Cteniogaster nana, Jan Bosselaers & Rudy Jocque, 2013
|
publication ID |
https://doi.org/10.5852/ejt.2013.40 |
|
publication LSID |
lsid:zoobank.org:pub:99B180D2-CCD2-4171-B640-E3EB68F94E2B |
|
DOI |
https://doi.org/10.5281/zenodo.6146945 |
|
persistent identifier |
https://treatment.plazi.org/id/B0321C9D-7120-407A-BAAF-BCF85186FDA0 |
|
taxon LSID |
lsid:zoobank.org:act:B0321C9D-7120-407A-BAAF-BCF85186FDA0 |
|
treatment provided by |
Jeremy |
|
scientific name |
Cteniogaster nana |
| status |
sp. nov. |
Cteniogaster nana View in CoL sp. nov.
urn:lsid:zoobank.org:act:B0321C9D-7120-407A-BAAF-BCF85186FDA0
Figs 3D-F View Fig. 3 , 12H-J, 14; Appendix 1B
Diagnosis
Cteniogaster nana sp. nov. differs from all other Cteniogaster gen. nov. species by its small size and by the male palp with a blunt, dorsally bent RTA, a relatively large and broad prolaterally inserted embolus, a small sclerotised apical conductor and a small and subtriangular retrolaterally inserted MA.
Etymology
The species name is derived from the Latin nanus, dwarf, and refers to the small size of the present species.
Type material
Holotype
Ƌ: TANZANIA, E. Usambara Mts., Amani , 5°5.7’S 38°38’E, 28 Oct.-9 Nov. 1995, 950 m asl, pitfalls, Griswold C., Scharff N. & Ubick D. ( CAS) GoogleMaps
Paratype
1 Ƌ: together with holotype GoogleMaps .
Description
Male (holotype)
TOTAL LENGTH. 1.74. Carapace length 0.74, width 0.58, unicoloured pale yellow, unbordered ( Fig. 3C View Fig. 3 ). Fovea pronounced, length 0.08, anterior end 0.53 from front end of carapace. MOQ length (when eight eyes present) 0.05, anterior width 0.02, posterior width 0.06. AER width 0.11, PER width 0.15. Median eyes with a strong tendency towards reduction, one specimen with reduced median eyes, the other having only four eyes ( Fig. 12J). AME very small (1/6 of ALE diameter) or absent, PME very pale and strongly reduced (1/8 of ALE diameter) or absent, PLE 2/3 of ALE diameter. Both eye rows (if ME are present) straight from above. Clypeus vertical, equal to 1/3 of ALE diameter. Chilum indistinct. Sternum yellow, unbordered ( Fig. 3D View Fig. 3 ), length 0.47, width 0.39. PCT indistinct.
ABDOMEN. Pale cream dorsally ( Fig. 3F View Fig. 3 ) with traces of a weak, diffuse anterior do scutum and ventrally with an oblong, sclerotised patch carrying strong modiFed setae in posterior half ( Fig. 3 E View Fig. 3 ). Spinnerets as for the genus in general. Legs pale yellow ( Fig. 3D, F View Fig. 3 ). Retrocoxal hymen pronounced, oval and white. Trochanter notch indistinct in legs I and II, pronounced in legs III and IV. Very sparse ve terminal preening brushes on mt III and IV. Tarsus IV slightly bent. Leg spination as in Appendix 1B.
LEG MEASUREMENTS:
| fe | pa | ti | mt | ta | Total | |
|---|---|---|---|---|---|---|
| I | 0.53 | 0.24 | 0.39 | 0.29 | 0.28 | 1.72 |
| II | 0.45 | 0.22 | 0.37 | 0.28 | 0.28 | 1.59 |
| III | 0.42 | 0.14 | 0.29 | 0.32 | 0.29 | 1.46 |
| IV | 0.55 | 0.21 | 0.47 | 0.50 | 0.39 | 2.13 |
MALE PALP. With a blunt, dorsally bent RTA, a relatively large and broad prolaterally inserted pointed embolus, a small sclerotised apical conductor, and a pointed, small and subtriangular retrolaterally inserted MA.
Female
Unknown.
Distribution
Tanzania, East Usambara mountains , 950 m asl.
