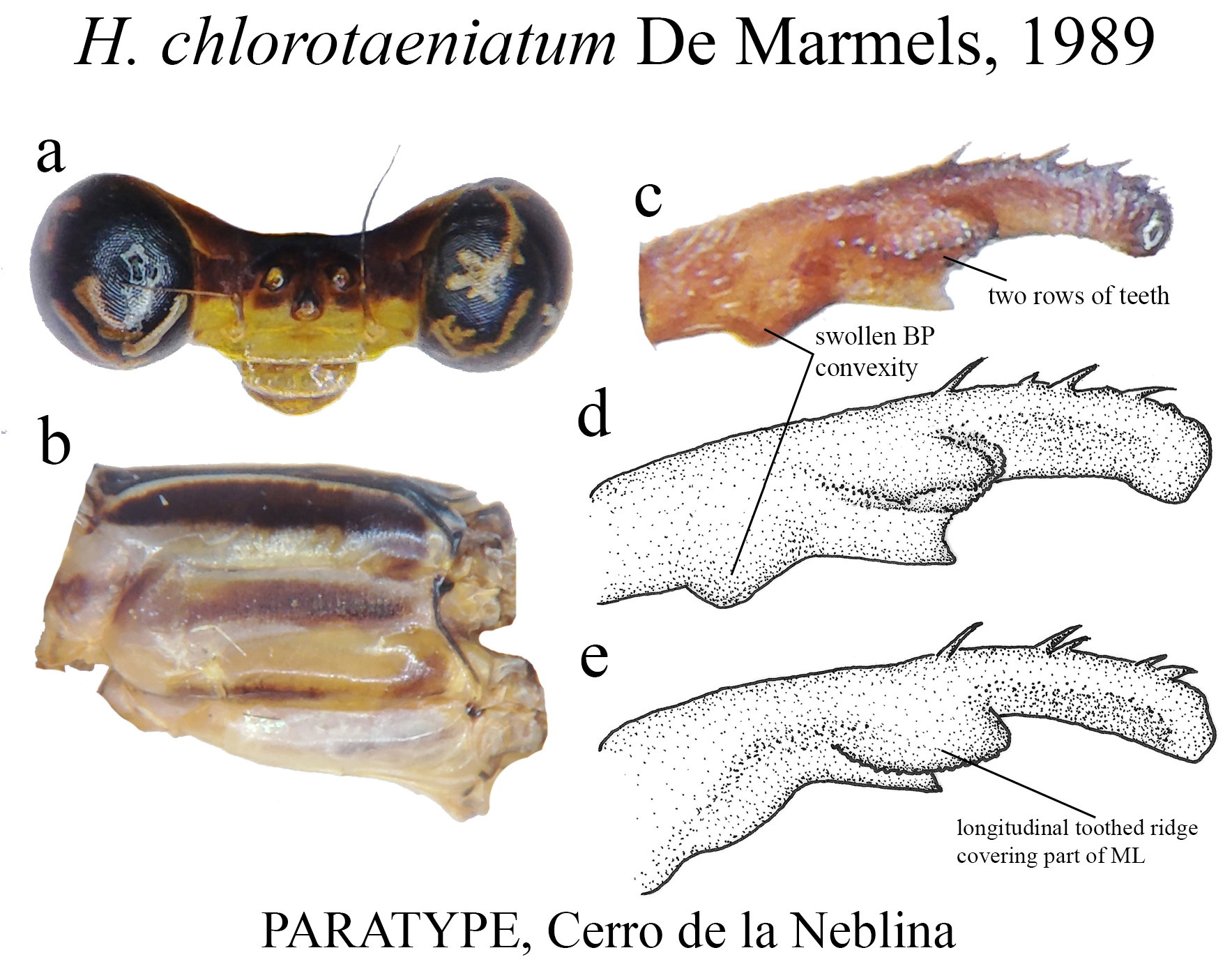
24 (23). Ridge above ML roughly bean-shaped ( Figs. 22c–e View FIGURE 22 )....................................... H. chlorotaeniatum View in CoL
24’. Ridge above ML nearly straight (e.g., Figs. 42e–i View FIGURE 42 , 29c View FIGURE 29 ).................................................. 26
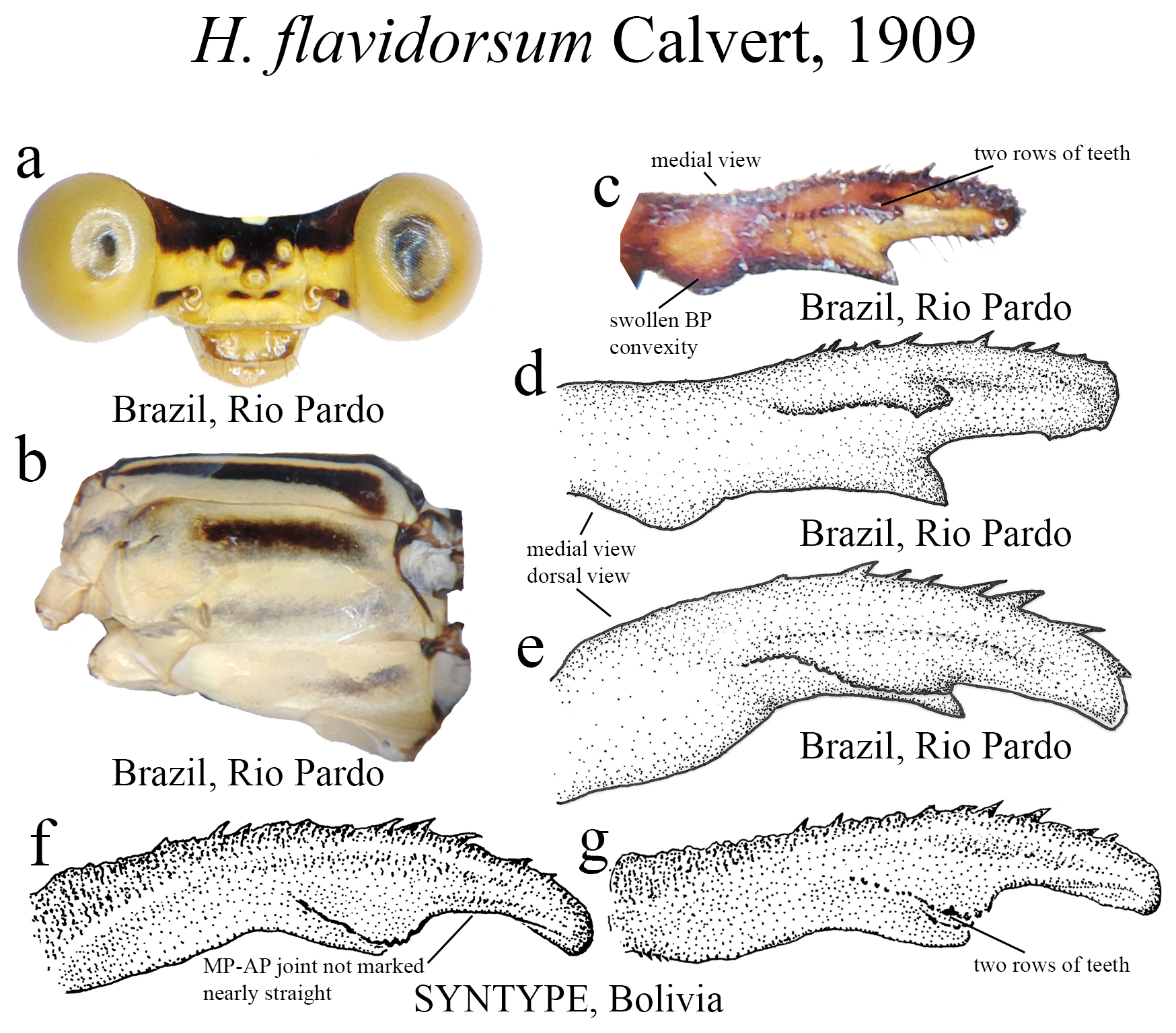
25 (23’). Ridge above ML nearly straight, with two parallel rows of teeth ( Fig. 33c View FIGURE 33 ); MP-AP junction not marked, nearly straight ( Fig. 33f View FIGURE 33 )............................................................................. H. flavidorsum View in CoL
