Americerura rivera (Schaus, 1901)
|
publication ID |
https://doi.org/ 10.1080/00222933.2023.2282624 |
|
DOI |
https://doi.org/10.5281/zenodo.10480435 |
|
persistent identifier |
https://treatment.plazi.org/id/733887BC-5837-FF88-3D9D-FC02FE032B07 |
|
treatment provided by |
Plazi |
|
scientific name |
Americerura rivera (Schaus, 1901) |
| status |
|
Americerura rivera (Schaus, 1901)
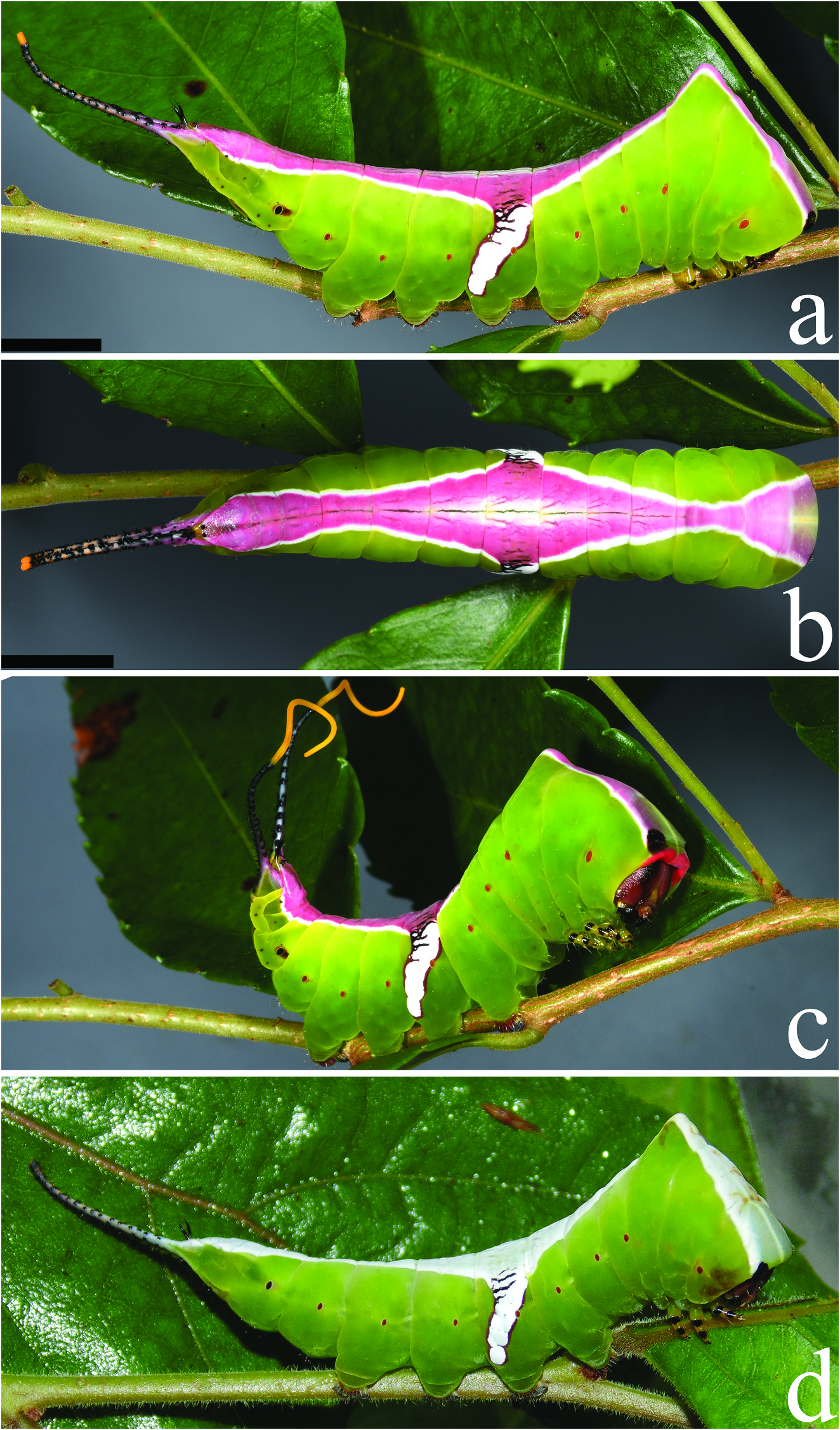
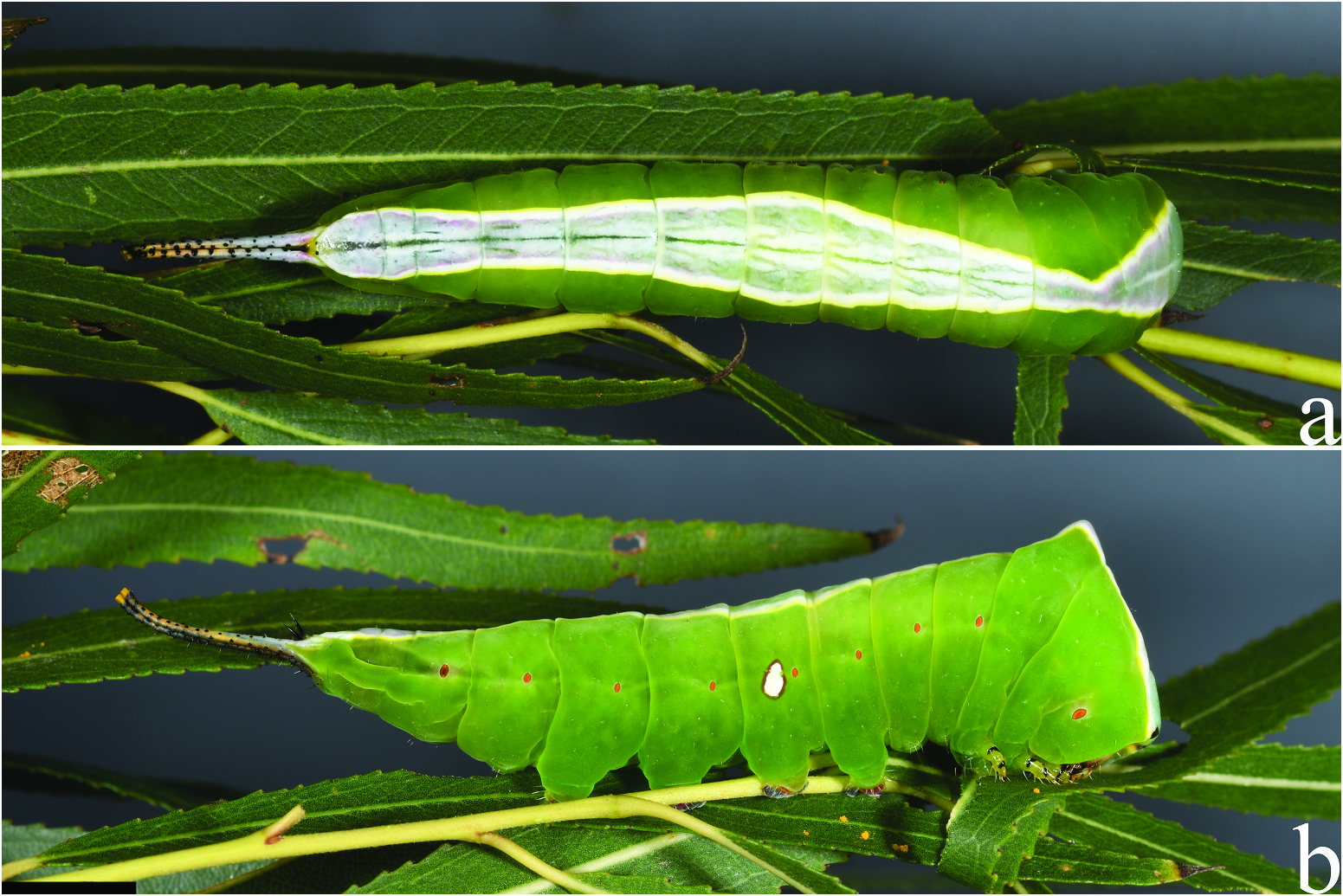
( Figures 9–11 View Figure 9 View Figure 10 View Figure 11 , 13 View Figure 13 , 19c,f View Figure 19 ); Accession numbers: DZUPIL 166
Tecmessa rivera (Schaus) in Schintlmeister (2013)
Tecmessa rivera (Schaus) in Becker (2014)
Americerura rivera (Schaus) in St Laurent et al. (2023)
Diagnosis
Larva. The first instar is not noticeably distinct from that of A. splendens , with only a minor alteration in stemapod spine spacing. The final instar is most similar to A. splendens due to a white lateral band on A4, but in A. rivera this band is usually not broken. The purple dorsal saddle form of A. rivera is so far known to be unique to this species.
Adult. Americerura rivera is most likely to be confused with A. argynnis , but A. rivera is larger, has less lustrous wings, and bears a black marking in the centre of the anal margin antemedial spot of the forewing, this spot (and others on the wing) are greenish-yellow rather than pure yellow as in A. argynnis .
Description of immature stages.
Egg. ( Figure 9a,b View Figure 9 ) Duration: Unknown, eggs hatched 5 days after discovery; 1.4 mm diameter (n = 1); circular, dorsally domed, base truncated and flattened. Overall colouration cream except for dark brown lateral band above truncated portion of egg, surface above the lateral ridge uniform in colour, lacking pattern except the dark brown micropyle at centre.
First instar. ( Figure 9c View Figure 9 ) Duration: ~5–6 days (n = 2); body length 6.2–6.3 mm without stemapods, 9.0– 9.2 mm with stemapods (n = 2); head capsule width 0.69–0.70 mm (n = 2), head hypognathous, glossy black. Body cylindrical, segments equal sized, T1 with pair of relatively large glossy black scoli at lateral apices of segment angled overhead, scoli with apparently fewer than 10 spines, each spine with single seta at apex, each seta longer than spine producing it; A10 with black dorsal shield and pair of glossy black paraprocts; upon hatching general colouration black but with feeding and growth colouration becomes deeper black-brown with light grey striations laterally and fine grey line supraspiracularly. Body naked except primary setae; thoracic legs black, A3–A6 prolegs coloured as for lateral portion of abdominal segments; crochets not examined; anal prolegs modified to form elongated black, robustly spined stemapods with maroon eversible component that is extruded when threatened, stemapod spines longest at terminus and four noticeably longer spines spaced roughly evenly along each stemapod.
Chaetotaxy. Not studied.
Second instar. ( Figure 9d View Figure 9 ) Duration: 4–5 days (n = 2); body length 9.3 mm without stemapods, 12.9 mm with stemapods (n = 1); head capsule width 1.15–1.20 mm (n = 2), gross morphology very similar to first instar, but T1 apical scoli smaller and stemapods more robust, thoracic segments noticeably defined, taller, and rectangular in overall shape, forming a blunt, lighter brown point at T3. Colouration similar to first instar except lighter red overall becoming pink laterally, thoracic, A7, and A8 segments lighter brown dorsally; stemapods with lighter brown banding near terminus.
Third instar. ( Figure 9e View Figure 9 ) Average duration: 6 days (n = 2); body length 14.4–14.9 mm without stemapods, 19.0– 20.8 mm with stemapods (n = 2); head capsule width 1.7 mm (n = 2) gross morphology and patterning as in second instar, thoracic segments more noticeably defined and rectangular with hump at T3 forming a more accentuated point, ventrum of A3–A6 truncated, base held flush with leaf surface at rest, concealing prolegs, paraprocts relatively longer. Colouration greener on thoracic segments and along terminal abdominal segments, dark brown dorsal saddle pattern (with medial area darkest) barely contrasts against brown-green supraspiracular lateral ground colour, saddle widest at A4 bleeding into pink lateral region; spiracles slightly more noticeable, orange; true legs and prolegs reddish brown; larger three to four spines of stemapods lighter brown in colour than remainder of black stemapods, spines tipped apically by darker brown, stemapods distally banded with light brown.
Fourth instar. ( Figure 9f View Figure 9 ) Duration: 5 days (n = 1); body length 20.3 mm without stemapods, 25.6 mm with stemapods (n = 1); head capsule width 2.88 mm (n = 1) head colouration lighter than in previous instars, overall gross morphology somewhat similar to third instar but proportionally larger and more robust, T1 apical scoli smaller relative to body, paraprocts larger relative to body, thoracic segments much more noticeably defined and rectangular, hump at T3 more accentuated, giving overall appearance of thorax an angled shape, appearing triangular viewed from above, ventrum of A3–A6 truncated as in third instar. Overall colouration vibrant apple green fading to bluish green lateral to ventrum, laterally speckled by light green flecks, dorsal saddle pattern well defined, widest at A4 where saddle forms point that extends to spiracle and faint brown hue present angled posteriorly below point, colouration of saddle deep chestnut brown and mostly uniform in colour along length except darkest mesally and thoracic saddle darker than abdominal, spiracles contrasting bright orange, true legs light yellow-green and brown, prolegs light green; stemapods covered in tooth-like spines three to four of which are larger than remainder, stemapods dark brown with faint lighter banding distally.
Fifth instar. ( Figures 10 View Figure 10 , 11 View Figure 11 , 13a View Figure 13 ) Duration: ~8 days (n = 1); body length ~ 31 mm without stemapods, ~ 39 mm with stemapods (n = 1); head capsule not measured, head colouration deep reddish brown to light grey. Overall morphology differs substantially in the final instar, but as in previous instars, thoracic segments enlarged and broad, forming a triangular shape when viewed from above, T1 lacks spined scoli and instead has a distinctly squared anterior appearance with black spots at the apices. When threatened bright red integument immediately behind and above the head of T1 is exposed. T3 with an acute hump; body overall apple green without specks or spots except for reddish brown splotch surrounding A8 spiracle, this splotch may be faint; spiracles bright orange except black (or dark orange) A8 spiracle. Dorsum from head to A10 with a well-defined saddle-like pattern that is sharply delimited from green ground colour of remainder of body. Colouration of saddle mostly concolorous over thoracic and abdominal segments but with greenish hue on T1 and black eyespots at anterior margin of T1 . Thoracic saddle widest at T1 becoming gradually narrower approaching the apical protuberance of T3 , thoracic component of saddle connected to abdominal saddle at T3 hump, saddle overall is smoothly maculated except for narrow longitudinal striations where darker ground colour breaks through, saddle widest at A4 , with dark-brown striations above spiracle and a bright white posteriorly angled, irregularly edged band reaches almost to base of A4 proleg, one larva lacked this band which was replaced by a pyriform white spot with a deep red margin; saddle displays three distinct colour forms, either bright white, vivid purple-pink that contrasts strongly against bright white outline of saddle and lateral band, or white at margins with blue-green and pink tinged medial area. True legs green with black at segment interfaces, prolegs green near body with deep red at terminus. Stemapods coloured dorsally as for saddle, but green invades laterally, covered in uniform black spines, extendable part of stemapods red and yellow early in 5th instar, shifting to all bright yellow later in the instar. Paraprocts shiny black.
Pupa. Duration: At least 24 days based on cocoon building to adult emergence. The first week of this period the larva is likely still prepupal inside cocoon. Morphology of pupa not examined.
Cocoon. ( Figure 13b View Figure 13 ) As in A. argynnis .
Life history. We observed two eggs in nature on a moderately sized (~ 1 m tall) X. pseudosalzmannii , and they hatched in the morning, between 8.00 and 10.00am. Interestingly, one egg was on a stem and the other on the adaxial surface of a leaf. Apart from the single egg on the stem, we have never observed any Americerura eggs deposited on anything other than the adaxial leaf surfaces of the host plants. Only one of the two larvae from these eggs survived to adult. We also observed larvae on a small plant of Xylosma ciliatifolia and on a large tree of Salix humboldtiana . Various sightings of Americerura larvae on the internet from Brazil and Paraguay seem to be this species, and all such sightings appear to be from various Xylosma species (St Laurent pers. obs.).
Regarding the larva found on Salix humboldtiana , we initially thought it could represent A. annulifera as it matched the figures of that species in Bourquin (1939) quite well and was found on the host plant of that species: compare our images of this A. rivera ( Figure 11 View Figure 11 ) to a preserved A. annulifera from Uruguay ( Figure 12 View Figure 12 ). When comparing our specimen to the figures in Bourquin (1939), the most obvious similarities are the presence of a single lateral spot and the well-defined dorsal stripes on the margins of the saddle, although these are also present in the other A. rivera colour forms to some degree. However, the resulting adult from this larva was clearly A. rivera (e.g. white ground colour, that of A. annulifera is brown, Figure 19i View Figure 19 ). We were aware of the sister relationship between A. rivera and A. annulifera based on the ongoing phylogenomic work with Argentinian and Uruguayan material (St Laurent unpublished) and so the similarity between the larvae of these two species was somewhat expected. As we have already illustrated in A. argynnis and A. splendens above, larvae of this genus are polymorphic and seemingly can be induced to produce different phenotypes due to environmental factors. Thus, host plant species may also play a role in phenotype, but this requires further study.
Host plants
Xylosma ciliatifolia , X. pseudosalzmannii and Salix humboldtiana ( Salicaceae ) in nature, which were also used to complete the rearing events in captivity. We offered B. parviflora to one of the larvae that were found as an egg on X. pseudosalzmannii ; it was not accepted and thus we transferred it back to X. pseudosalzmannii for the remainder of the rearing.
Distribution
This widespread species is known to us from Argentina (Chaco and Missiones ), Brazil (Paraíba, Bahia, Minas Gerais, Rio de Janeiro, São Paulo, Paraná, Santa Catarina, Rio Grande do Sul), Paraguay and Uruguay. A specimen from Bolivia in the CMNH may be this species but has slightly different maculation. We are also aware of an apparently undescribed species very similar to A . rivera from Venezuela. The distribution of A . rivera in the Pampa is currently unclear as we are aware of specimens seemingly intermediate between widespread A. rivera and the Pampa species A. annulifera . See the Discussion for further examination of this topic.
| A |
Harvard University - Arnold Arboretum |
| CMNH |
The Cleveland Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Americerura rivera (Schaus, 1901)
| St Laurent, Ryan A., Carvalho, Ana Paula S., Orlandin, Elton & Carneiro, Eduardo 2024 |
Cerura rivera
| Schaus 1901 |