Discussion
Phylogenetic analyses
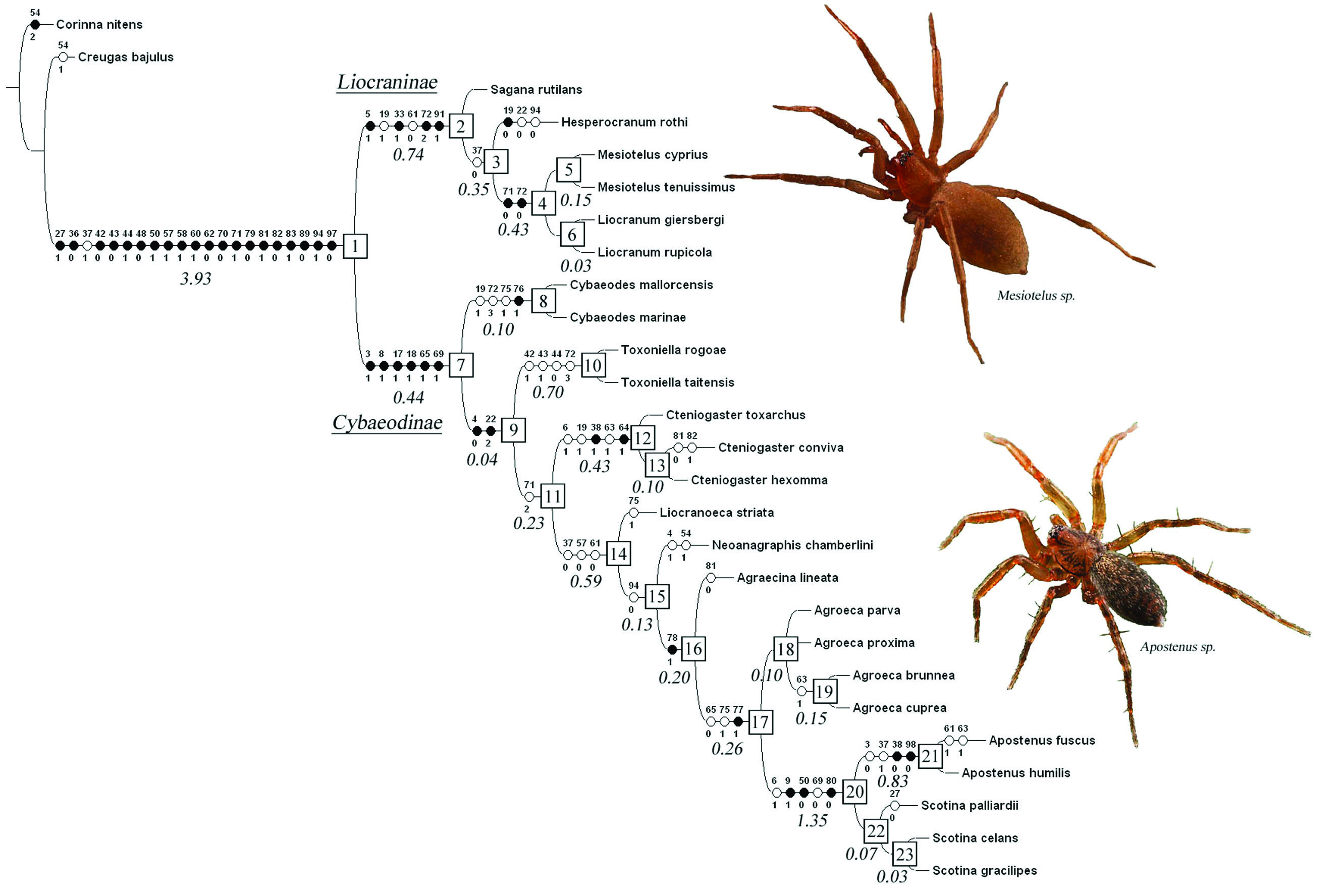
The preferred consensus tree for 27 species known from both sexes, with node numbers, state changes for 47 characters and Goloboff Ft Bremer support values (as reported in TNT) in italics below branches is illustrated in Fig. 1 View Fig. 1 . Each ambiguity on the tree was optimized in isolation, in order to avoid scoring character states for absent structures, and also because only a combination of ACCTRAN and DELTRAN optimisation can produce the most robust proposal for a supposed homology. Indeed, the use of ACCTRAN only, as is often preferred, does not always maximize parallel loss of complex traits over convergent gains ( Agnarsson & Miller 2008). Of the Fve ambiguous characters shown on the preferred tree in Fig. 1 View Fig. 1 , DELTRAN optimisation was preferred for characters 19, 38 and 72, ACCTRAN for character 37, and only unambiguous changes are shown for character 22.
