Glossidium pedatum Looss, 1899
|
publication ID |
https://doi.org/10.11646/zootaxa.5284.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:DA6684D9-508D-47A3-ACD9-D36A201086C3 |
|
DOI |
https://doi.org/10.5281/zenodo.7937292 |
|
persistent identifier |
https://treatment.plazi.org/id/6E5B321F-FFAE-FFFB-74EC-FF71C7AFFC66 |
|
treatment provided by |
Plazi |
|
scientific name |
Glossidium pedatum Looss, 1899 |
| status |
|
Glossidium pedatum Looss, 1899 View in CoL View at ENA
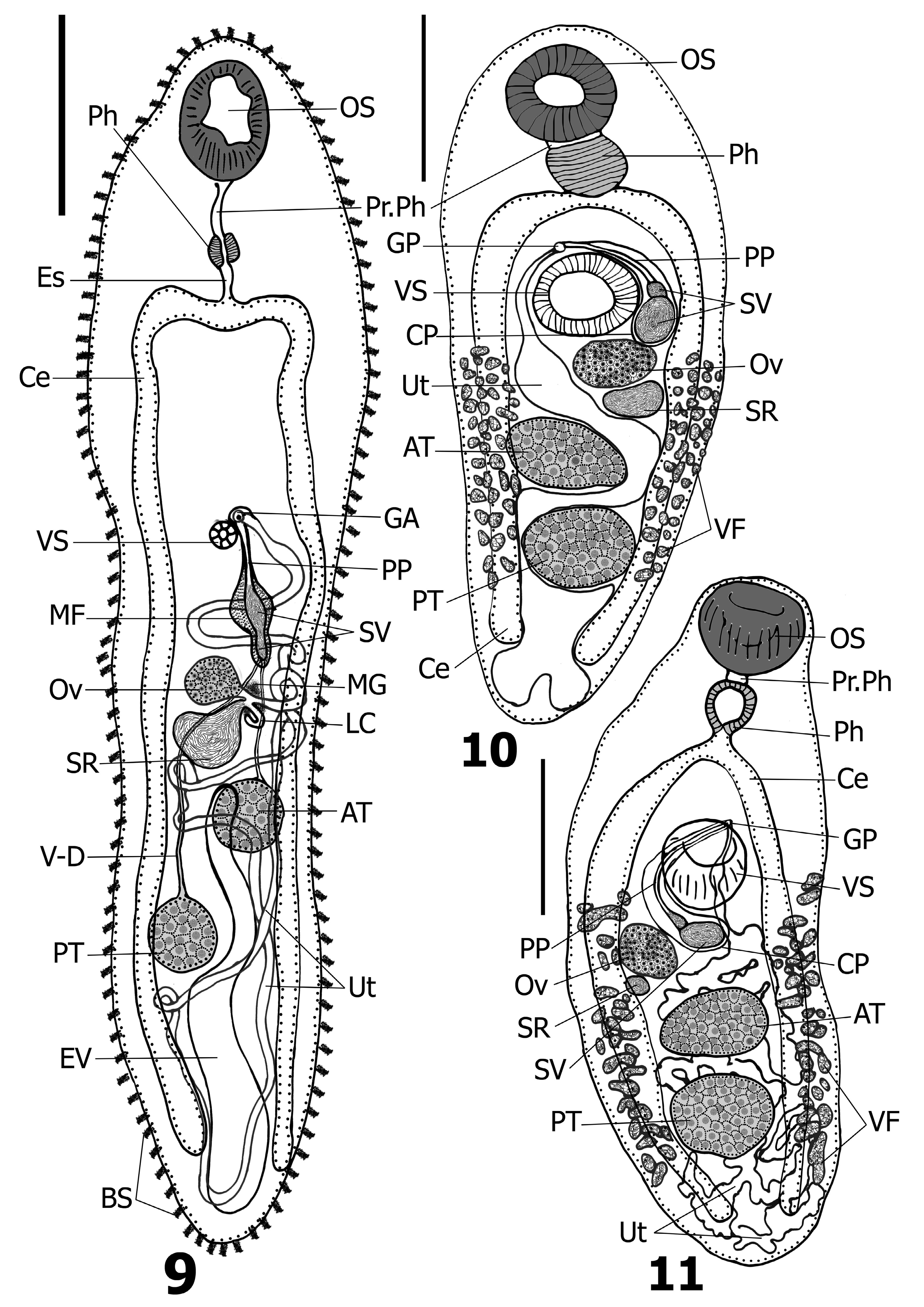
( Figs. 10 & 11 View FIGURES 9–11 )
(Syn. Astiotrema lazeri El-Naffar, Saoud & Hassan, 1984 n. synonym)
Records. 1. Looss (1899); 2. Beverley-Burton (1962); 3. Fischthal (1973); 4. Mashego (1977); 5. Moravec (1977); 6. El-Naffar et al. (1984); 7. Mashego & Saayman (1989); 8. El-Naggar et al. (1991, 1993a, 1993b, 1993c); 9. Imam et al. (1991); 10. El-Shahawi & Al-Bassel (1992); 11. Arafa & Reda (2002); 12. van Rensburg et al. (2003); 13. Ibraheem (2007); 14. Abdel-Gaber et al. (2016); 15. Zhokhov et al. (2017); 16. Dumbo et al. (2019).
