Americerura argynnis (Schaus, 1901)
|
publication ID |
https://doi.org/ 10.1080/00222933.2023.2282624 |
|
DOI |
https://doi.org/10.5281/zenodo.10480431 |
|
persistent identifier |
https://treatment.plazi.org/id/733887BC-5827-FF9F-3DEC-FA21FB4728D7 |
|
treatment provided by |
Plazi |
|
scientific name |
Americerura argynnis (Schaus, 1901) |
| status |
|
Americerura argynnis (Schaus, 1901)
( Figures 1–5 View Figure 1 View Figure 2 View Figure 3 View Figure 4 View Figure 5 , 19a,d View Figure 19 ); Accession numbers: DZUPIL 167
Tecmessa argynnis (Schaus) in Schintlmeister (2013)
Tecmessa argynnis (Schaus) in Becker (2014)
Americerura argynnis (Schaus) in St Laurent et al. (2023)
Americerura argentina (Dognin, 1911) , synonymised by St Laurent et al. (2023)
Diagnosis
Larva. In early instars A. argynnis can be recognised by the reduced size of the stemapod spines. In all other species treated here, the stemapods bear at least three noticeably larger spines on each stemapod (larger than the largest spines in Figure 1 View Figure 1 ). Last-instar larvae have brown dorsal saddles, distinguishing them from A. rivera and A. splendens , and are less heavily speckled laterally than A. brasiliensis sp. n., which is another brown-saddled species. The white-saddled form of A. argynnis is similar to A. rivera and A. splendens , but A. argynnis always lacks the lateral band at A4 that is usually present in the other two species.
Adult. In south-eastern Brazil and adjacent countries, A. argynnis is easily recognised by its reflective white ground colour, zigzagging black lines in the antemedial, medial and submarginal areas, with two yellow spots on the costa and one on the anal angle of the antemedial region of the forewing. These yellow spots lack any internal markings but are variable in thickness.