Homoplasy in the data matrix which produced the preferred tree is quite acceptable: 22 out of 99 characters are completely free of homoplasy. Sanderson & Donoghue (1989: 1785, Fg. 1) performed a polynomial regression analysis on data from 60 cladistic analyses, and derived the following equation based on them: ci = 0.90 - 0.022×(number of taxa) + 0.000213×(number of taxa)2. Applying this equation, 27 taxa would yield a ci value of 0.461, quite similar to the actual ci value of 0.412 obtained for the consensus tree in the present analysis.
The ingroup (clade 1) is supported on the preferred tree by the absence of apical do spines on fe III and IV (11:0, not shown in Fig. 1 View Fig. 1 , reversed in Cybaeodes marinae Di Franco, 1989 and in clade 14), the presence of a distal spine on the male palpal pl edge [27:1, absent in Scotina palliardii (L. Koch, 1881)], absence of true claw tufts (36:0), the presence of tenent hairs at the tip of tarsi (37:1, reversed in clades 3 and 14, but present in Apostenus), a simple sternal border (42:0, reversed in Toxoniella), two retromarginal cheliceral teeth (43:0, more than two in Toxoniella), a shaggy hair in front of the fang base (44:1, reversed in Toxoniella), a conspicuous serrula on the endites (48:0), a Fat carapace (50:1, changed to slanting in clade 20), modiFed PME (57:1, reversed in clade 14), the MOQ widest posteriorly (58:1), curved strong hairs frontally on abdomen (60:1), no epigastric sclerite (62:0, 70:0), laterally compressed female PMS (71:1, changed to slender in clade 4 and to stout and subtriangular in clade 11), absence of a coiled sperm duct (79:0), a membranous conductor (81:1, reversed in Cteniogaster conviva sp. nov. and Agraecina lineata (Simon, 1878), unapplicable in clade 20), a simple conductor (82:0, complex in C. conviva sp. nov., unapplicable in clade 20), MA present (83:1), subtegulum pl (89:0), anterior hood present in epigyne (94:1, reversed in Hesperocranum rothi Ubick & Platnick, 1991, and in clade 15) and anterior epigynal entrances (97:0). We consider clade 1 to represent the family Liocranidae . It is divided in two sister clades in the present analysis: clade 2 and clade 7 ( Fig. 1 View Fig. 1 ).
Clade 2 is interpreted as subfamily Liocraninae and is supported on the preferred tree by the presence of large erectile bristles in the ve scopulae of legs I and II (5:1), ventral scopulae on ti I and II (33:1) and a pl terminal lobe on the male palpal ti (91:1). Clade 4 ( Mesiotelus Simon, 1897 and Liocranum L. Koch, 1866) is further characterised by slender female PMS carrying only a single large spigot (71:0, 72:0, contra Wunderlich 2008: 489).