Remarks. Glossidium was erected for the type-species G. pedatum gathered from the intestine of the bayad, Bagrus bajad (Forsskål) , and the semutundu, Bagrus docmak (Forsskål) ( Siluriformes : Bagridae ), from the River Nile at Cairo ( Looss 1899). Subsequent records of G. pedatum revealed that the North African catfish, Clarias gariepinus (Burchell) (syns. Clarias lazera Valenciennes and Clarias mossambicus Peters ) ( Siluriformes : Clariidae ), represented the most frequently reported hosts of this parasite ( Fischthal 1973; Mashego 1977; Mashego & Saayman 1989; Imam et al. 1991; El-Shahawi & Al-Bassel 1992; van Rensburg et al. 2003; Bray & Hendrix 2007; Abdel-Gaber et al. 2016; Dumbo et al. 2019). We found only one other record of G. pedatum from B. bajad and B. docmak (see Ibraheem 2007). In addition, the distribution of G. pedatum is restricted to freshwater localities across the continent of Africa including Botswana ( van Rensburg et al. 2003), Egypt ( Looss 1899; Imam et al. 1991; El-Shahawi & Al-Bassel 1992; Ibraheem 2007; Abdel-Gaber et al. 2016), Ethiopia ( Fischthal 1973), Lake Malawi ( Bray & Hendrix 2007), Mozambique ( Dumbo et al. 2019) and South Africa ( Mashego 1977; Mashego & Saayman 1989; Bray et al. 2006). Some morphological features in G. pedatum exhibited a slight range of variability which we feel falls within the range of intra-specific variation including: (i) prepharynx length varied from shorter than the pharynx ( Looss 1899; Abdel-Gaber et al. 2016; Dumbo et al. 2019), equal in length to the pharynx ( Fischthal 1973) or longer than the pharynx ( van Rensburg et al. 2003); (ii) pharynx shape either cylindrical ( Looss 1899; van Rensburg et al. 2003; Dumbo et al. 2019), papillate or four-lobed ( Fischthal 1973; Dumbo et al. 2019), or tulip-shaped ( van Rensburg et al. 2003; Dumbo et al. 2019); (iii) excretory pore positioned either subterminally ( Looss 1899; Fischthal 1973; Dumbo et al. 2019) or terminally ( Ibraheem 2007; Abdel-Gaber et al. 2016); and (iv) cecal ends terminate at either the posterior level of the posterior testis ( Looss 1899; Fischthal 1973; van Rensburg et al. 2003; Dumbo et al. 2019) or slightly posterior to it without reaching the posterior extremity ( Ibraheem 2007; Abdel-Gaber et al. 2016; Dumbo et al. 2019, fig. 2a). From all previous records, it can be concluded that the key combination of diagnostic morphological characteristics for G. pedatum include: possessing suckers of roughly equal size, a short or absent esophagus, an intestinal bifurcation positioned in the mid-forebody, a moderately-sized and bipartite seminal vesicle occupying about 1/3 of the cirrus-pouch with a longer and tubular pars prostatica and ejaculatory duct, an ovary very close to or adjacent to the ventral sucker, tandem testes or nearly so, ceca that reach well into the anterior portion of the post-testicular region, vitellarium extending from the level of the anterior margin of the ventral sucker to the level of the posterior testis, and G. pedatum is endemic to Africa infecting freshwater fish of the Clariidae Bonaparte and the Bagridae Bleeker.
Investigation and reexamination of Afromacroderoides and its type species, A. lazerae , by Mashego & Saayman (1989) revealed that the morphological features of this genus were more typical of the Plagiorchiidae than the Allocreadiidae and that A. lazerae and G. pedatum share the same host species ( C. gariepinus ) and are both found in Africa (White Nile River near Khartoum, Sudan and what was Lebowa, South Africa) (see Khalil 1972). Thus, Mashego & Saayman (1989) synonymized Afromacroderoides with Glossidium as well as reassigned its type-species, A. lazerae , as a synonym of G. pedatum . Subsequent authors adopted the synonymy of Afromacroderoides with Glossidium but they have retained A. lazerae (now considered to be Glossidium lazerae [ Khalil, 1972] Pojmańska, Tkach & Gibson, 2008) as a distinct species separate from G. pedatum ( Bray et al. 2006; Pojmańska et al. 2008; Dumbo et al. 2019). This is based on the former species possessing longer ceca that terminate near the posterior extremity of the body, a distinctly long esophagus, and G. lazerae has a bipartite seminal vesicle that is larger, occupying almost the entire cirrus-pouch (approximately 3/4 of it) with a shorter pars prostatica and ejaculatory duct (see Khalil 1972; Bray et al. 2006; Pojmańska et al. 2008, figs. 66.8 & 66.9; Dumbo et al. 2019).
Astiotrema lazeri was described for specimens collected from the intestine of the North African catfish, C. gariepinus (syn. C. lazera ), from Lake Nasser in Aswan, Egypt ( El-Naffar et al. 1984). One of the most interesting features mentioned in the original description of A. lazeri is that it possesses a bipartite seminal vesicle in contrast to other known species of Astiotrema . However, El-Naffar et al. (1984) did not use this feature in their comparisons with other taxa of Astiotrema at that time; they depended on features such as sucker size, presence or absence of an esophagus, host and geographical distribution, slight differences in vitellarium extent, egg size and location of the cecal ends. The nature of the seminal vesicle (i.e., unipartite vs bipartite; saccular vs tubular; straight vs coiled) is a strong morphological character for differentiating taxa at the genus, family and even superfamily levels as we have experienced. Accordingly, A. lazeri does not belong within Astiotrema (sensu stricto) nor its closely related genera (i.e., Homeoastiotrema , Ichthyastiotrema and Plesioastiotrema ). Our analysis of the diagnostic features of A. lazeri in combination with its reported host showed that it clearly belongs within Glossidium . Based on the previously mentioned morphological comparison between G. lazerae and G. pedatum , specimens from El-Naffar et al. (1984) appear identical with G. pedatum in all morphological features as well as have the same host species ( C. gariepinus ) and locality ( Egypt); accordingly, A. lazeri represents a synonym of G. pedatum . This same conclusion was suggested by Bray et al. (2006), who also pointed out the close morphological relationship between the specimens of A. lazeri of El-Naffar et al. (1984) and that of G. pedatum .
Bray et al. (2006) morphologically compared A. reniferum and G. pedatum and noted that both species have been frequently reported from the North African catfish, C. gariepinus , in Africa (see Khalil 1959; Beverley-Burton 1962; Fischthal 1973; Moravec 1977; Mashego & Saayman 1989; El-Naggar et al. 1991; van Rensburg et al. 2003; Dumbo et al. 2019). Bray et al. (2006) also indicated that specimens reported as A. reniferum by Beverley-Burton (1962), Moravec (1977) and El-Naggar et al. (1991) appear morphologically closer to G. pedatum than to A. reniferum . We concur with Bray et al. (2006) regarding these particular reports of A. reniferum based on the latter possessing all the characteristics of G. pedatum , particularly the bipartite seminal vesicle. The same previous considerations also indicate the erroneous identification of specimens reported by El-Naggar et al. (1993a, 1993b, 1993c), Arafa & Reda (2002) and Zhokhov et al. (2017) as A. reniferum to also be G. pedatum . Thus, our conclusion supports the opinion of Bray et al. (2006) that A. reniferum does not commonly occur in African freshwater fishes, having usually been mistaken for G. pedatum .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Opisthorchioidea |
|
Family |
|
|
Genus |