Description of immature stages
Egg. ( Figure 3a,b View Figure 3 ) Average duration: ~13 days (n = ~74); 1.2 mm diameter (n = 8); circular, dorsally domed, base truncated and flattened. Colouration pinkish-red to tan, outer margin uniform dark pink-brown continuous with basal truncated part; inner margin of dorsal aspect lighter pink-brown with irregular central edge encircling dark central region that becomes darker as eclosion nears.
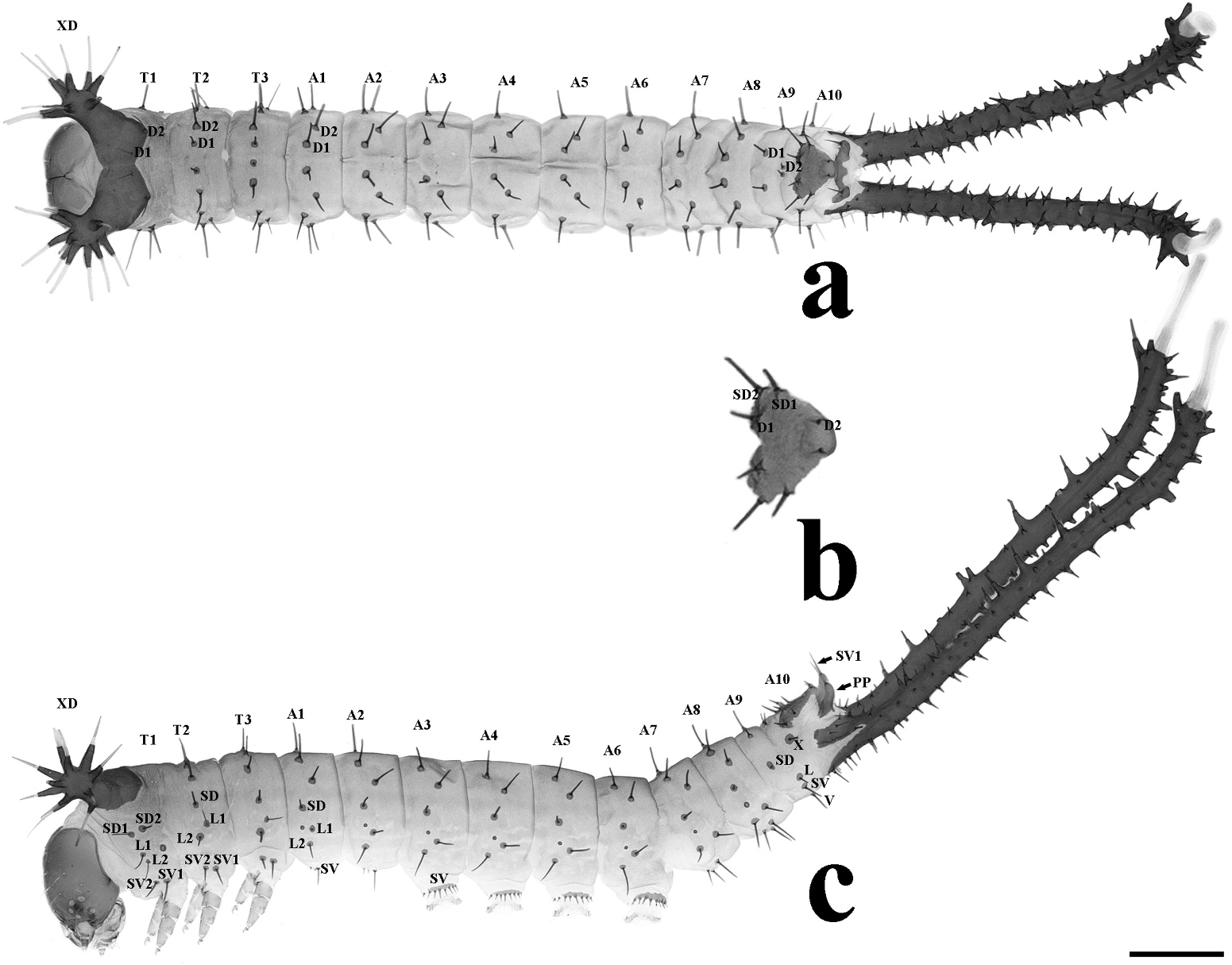
First instar. ( Figures 1 View Figure 1 , 2 View Figure 2 , 3c View Figure 3 ) Average duration: ~4 days (n = 15); body length 5.7– 6.6 mm without stemapods, 8.5–9.4 mm with stemapods (n = 6); head capsule width 0.60–0.62 mm (n = 8, Figure 2a,b View Figure 2 ), head hypognathous, glossy black. Body cylindrical, segments generally equal sized although thoracic segments slightly taller, T1 with pair of relatively large scoli at lateral apices of segment angled overhead, scoli black with about 10 spines, each spine with single seta at apex, each seta longer than spine producing it; A10 with black dorsal shield ( Figure 1b View Figure 1 ) and pair of glossy black paraprocts; upon hatching general colouration black but with feeding and when grown, colouration more deep red-brown with light grey striations laterally and fine grey supraspiracular lines. Body naked except primary setae, all on chalazae; thoracic legs black, A3–A6 prolegs coloured as for lateral portion of abdominal segments; crochets uniserial uniordinal; anal prolegs modified to form elongated black, spiny stemapods with maroon eversible component that is extruded when threatened, stemapod spines longest at terminus.
Chaetotaxy
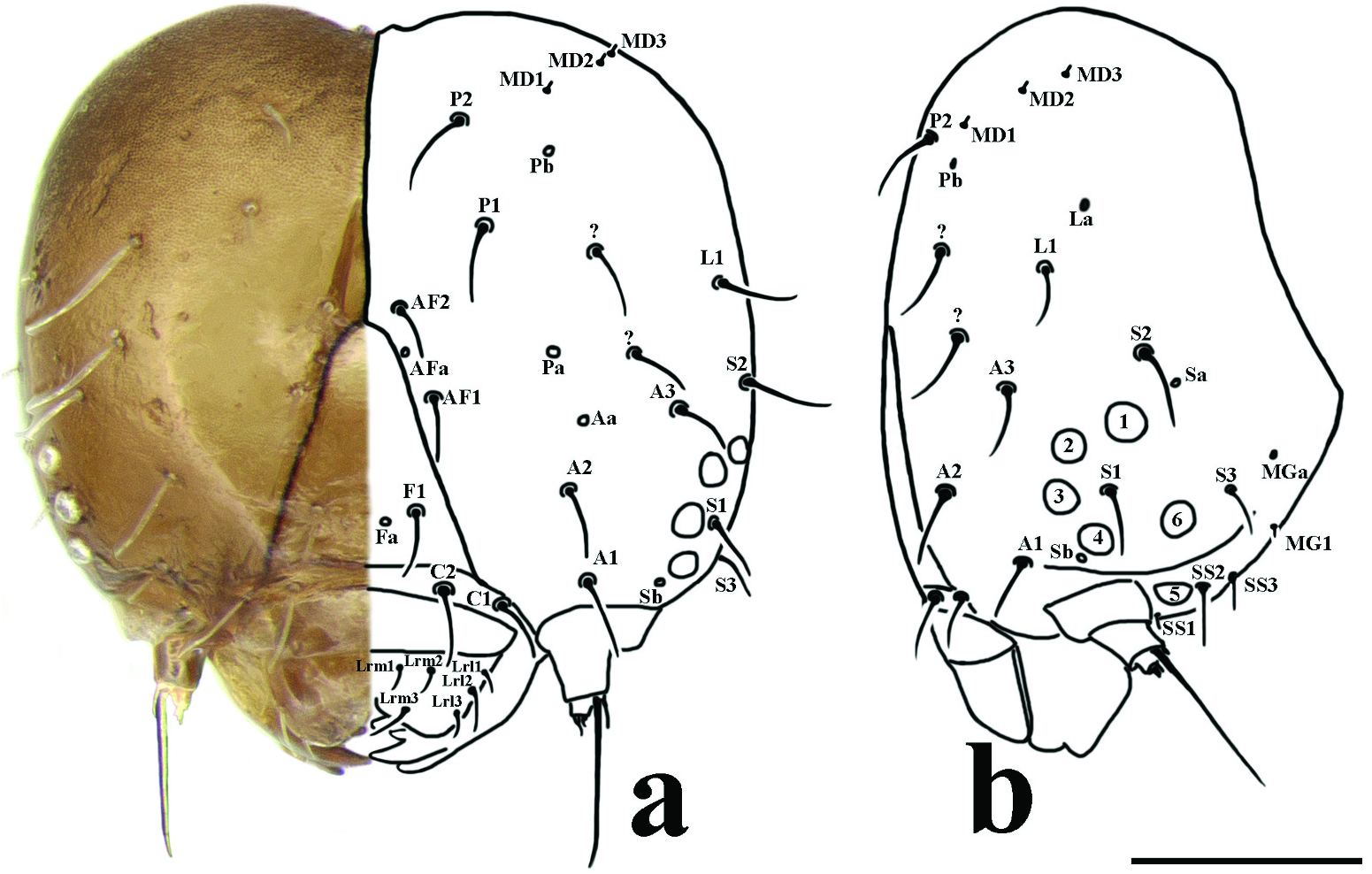
Head. ( Figure 2 View Figure 2 ) One pair of frontal setae (F1), between and below them one pair of frontal pores (Fa); two pairs of adfrontal setae (AF1, AF2), between them one pair of adfrontal pores (Afa), Afa closer to AF2 than AF1; three anterior pairs of setae (A1, A2, A3) and one pair of anterior pores (Aa) above A2; two pairs of posterior setae (P1 and P2) and two pairs of pores (Pa, Pb), Pa below P1 and Pb below and lateral to P2; two pairs of extra setae, above A3; three pairs of microdorsal setae (MD1, MD2, MD3), microdorsal pore (Mda) not visible. Laterally, one lateral seta (L1) and one pore (La); three stemmatal setae (S1, S2, S3), S1 posterior of stemma 3, S2 below stemma 1 and S3 posterior of stemma 6, two pairs of stemmatal pores (Sa, Sb), Sa posterior to and above stemma 1 and S3 setae, Sb anterior to and below stemma 4; three pairs of substemmatal setae (SS1, SS2, and SS3), SS1 behind antennae, SS2 behind stemma 5, and SS3 behind SS2. On clypeus two pairs of setae (C1, C2). On labrum three central pairs of setae (Lrm1, Lrm2, Lrm3), Lrm1 parallel to Lrm2 and Lrm3 below Lrm2; three lateral pairs of setae (Lrl1, Lrl2, Lrl3), Lrl2 below Lrl1 and Lrl3 below Lrl2.
Body. ( Figures 1 View Figure 1 , 3c View Figure 3 ) T1: XD a pair of scoli (called T1 apical scoli throughout rest of text) with apex with 10 spines; D1 and D2 setae on pronotal plate; SD1 and SD2 setae below pronotal plate, SD1 seta anterior to SD2 seta; L1 and L2 setae anterior to spiracle, L2 seta below L1 seta; SV1 and SV2 setae below L2 seta, SV2 seta anterior to SV1 seta. T2–T3: D2 seta lateral to D1 seta; SD seta single; L2 seta below SD seta and L1 seta posterior and above L2 seta; SV1 and SV2 setae as on T1. A1–A2, A7–A8: D2 seta lateral and posterior to D1 seta; SD seta single; L1 seta posterior to spiracle, L2 seta below and anterior to L1 seta; SV1 and SV2 setae next to V setae. A3–A6: D1, D2, SD, L1 and L2 setae as on A1–A2, A7–A8; SV and V setae on prolegs on a sclerotised plate with some secondary setae. A9: D2 shorter than D1, above and posterior to D1; SD, L, SV and V setae single. A10: D1, D2, SD1 and SD2 setae on anal plate; SV1 seta on PP (paraproct); X seta on the antero-lateral corner of the anal shield.
Second instar. ( Figure 3d View Figure 3 ) Average duration: ~4–5 days (n = 11); body length 8.1– 9.1 mm without stemapods, 11.9–13.3 with stemapods (n = 4); head capsule width 0.95–0.99 mm (n = 11); overall gross morphology very similar to first instar, but T1 apical scoli and stemapods each slightly smaller in proportion to rest of body, paraprocts larger proportionally, and thoracic segments more noticeably defined and somewhat more rectangular in overall shape, forming a blunt point at T3. Colouration light to dark brown dorsally with faint light brown to green-brown lines and very faint darker brown dorsal saddle-like pattern extending along length medially, laterally light grey becoming lighter proximal to ventrum; in some individuals thoracic segments with triangular light brown pattern dorsally and A8–A10 contrastingly lighter brown against darker brown ground colour, in those forms T1 scoli and stemapods lighter brown overall; stemapods in both forms covered in minute, mostly uniform length spines and with lighter brown banding, especially near terminus.
Third instar. ( Figure 3e View Figure 3 ) Average duration: ~4–5 days (n = 12); body length 10.6– 13.2 mm without stemapods, 16.9–18.6 mm with stemapods (n = 4); head capsule width 1.39–1.47 mm (n = 9) head colouration dark brown, no longer glossy; overall gross morphology very similar to second instar, but T1 apical scoli more robust, paraprocts slightly larger proportionally, thoracic segments still more noticeably defined and rectangular, hump at T3 more accentuated, ventrum of A3–A6 truncated, base held flush with leaf surface at rest, concealing prolegs. Colouration as for second instar when young becoming greener overall, well-defined dorsal pattern more noticeable on green larvae, saddle widest at A4, contrast between brown dorsum and silvery grey lateral aspect more defined, spiracles more noticeable, spiracle colouration as for lateral ground colour centrally but ringed in brown; true legs greenish brown, prolegs light brown; stemapods covered in mostly uniform short spines, banding medially and near terminus lighter and more defined.
Fourth instar. ( Figure 3f View Figure 3 ) Average duration: ~5 days (n = 9); body length from 15.4– 19.5 mm without stemapods, 21.9–26.6 mm with stemapods (n = 8); head capsule width 2.00– 2.05 mm (n = 11) head colouration and surface similar to that of third instar; overall gross morphology very similar to third instar but significantly larger and more robust overall, T1 apical scoli much smaller relative to body, paraprocts larger relative to body, thoracic segments more noticeably defined and rectangular, hump at T3 more accentuated giving overall appearance of thorax an angled shape, ventrum of A3–A6 truncated as in third instar. Colouration somewhat similar to third instar but lighter apple green, well-defined dorsal saddle pattern more uniformly brown, widest at A4, silvery grey lateral colouration very faint giving underlying green colouration a yellowish hue, but still lighter green-yellow near proleg bases, spiracles more noticeable with bright orange colouration, except for spiracle on A8 which is dark brown with faint brown smudge encircling it, true legs striped green and brown, prolegs light yellow; stemapods covered in mostly uniform short spines as in previous instars, banding medially and near terminus lighter and more defined than in previous instars.
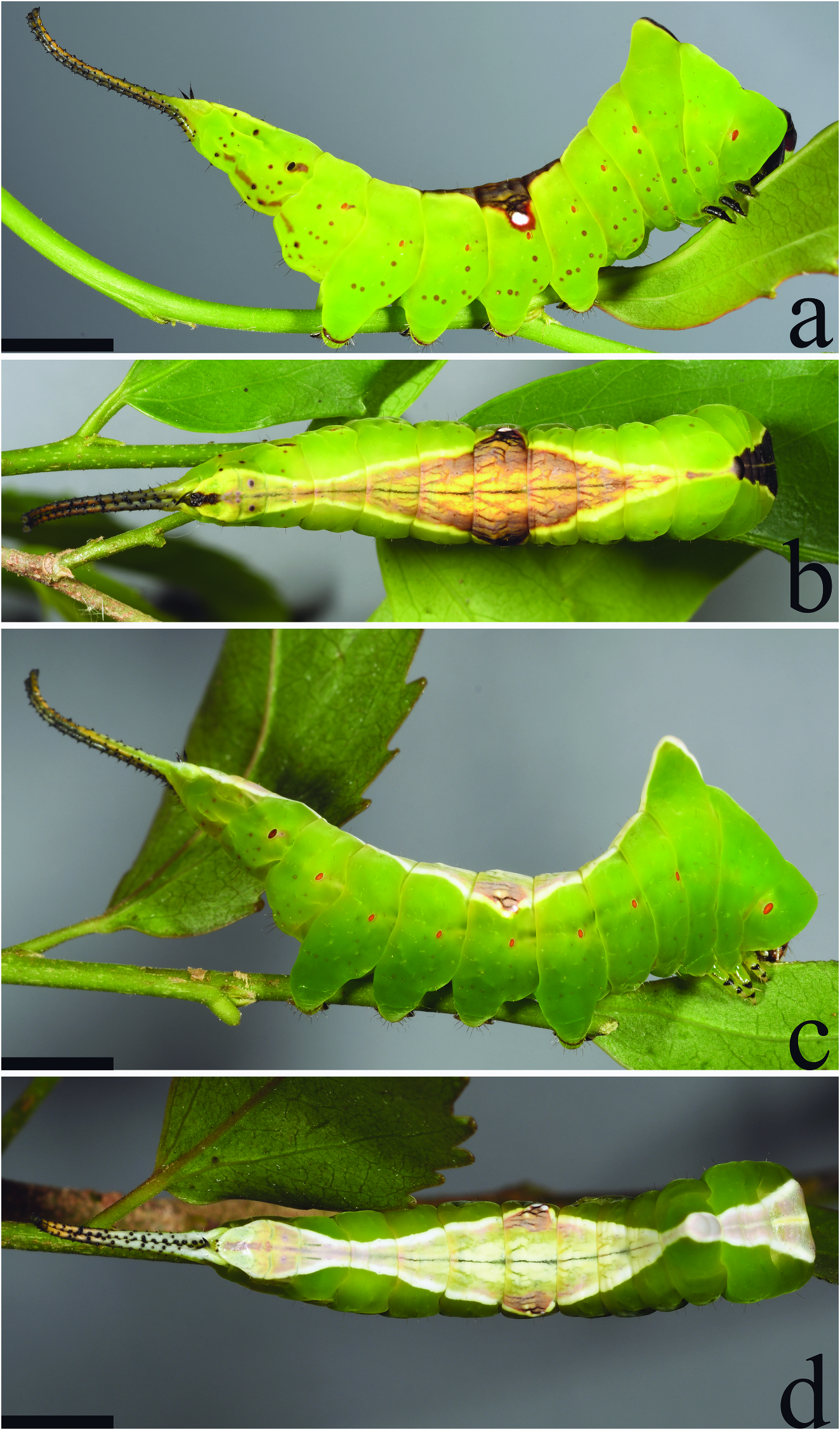
Fifth instar. ( Figures 4a–d View Figure 4 , 5a–c View Figure 5 ) Average duration: ~5–6 days (n = 7); body length 20– 35 mm without stemapods, 37–40 mm with stemapods (n = 8); head capsule width 3.1– 3.2 mm (n = 3). Morphology differs substantially in the final instar and two distinct colour forms exist, which are distinguished below. Gross morphology of the two forms similar and described together but see below for differences in patterning: as in previous instars, thoracic segments enlarged and broad, forming a triangular shape when viewed from dorsum, T1 lacks spined scoli and instead has slight protuberances giving the anterior of T1 a squared appearance into which the head can be retracted at rest and when threatened. When threatened bright pink integument of T1 immediately behind and above the head exposed and somewhat inflated. T3 with a pronounced dorsal hump. Stemapods as in previous instars but thicker and uniformly spined along length. Paraprocts robust and black.
Brown-saddled form ( Figures 4a,b View Figure 4 , 5a–c View Figure 5 ): Head shiny black to dark brown, body overall apple green with irregular brown specks that range from mostly uniform in coverage to mostly absent; spiracles bright orange, except for spiracle on A8 which is dark brown and surrounded by a brown blotch as in fourth instar. Dorsum from head to A10 with a well-defined saddle-like pattern. Thoracic segments̾ saddle very dark brown with lighter central stripe, saddle widest at T1 becoming gradually narrower approaching the apical protuberance of T3, thoracic component of saddle connected by narrow yellowish-cream stripe to abdominal saddle which is much lighter brown than thoracic saddle, abdominal saddle light yellow medially, becoming light brown outwardly, darker brown longitudinal striations present over entire saddle giving it a wooden appearance, saddle widest at A4, lateral extent of saddle somewhat variable in maculation, either with one or two large white spots immediately above A4 spiracle or lacking white spots entirely; regardless of presence of lateral spot(s), outer margins of thoracic and abdominal saddle outlined by yellow-cream which becomes primary colouration along posterior extreme of saddle which mostly lacks wooden brown striations and widens again over A9 and A10 after narrowing considerably from A5 to A9. True legs black and green, prolegs apple green. Stemapods mostly black dorsally, but may be yellowish-green laterally, extendable part of stemapods bright yellow except immediately after moult to fifth instar when these are red and yellow banded.
White-saddled form: Gross morphology as for brown-saddled form, but differing in maculation, namely the light grey head colour, lateral regions of body lacking speckles and the thoracic and dorsal saddles white rather than yellow and brown, the thoracic saddle with a pinkish hue. Stemapods white dorsally. As in brown-saddled form, presence of a white spot or spots above A4 spiracle is variable. Extendable part of stemapods as in brown-saddled form.
Pupa. ( Figure 5d View Figure 5 ) Average duration: 23 days (n = 5, non-diapause). Length: 16 mm (n = 1, male). Colouration light brown, obtect, relatively lightly sclerotised; head with mesal projected edge, proboscis not visible, antenna of examined male pupa widest at base gradually narrowing along length, terminating more than halfway along costa, eyes occupying roughly two-thirds of head; three pairs of legs, all visible, wings do not extend beyond distal margin of A5; spiracles narrow; terminus of abdomen without cremaster but rugged, slightly spined.
Cocoon. ( Figure 5e View Figure 5 ) Formed along the length of dried wood, appearing well camouflaged due to interweaving of chewed wood, covering of moss, and any other materials originally on the wood that the larva incorporates onto cocoon. Silk is dark brown. Some cocoons are covered by a shiny grey hardened foam that is excreted orally by the larva during the process of spinning the cocoon.
Life history
Eggs are laid singly on the adaxial surface of the leaf, and regularly hatch in the morning, generally before 8.00am. Larvae are almost always found on very small saplings of the host plant, less than 0.5 m above the ground. Larvae chew through but do not consume the entire egg chorion. First-instar larvae sit atop a silken platform exposed on the adaxial surface and scrape the adaxial surface of the leaf to feed, leaving behind patches of scraped surface (usually not breaking through the leaf, thus not fully skeletonising the leaf area). All subsequent instars feed from the edge of the leaf. All larval instars rest atop the leaf surface, except the final instar which, due to size, usually holds on to host plant branches or the petiole. Time spent in each instar and overall larval progression and growth is relatively quick, generally only spending a few days in each instar. If harassed, early instar larvae violently thrash thoracic segments and head side to side while whipping about stemapods, extruding a maroon filamentous component. The threat display in the final-instar larvae differs slightly, as they angle their head forward, exposing pink integument at the head-prothorax interface, splay their shiny black mandibles, and whip their stemapods over their head or angled downward, everting a yellow filament. The yellow filament may be extended in and out multiple times, sometimes curling. Larvae are easily conditioned to threat responses and generally only present their full response when first agitated; captive larvae typically do not exhibit much response if reared from early stages.
We observed two distinct last-instar colour forms, a brown-saddled form which was most common (all but one wild-collected larvae and all but one from the cohort of eggs) and a rare, white-saddled form (one wild-caught larva from EEA and one of ~ 20 larvae reared from eggs derived from a single female). We hypothesise that this white-saddled form may be environmentally induced because the single wild-caught larva that exhibited this form was found as a final instar in mid-September (early spring) when the preceding weeks were quite cold, all other immature stages observed at the site during this time were eggs and first instars. Thus, the white-saddled form would have been from a previous late winter brood. The single individual from our reared cohort that showed this form was reared outdoors and experienced much cooler and more variable temperatures than the lab-reared larvae. Certainly, more research is warranted to understand the phenotypic plasticity in A. argynnis , and other Americerura since we also observed multiple colour forms in A. rivera larvae (and of A. scitiscripta (Walker) in the United States).
Final-instar larvae darken and wander in the evening until dried wood (we provided sticks of various sizes) is available, upon which the larvae commence cocoon building, which consists of chewed wood and silk integrated into a matrix that becomes quite hard with time. The surface of the cocoon can be glossy and light grey but is usually extremely well camouflaged due to the integration of the surface medium which the larvae strip from the branch surface under which the cocoon is built. If moss is present on the branch, larvae will cut it and go underneath to build the cocoon with the moss situated above it.
Adults hatch in the evening hours. Females begin releasing pheromones at ~5.00pm but we did not observe the extent of mating behaviour or wild male response in multiple trials. We have not observed females at lights, despite the common presence of males at many localities; males typically fly after 10.00pm. Females are also much less common in collections than for other Americerura species.
Of our reared A. argynnis material, pupae of about half hatched within roughly 3 weeks of becoming prepupal with the remaining half apparently entering a summer diapause. Based on collecting data and our field work, adults of this species are not as prevalent in the southern hemisphere summer as they are in the spring and autumn months. As of this writing (March 2023) the remaining pupae have not hatched despite remaining viable.
Host plants
Banara parviflora , B. tomentosa and Xylosma pseudosalzmannii (all Salicaceae ), in captivity and in nature. We found larvae of A. argynnis at EEA on B. parviflora and B. tomentosa and at JdC we found them on B. parviflora and X. pseudosalzmannii .
Distribution
Americerura argynnis is known from Paraguay (Alto Paraná), Argentina (Chaco, Missiones, Tucumán) and Brazil (Espírito Santo, Rio de Janeiro, Paraná, Santa Catarina, Rio Grande do Sul).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Cerurinae |
|
Genus |
Americerura argynnis (Schaus, 1901)
| St Laurent, Ryan A., Carvalho, Ana Paula S., Orlandin, Elton & Carneiro, Eduardo 2024 |
Cerura argynnis
| Schaus 1901 |