Clade 7 is interpreted as subfamily Cybaeodinae and is supported on the preferred tree by the presence of a trochanter notch (3:1, reversed in Apostenus), the presence of bent male tarsi (8:1), presence of do spines on ti III (at least in females) and IV (17:1, 18:1), cylindrical ALS in males (65:1, reversed in clade 17), and presence of EPGS in males (69:1, reversed in clade 20). Although present in some Clubionidae as well (see discussion of character 69, above), the presence of EPGS seems to be an interesting apomorphy for the subfamily Cybaeodinae. The character is lost in the genera Apostenus and Scotina, most probably due to their small size. Cybaeodes Simon, 1878 (clade 8) holds a basal position within Cybaeodinae in the present analysis, supported by two rows of more than Fve large spigots on female PMS (paralleled in Toxoniella) and a small dorsal bristle mat on the male palpal cymbium (76:1). Toxoniella (clade 10) differs from all other members of clade 1 by a number of reversals, being a rebordered sternum (42:1), more than two retromarginal cheliceral teeth (43:1) and absence of a shaggy hair in front of the cheliceral fangs (44:0), but the genus Fts within the clade for all other important characters, including the presence of an epigynal anterior hood (94:1). Toxoniella shares with Cybaeodes, Hesperocranum Ubick & Platnick, 1991 and Sagana Thorell, 1875 the laterally compressed female PMS (71:1), and with Cybaeodes and Sagana a large number of tarsal tenent hairs (38:2). Both characters appear plesiomorphic within the family. Within Cybaeodinae, Toxoniella shares the presence of EPGS (69:1) with most other genera. Toxoniella is herewith transferred to Liocranidae . None of the other genera currently included in Gallieniellidae is reported to possess EPGS in males.
Cteniogaster gen. nov. also Fts well within Cybaeodinae, but differs from related genera by the wide patellar indentations (6:1, 7:1, paralleled in Apostenus), the presence of one mt IV plv and rlv spine (21:1), two pairs of tenent hairs (38:1), and the presence of ve abdominal setae in males (63:1, paralleled in Agroeca brunnea (Blackwall, 1833) and Agrocea cuprea Menge, 1873 (clade 19) and in Apostenus fuscus Westring, 1851). The fact that these male ve abdominal setae occupy a well delimited small oval area (64:1) is an autapomorphy for the genus. Clade 14 is supported by the absence of mt III and IV vt spines (23:0, reversed in clade 20) and the presence of circular PME (57:0). Clade 16 is supported by the presence of a subtegular locking lobe (78:1), complemented in clade 17 (the genera Agroeca, Apostenus and Scotina) by a tegular locking lobe (77:1) and conical ALS in males (65:0). The genus Agroeca (clade 18) is supported by a biFd MA (84:1, paralleled in Sagana rutilans Thorell, 1875) and a Fat, ribbonshaped embolus (87:4). Clade 20, consisting of small, derived Cybaeodinae, is distinguished by plv spines on fe I (9:1), a slanting carapace (50:0), and absence of a conductor (80:0) and of EPGS (69:0). Apostenus is further characterised by the presence of one pair of tenent hairs (38:0), widely separated PME (59:2) and the absence of ST2 (98:0). Its sister genus Scotina features equidistant PE (59:1) and a whip-shaped embolus (87:5).
While some characters (36:0, 48:0, 58:1, 60:1, 62:0, 70:0, 79:0, 83:1, 89:0, 97:0) are constant throughout Liocranidae , others (44:1, 57:1, 65:1, 69:1, 94:1) show reversal in some clades.
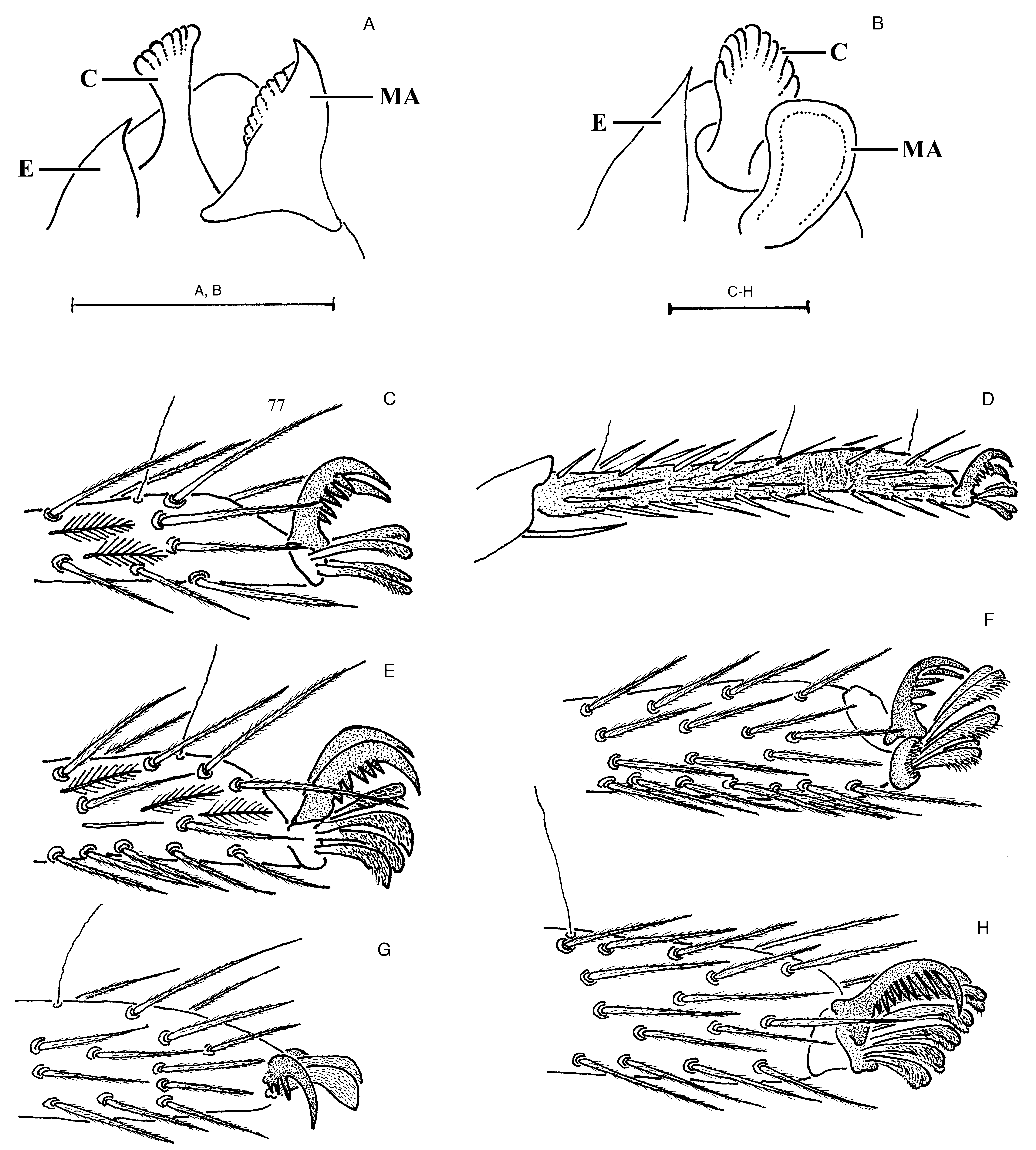
Reversals and secondary losses of characters are particularly common on the preferred tree in the genus Toxoniella and in clade 20, which groups the genera Apostenus and Scotina. Apart from the three reversals mentioned above, Toxoniella has no plv and rlv spines on female ti I (14:0) and has male and female PLS close together (68:0 and 73:0, paralleled in clade 3 and in clade 4, respectively). In clade 20, apart from the already mentioned absence of EPGS (69:0) and a conductor (80:0), the number of mt III plv and rlv spines is reduced (20:0, paralleled in Cteniogaster gen. nov. and Hesperocranum), mt III and IV have ve terminal spines (23:1), feathery hairs are absent (30:0, paralleled in Hesperocranum, Liocranoeca and Agraecina), there is no apical maxillar hair tuft (49:0, paralleled in Cteniogaster gen. nov., Hesperocranum and Liocranoeca), and PME and PLE are close to each other (59:1 or 59:2). Moreover, Apostenus has no trochanter notch (3:0) and no ST2 (98:0). The presence of male modiFed ve abdominal setae (63:1) appears to have evolved convergently in Cteniogaster gen. nov. and in some Agroeca and Apostenus ( Wunderlich 2008: 489). The character was already present in the eocene Apostenus spinimanus. The presence of tenent hairs (37:1) also shows a peculiar distribution on the preferred tree, being restricted to the genera Sagana, Cybaeodes, Toxoniella, Cteniogaster gen. nov., and Apostenus. While Sagana has 9 and Cybaeodes (see Bosselaers 2009) and Toxoniella 5 pairs of tenent hairs, Cteniogaster gen. nov. has only two pairs ( Fig. 11C-E View Fig. 11 ) and recent Apostenus only one ( Fig. 11G View Fig. 11 ). However, the extinct Apostenus spinimanus, most probably congeneric with recent species, has three pairs ( Fig. 11F View Fig. 11 , contra Wunderlich 2004: 1627). Based on the presence of tenent hairs, modiFed male ve abdominal setae, an epigynal anterior hood and the other characters listed above for clade 1 (with the exception of a sclerotised conductor for Cteniogaster conviva sp. nov.), the new genus Cteniogaster gen. nov. is placed in Liocranidae, Cybaeodinae.
The present phylogenetic analysis does not produce an unequivocal autapomorphy for Liocranidae . However, a combination of a number of non-homoplasious character changes mentioned above for clade 1 in the discussion of phylogenetic Fndings, offers signiFcant potential for recognising genera as Liocranidae . No doubt, most of these character states (11:0, 27:1, 42:0, 43:0, 44:1, 48:0, 57:1, 58:1, 60:1, 89:0, 97:0) are not unique to Liocranidae and probably plesiomorphic on a wide scale or only apomorphic to a larger clade in which Liocranidae will prove to be embedded. Nevertheless, we consider a combination of a sizable number of these character states as the best approach available to date towards recognising Liocranidae : the absence of true claw tufts (36:0), the presence of tenent hairs (37:1), the absence of extensive abdominal ve sclerotisation, at least in females (70:0), the presence of a simple, membranous conductor as well as a MA in the male palp (81:1, 82:0, 83:1) and an epigyne with an anterior hood (94:1). The attribution of the liocranid genera included in the present analysis to subfamilies Liocraninae and Cybaeodinae can be considered robust, as both supraspeciFc taxa are supported by apomorphies within Liocranidae (5:1, 91:1 and 3:1, 8:1, 17:1, 18:1, 69:1, respectively). The presence of EPGS in males (69:1) is the main character on which the transfer of Toxoniella to Liocranidae, Cybaeodinae is based. The family Gallieniellidae is poorly deFned: porrect chelicerae, the only synapomorphy found in Platnick’s cladogram ( 2002: 9), also occur in other araneomorph spider families, such as Clubionidae and Theridiidae Sundevall, 1833. In addition, Haddad et al. (2009: 16) mention small male AME, a recurved PER, conical ALS and a short cymbium tip, but most of these characters reverse somewhere within the Gallieniellidae clade and none is unique to the family. The presence of tenent hairs (37:1), a character found in the majority of Liocranidae , is also encountered in the gallieniellid genera Drassodella Hewitt, 1916 ( Warui & Jocqué 2002: 314) and Austrachelas Lawrence, 1938, although in the latter they are only present on the posterior two pairs of legs, similar to what is described in Raven & Stumkat (2002) for some Clubionidae . However, no other gallieniellid has cylindrical ALS provided with EPGS in males, and this character, in combination with the above mentioned set of characters commonly encountered in Liocranidae , is judged sufFcient to justify the transfer.