25’. Ridge above ML with a single row of teeth; MP-AP junction marked, being rounded or angled ( Figs. 14d–g View FIGURE 14 , 50c–e View FIGURE 50 ).... .............................................................................................. 27
26 (24’). Ridge above ML bearing small teeth (e.g., Figs. 42e–i View FIGURE 42 , 29c View FIGURE 29 )............................................... 28
26’. Ridge above ML bearing strong teeth ( Figs. 7g –h View FIGURE 7 ; Fig. 10g View FIGURE 10 ).............................................. 29
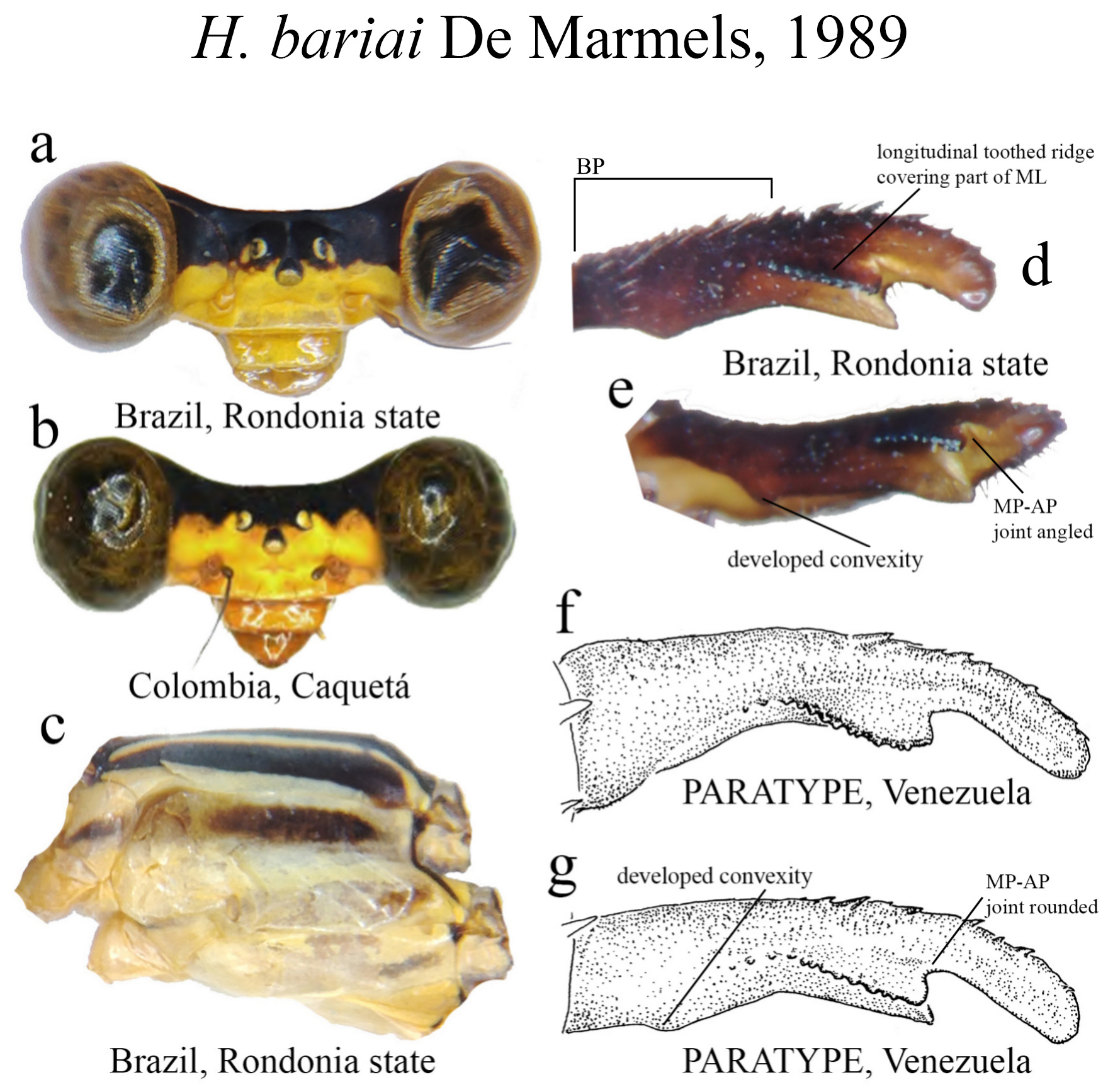
27 (25’). BP the longest cercus section ( Fig. 14d View FIGURE 14 )......................................................... H. bariai View in CoL
27’. All portions of cercus subequal in length ( Fig. 50c View FIGURE 50 )............................................. H. mitratum View in CoL
28 (26). Ridge above ML with a single row of teeth ( Figs. 29c–d View FIGURE 29 ); male postclypeus yellow with brown transverse line ( Fig. 29a View FIGURE 29 ); in lateral view, cercus slightly elbowed along midlength ( Fig. 29e View FIGURE 29 ); restricted to the Colombian Amazon................................................................................................. H. demarmelsi
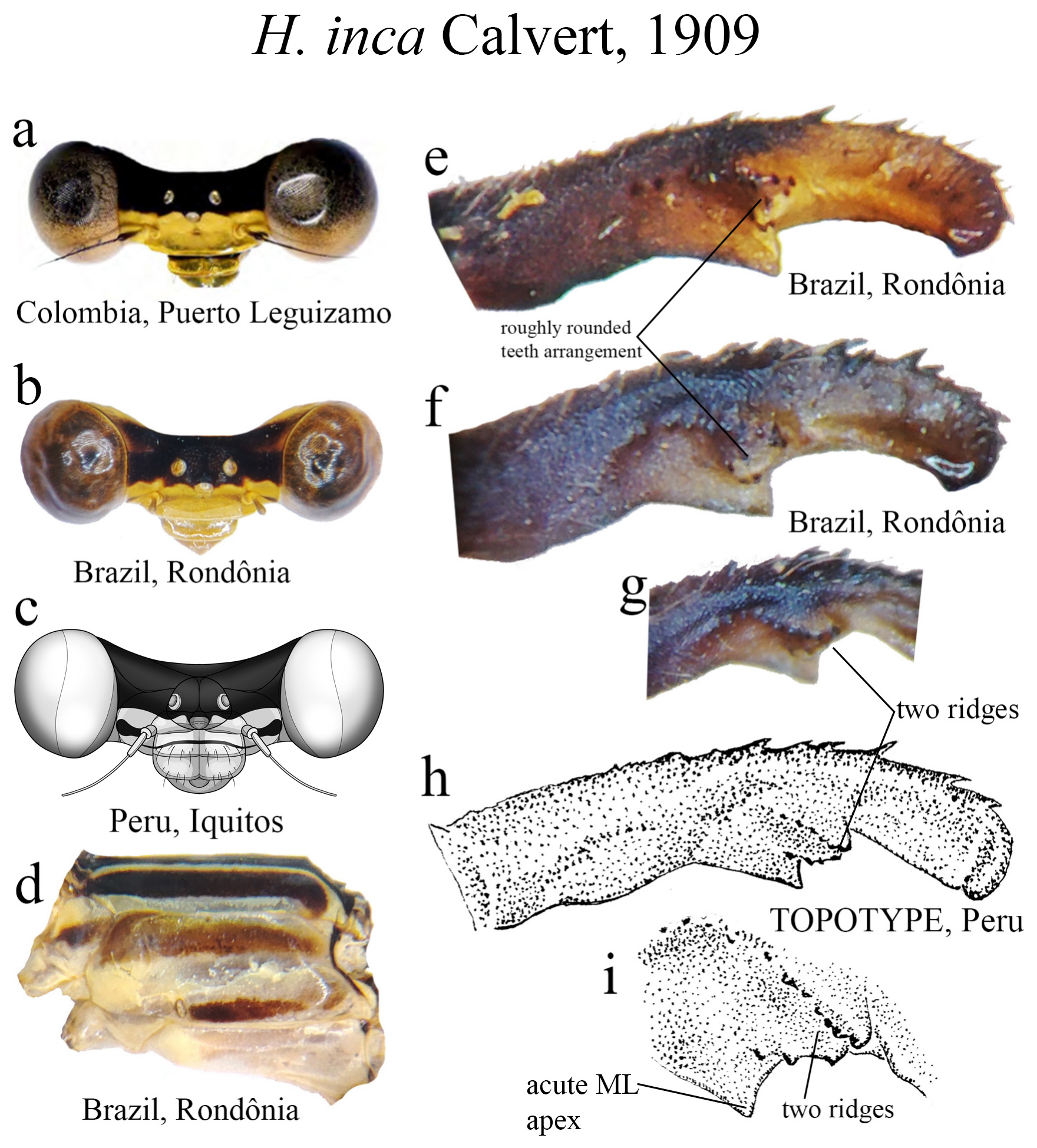
28’. Ridge above ML forming a roughly rounded teeth arrangement between the two rows ( Figs. 42e–f View FIGURE 42 ); male postclypeus yellow with a black transversal line ( Figs. 42a, 42c View FIGURE 42 ), or lacking a transversal line ( Fig. 42b View FIGURE 42 ); in lateral view, cercus not elbowed along midlength; Colombian Amazon, Ecuador, Peru, Brazil, and Bolivia........................ H. inca View in CoL
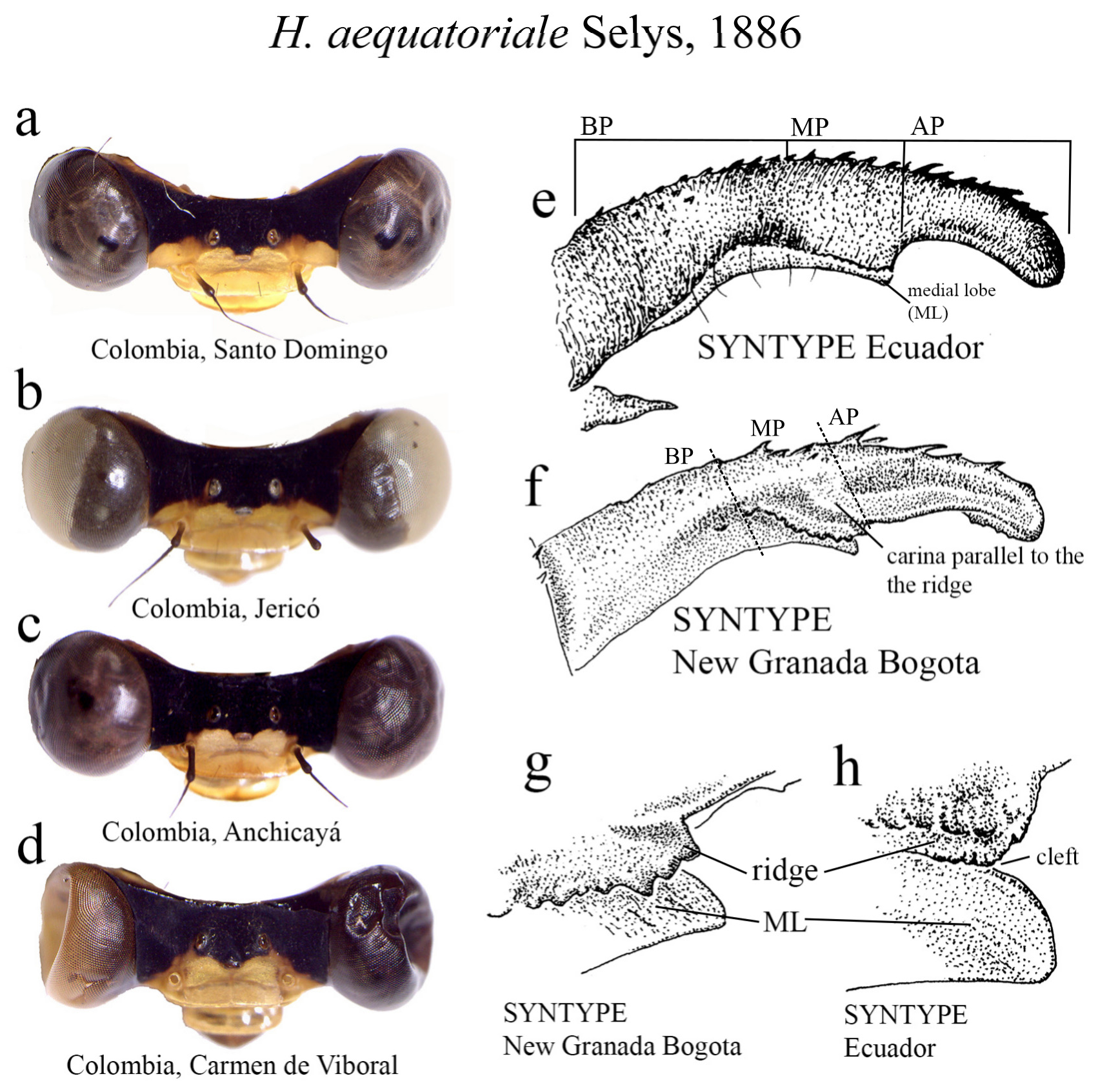
29 (26’). Ridge above ML sinuous, bearing strong teeth, with a carina parallel to the ridge ( Figs. 7e–f View FIGURE 7 ); ML apex blunt ( Figs. 7g –h View FIGURE 7 ).............................................................................. H. aequatoriale View in CoL
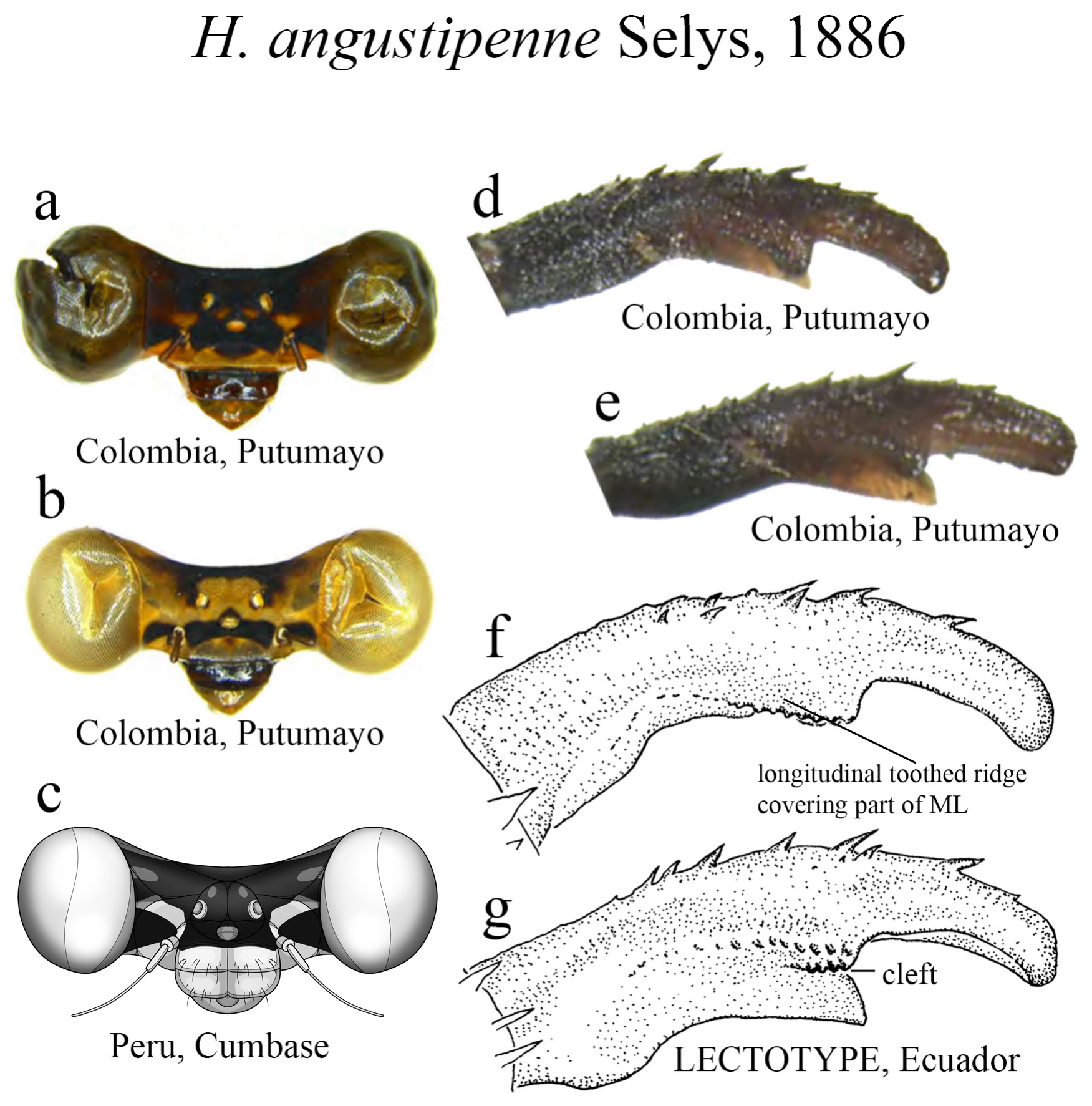
29’. Ridge above ML nearly straight, with an additional row of teeth below the main ridge ( Figs. 10d–g View FIGURE 10 ); ML apex acute ( Figs. 10d–e, g View FIGURE 10 )........................................................................... H. angustipenne View in CoL
Species Accounts
Lencioni (2013), Bota-Sierra et al. (2017), and Stand-Pérez et al. (2019) extensively commented on the taxonomy and ecology of most of the species listed. Thus, in the following accounts we provide complementary information. We made no additional conservation comments on species assessed as Least Concern in the IUCN Red List.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |