Americerura splendens (Jones, 1908)
|
publication ID |
https://doi.org/ 10.1080/00222933.2023.2282624 |
|
DOI |
https://doi.org/10.5281/zenodo.10480433 |
|
persistent identifier |
https://treatment.plazi.org/id/733887BC-5829-FF81-3DEF-FE9DFB472B64 |
|
treatment provided by |
Plazi |
|
scientific name |
Americerura splendens (Jones, 1908) |
| status |
|
Americerura splendens (Jones, 1908)
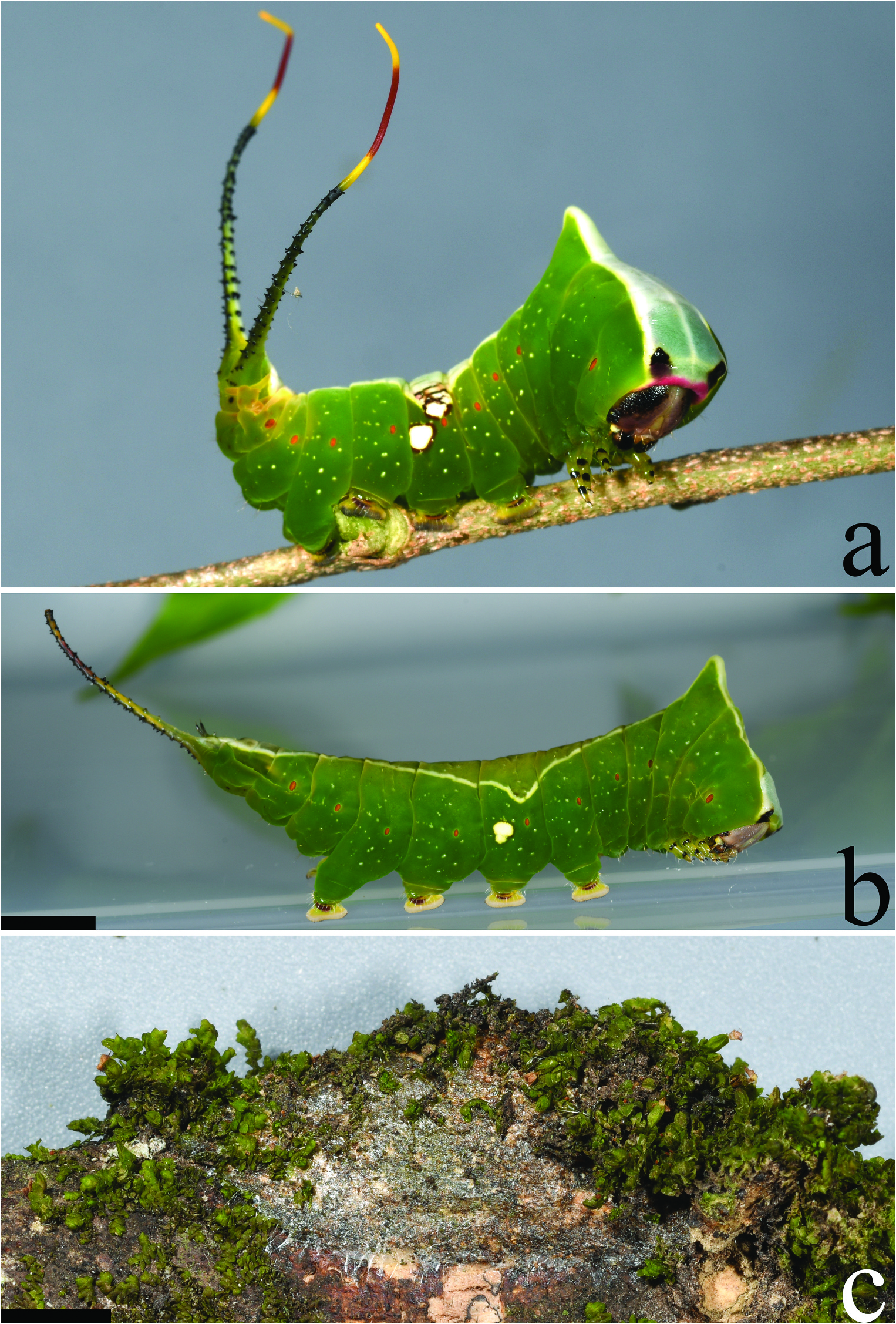
( Figures 6–8 View Figure 6 View Figure 7 View Figure 8 , 19b, e View Figure 19 ); Accession numbers: DZUPIL 168
Tecmessa splendens (Jones) in Schintlmeister (2013)
Tecmessa splendens (Jones) in Becker (2014)
Americerura splendens (Jones) in St Laurent et al. (2023)
Diagnosis
Larva. The first instar is very similar to that of A. argynnis but has noticeably longer stemapod spines. The last-instar larvae are recognisable by the white saddle and the broken longitudinal lateral band on A4. There is some intraspecific variation in A. splendens where the lateral band may be absent, and therefore giving a similar appearance to the white-saddled A. argynnis , but A. splendens larvae are always speckled with white overall laterally, regardless of form, whereas white-saddled A. argynnis lack speckles and never bear a white lateral band on A4. Americerura splendens larvae can also be recognised by their extremely narrow T3 protuberance which is narrower and more pointed than in other Americerura species discussed in the present article.
Adult. Americerura splendens is the among the most readily diagnosable Americerura in South America due to the complete absence of the otherwise typical black zigzagging lines on the forewings, except for the postmedial line which is present. Somewhat similar species lacking most black markings are present in the Amazon ( A. purusa (Schaus)) and Mexico ( A. trigonostigma (Dyar)) . The typical yellow spots near the base of the forewing in A. splendens , as in A. argynnis and A. rivera , are present. Like in A. argynnis , these yellow spots never have internal markings, but unlike in that species the colour of the spots is greener.
Description of immature stages
Egg. ( Figure 6a,b View Figure 6 ) Average duration: ~10 days (n = ~7; the captive female laid very few eggs most nights, thus hatching was highly staggered); 1.4 mm diameter (n = 5); circular, dorsally domed, base truncated and flattened. Upon laying eggs greenish, becoming brown basally with the dorsal surface appearing lighter pink due to a reticulated pattern that covers the entire dorsal egg surface above the lateral ridge, except micropyle dark brown; egg colouration darkens to purplish-pink with age.
First instar. ( Figure 6c View Figure 6 ) Average duration: ~5 days (n = 2); body length 6.0– 6.7 mm without stemapods, 8.8–10.2 mm with stemapods (n = 6); head capsule width 0.59– 0.61 mm (n = 10), head hypognathous, glossy black. Body cylindrical, segments generally equal sized although thoracic segments slightly taller, T1 with pair of relatively large glossy black scoli at apices of segment angled overhead, scoli with about 10 spines, each spine with single seta at apex, each seta longer than spine producing it; A10 with black dorsal shield and pair of glossy black paraprocts; upon hatching general colouration black but with feeding and when grown colouration more deep black-brown with light grey striations laterally and fine grey supraspiracular lines. Body naked except primary setae; thoracic legs black, A3–A6 prolegs coloured as for lateral portion of abdominal segments; crochets uniserial uniordinal; anal prolegs modified to form elongated black, spiny stemapods with maroon eversible component that is extruded when threatened, stemapod spines longest at terminus and three to four noticeably longer spines spaced roughly evenly along each stemapod.
Chaetotaxy. As in A. argynnis .
Second instar. ( Figure 6d View Figure 6 ) Average duration: ~4–5 days (n = 5); body length 8.6–10.9 mm without stemapods, 12.2–14.3 mm with stemapods (n = 6); head capsule width 1.00– 1.05 mm (n = 6), gross morphology similar to first instar, but T1 apical protuberances and stemapods more robust, thoracic segments more noticeably defined and somewhat more rectangular in overall shape, forming a blunt, lighter brown point at T3. Colouration as for first instar except thoracic and A7 segments lighter brown dorsally, body overall laterally silvery grey; stemapods with lighter brown banding near terminus.
Third instar. ( Figure 6e View Figure 6 ) Average duration: ~5 days (n = 3); body length 13.8–15.7 mm without stemapods, 19.9–22.8 mm with stemapods (n = 4); head capsule width 1.56– 1.65 mm (n = 10) colouration lighter brown than in previous instars, gross morphology as in second instar, thoracic segments still more noticeably defined and rectangular, hump at T3 forming a sharper point, ventrum of A3–A6 truncated, base held flush with leaf surface at rest, concealing prolegs, paraprocts relatively larger. Colouration greener overall than second instar, dark brown to light brown dorsal saddle pattern with medial area darkest, contrasts against brown-green to apple green ground colour and silvery-white lateral surface of body, saddle widest at A4, between silvery sides and brown dorsum lies faint, darker brown-green stripe, lateral region overall lightly speckled with grey-green; spiracles more noticeable, orange; true legs yellow-green, prolegs light yellow-green; larger three to four spines of stemapods lighter brown in colour than remainder of stemapods, spines tipped apically by darker brown. Eversible part of stemapods more red in colour.
Fourth instar. ( Figures 6f View Figure 6 , 7a View Figure 7 ) Average duration: ~5 days (n = 9); body length 18.0– 20.4 mm without stemapods, 27.0–31.0 mm with stemapods (n = 5); head capsule width 2.35–2.50 mm (n = 9) head colouration lighter than in previous instar, overall gross morphology similar to third instar but significantly larger and more robust overall, T1 apical scoli smaller relative to body, paraprocts larger relative to body, thoracic segments much more noticeably defined and rectangular, hump at T3 highly accentuated and pointed, giving overall appearance of thorax an angled shape, appearing triangular when viewed from above, ventrum of A3–A6 truncated as in third instar. Overall colouration vibrant apple green fading to bluish green lateral to ventrum, laterally speckled with light blue-green flecks, dorsal saddle pattern well defined, widest at A4, colouration of saddle variable, ranging from deep chestnut brown ( Figure 6f View Figure 6 ) to pinkish cream ( Figure 7a View Figure 7 ), but always darkest mesally and on thoracic segments and with light cream-coloured margins, spiracles contrasting bright orange, true legs light yellow-green, prolegs light green, matching colouration of apple-green region above blue-green lateral side and below saddle; stemapods covered in tooth-like spines with three to four spines larger than remainder, stemapods light brown or dark brown with lighter banding.
Fifth instar. ( Figures 7b–d View Figure 7 , 8a,b View Figure 8 ) Average duration: ~5 days (n = 4); body length 30– 40 mm without stemapods, 40–47 mm with stemapods (n = 8); head capsule width 3.4 mm (n = 1). Morphology differs substantially in final instar, but as in previous instars, thoracic segments enlarged and broad, forming a triangular shape when viewed from dorsum, T1 lacks spined scoli and instead has a distinctly squared anterior appearance into which the head can be retracted. When threatened the bright rosepink integument immediately behind and above the head of T1 is exposed. T3 with an exaggerated, sharply acute hump having a height almost equivalent to width of T2. Head shiny purplish-grey with black splotches anteriorly, size of splotches somewhat variable and may cover most of anterior area of head, overall body apple green with irregular white specks overall giving body a ̍starry̾ appearance laterally; spiracles bright orange. Dorsum from head to A10 with a well-defined saddle-like pattern that is sharply delimited from green ground colour of remainder of body. Colouration of saddle mostly concolorous over thoracic and abdominal segments but with bluish hue on T1 and black eyespots at anterior margin of T1. Thoracic saddle widest at T1 becoming gradually narrower approaching the apical protuberance of T3, thoracic component of saddle connected by narrow stripe to abdominal saddle which is smoothly maculated except for narrow longitudinal striations where green ground colour breaks through the white, saddle widest at A4, saddle usually disrupted by dark brown above spiracle at A4 with variable presence of large white spots above and below A4 spiracle giving appearance of longitudinal broken white lateral band, if white spot present below spiracle it is situated posterior to spiracle, in some individuals the upper spot or both lower and upper spots are absent; regardless of presence of spot(s), outer margins of thoracic and abdominal saddle outlined in white. True legs green with black at segment interfaces, prolegs apple green with deep red distally. Stemapods mostly bluish cream-coloured dorsally but green invades laterally, covered in uniform black spines, extendable part of stemapods bright yellow at base and apically with expansive red region between yellow regions. Paraprocts shiny black.
Pupa. Not figured but very similar to previous species. Average duration: 27 days (n = 2, non-diapause).
Cocoon. ( Figure 8c View Figure 8 ) As in A. argynnis , illustrated cocoon an example of one in which moss was placed by larva.
Life history
Eggs were not observed in nature, but in captivity they regularly hatch in the morning, generally between 8.00 and 10.00am. A single observed wild larva was found in a large B. parviflora tree, unlike the small saplings that typically host A. argynnis . First-instar larval feeding behaviour is as in A. argynnis except they tend to wander and do not stay at a single location on leaves for very long; later instars behave like A. argynnis . Threat displays are also similar, but in A. splendens the extrudable parts of the stemapods are alternating red and yellow banded. Such alternating patterns only occur in freshly moulted A. argynnis , which otherwise have solid yellow extrudable parts as the larva matures. Prepupal and cocoon building behaviour is as for A. argynnis . As in A. argynnis , adult A. splendens hatch late in the evening, from 8.00 to 11.00pm, although we observed occasional midday hatching.
Like A. argynnis , we noted adults hatching just 3–4 weeks after larvae were prepupal, but then a decline in emergence throughout the summer months. We artificially exposed pupae to cooler (~22°C) temperatures and periodical water spraying and were able to influence some moths to hatch during the summer. Our fieldwork also noted fewer adults of A. splendens at lights outside of the typical spring/autumn flight periods for this species. In general, the species discussed in the present work can be anticipated in the proper habitat during most months when weather is mild, but with clear peaks in activity in the spring and autumn.
Host plants
Banara parviflora ( Salicaceae ) in captivity and in nature (based on one observation in Nova Petrópolis, RS, Brazil).
Distribution
So far, we are only aware of this species from Brazil, from the following states: Bahia, Minas Gerais, Espírito Santo, Rio de Janeiro, São Paulo, Paraná, Santa Catarina,and Rio Grande do Sul.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Cerurinae |
|
Genus |
Americerura splendens (Jones, 1908)
| St Laurent, Ryan A., Carvalho, Ana Paula S., Orlandin, Elton & Carneiro, Eduardo 2024 |
Cerura splendens
| Jones 1908 |