As far as the other liocranid genera listed in Platnick (2012) are concerned, some conclusions can be drawn and a few transfers proposed. Argistes Simon, 1897 and Sphingius Thorell, 1890 most probably belong in Liocranidae , given the presence of tenent hairs ( Deeleman-Reinhold 2001: Fgs. 639; personal observation), a simple conductor and a MA. As long as the conspeciFcity of the male described by Bosmans (2011: 20, Fgs. 15-16) with females of Arabelia Bosselaers, 2009 is not proved beyond doubt, it seems better to keep the genus as Liocranidae incertae sedis, due to the presence of Fve pairs of tenent hairs and an anterior epigynal hood, as well as the complete absence of abdominal sclerotisation. Pending a generic revision, the same classiFcation is defended for Rhaeboctesis Simon, 1897, given the absence of true claw tufts and abdominal sclerotisation, as well as the presence of a simple conductor and a MA. Andromma Simon, 1893 does not Ft well in Liocranidae as deFned here, due to the presence of true claw tufts and the absence of a MA and anterior epigynal hood. It is likely to belong in Corinnidae , but since a comprehensive cladistic analysis of a larger number of liocranid and corinnid genera is not yet available, it is best to keep it in Liocranidae as incertae sedis. Paratus Simon, 1898, which lacks a MA ( Marusik et al. 2008; Zapata & Ramírez 2010) was placed in a subfamily of its own by Marusik et al., based, apart from the general character states already mentioned above for clade 1, on an insufFent number of characters ( 2008: 51), such as absence of a retrocoxal hymen (also absent in Cybaeodes, Liocranoeca, Neoanagraphis and Sagana, see Appendix 3), embolus inserted centrally on tegulum, very simple epigyne and abdomen with guanine spots. It seems best to keep the genus as Liocranidae incertae sedis until a thorough analysis has been performed. Sudharmia Deeleman-Reinhold, 2001, with its almost unsclerotised female abdomen and male palp with pl subtegulum and simple, membranous conductor (but lacking MA) is also considered Liocranidae incertae sedis here. Literature data on Heterochemnis F.O.P. Cambridge, 1900, Laudetia Gertsch, 1941, Liparochrysis Simon, 1909 and Mesobria Simon, 1897, genera that have never been thorougly diagnosed or revised, are insufFcient to judge on their afFnities: these four genera are best kept in Liocranidae incertae sedis for the time being. Sesieutes Simon, 1897, will be transferred to Corinnidae, Phrurolithinae Simon, 1903 by Dankittipakul & Deeleman-Reinhold (in press), and we propose the same transfer for the genera Jacaena Thorell, 1897, Plynnon Deeleman-Reinhold, 2001 and Teutamus Thorell, 1890, based on their inFated tegulum, absence of MA, modiFed male palpal fe, simple epigyne without anterior hood and abdominal sclerotisation ( Deeleman-Reinhold 2001). A comparison of Simon’s specimens of Prochora lycosiformis (O.P.-Cambridge, 1872) with the published illustrations of the male and female copulatory organs of Itatsina praticola (Bösenberg & Strand, 1906) clearly shows that the monospeciFc genus Itatsina Kishida, 1930 is congeneric with the equally monospeciFc Prochora Simon, 1886, although both species are not identical. Consequently, Prochora praticola comb. nov. is transferred to Miturgidae Simon, 1885 here, because of its biFd RTA, combined with a cymbium with a rl groove lined with a fringe of setae and a basally inserted embolus encircling the tegulum ( Song et al. 1999: Fg. 238J-L). Coryssiphus Simon, 1903 and Donuea Strand, 1932 will be transferred to other families in forthcoming publications. The holotype of Montebello tenuis Hogg, 1914 was studied by Ovtsharenko (personal communication) and turned out to be a juvenile, damaged specimen with eyes and spinnerets reminiscent of Gnaphosidae Pocock, 1898. The genus is transferred to Gnaphosidae incertae sedis here.
Wunderlich’s (2011: 108) proposal to include Liocranidae in Zoridae O.P.-Cambridge, 1893 and transfer Cybaeodes to Gnaphosidae is rejected because it is based on insufFcient data and is not supported by a cladistic analysis. Moreover, the four zorid species studied by the authors, Zora spinimana (Sundevall, 1833), Tuxoctenus gloverae Raven, 2008, Argoctenus sp. and Hestimodema sp., apart from lacking tenent hairs and male EPGS, all share a set of characters not encountered in Liocranidae : a PER with large eyes in two rows, as in Ctenidae and Lycosidae Sundevall, 1833, PME close together, thick claw tufts, an anteriorly strongly narrowed carapace, a male palpal cymbial tip with modiFed thick setae as described in Raven (2008: 352), a RTA with a Fattened basal membranous wing, an epigyne without anterior hood and a vulva without ST2 and with posterior globular ST1 connected to tortuous insemination ducts.
The proposals formulated here limit Liocranidae to 25 genera, including Cteniogaster gen. nov., Toxoniella and the recently described genus Vankeeria Bosselaers, 2012, of which the latter can be considered incertae sedis ( Bosselaers 2012). Of these genera, four belong in Liocraninae, nine can be attributed to Cybaeodinae, and twelve remain incertae sedis, stressing the need for additional revisions and a more thourough analysis of Liocranidae and related dionychan ground spiders.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |