Monstrilla aff. grandis Giesbrecht, 1891
|
publication ID |
https://doi.org/ 10.1080/00222933.2012.742933 |
|
publication LSID |
lsid:zoobank.org:pub:F3B9C990-9318-4B91-A28B-201C4426C67B |
|
DOI |
https://doi.org/10.5281/zenodo.5198377 |
|
persistent identifier |
https://treatment.plazi.org/id/6378878D-703B-B35A-5D91-F9FDFF60AAEE |
|
treatment provided by |
Felipe |
|
scientific name |
Monstrilla aff. grandis Giesbrecht, 1891 |
| status |
|
Monstrilla aff. grandis Giesbrecht, 1891
( Figures 2 View Figure 2 , 3 View Figure 3 )
Material examined
Adult male from Cahuita National Park (sta. cresta externa: 9 ◦ 45 ′ N, 82 ◦ 49 ′ W), Limón, Costa Rica, Central America , partially dissected, ethanol-preserved, vial. Selected appendages on slide mounted on glycerine, sealed with Entellan ®. Date and hour of collection: 10 June 2011, 07:00 h. Plankton sample, depth to bottom 10 m. Vial and slide deposited in the collection of Zooplankton at El Colegio de la Frontera Sur ( ECOSUR), in Chetumal, Mexico (ECO-CHZ-07568) GoogleMaps .
Description
Total body length of adult male: 0.68 mm, measured from anterior end of cephalothorax to posterior end of anal somite. Cephalothorax 0.31 mm long, representing up to 44.2% of total body length. Oral papilla protuberant ( Figure 2A,D View Figure 2 ), located at about midway (0.46) back along ventral surface of cephalothorax. Pair of relatively small lateral pigment cups well developed, separated by length of less than one eye diameter, moderately pigmented; ventral cup slightly larger than lateral cups. Forehead with moderate medial depression ( Figure 2E View Figure 2 ). Wide-based, low rounded process on ventral surface protruding between bases of antennules (arrowed in Figure 2D View Figure 2 ). Two pairs of nipple-like cuticular processes on anterior ventral surface between antennule bases and oral papilla (arrowed in Figure 2D View Figure 2 ); additional triplet of nipple-like processes posterior to latter pairs ( Figure 2D,E View Figure 2 ).
Antennule elongate, five-segmented, segment 4 longest. Antennule 0.36 mm long, representing 55% of the body length and c.120% as long as cephalothorax ( Figure 2C View Figure 2 ). In terms of pattern described by Grygier and Ohtsuka (1995) for female monstrilloid antennular armature and complemented with nomenclature by Huys et al. (2007) for elements on distal segment of males, element 1 present on first segment; elements 2d 1, 2d 2, 2v 1, 2v 2, 2v 3 and IId on second segment. Third segment with elements 3, IIId and IIIv. Segment four bearing normally developed elements 4d 1 and 4v 1–3 as well as setae IVd and IVv. Small aesthetasc 4aes on ventral surface. Terminal segment armed as follows: elements 1–7 present on anterior margin, with three branched setal elements; small subterminal aesthetasc. Terminal antennular segment unmodified ( Figure 2C View Figure 2 ).
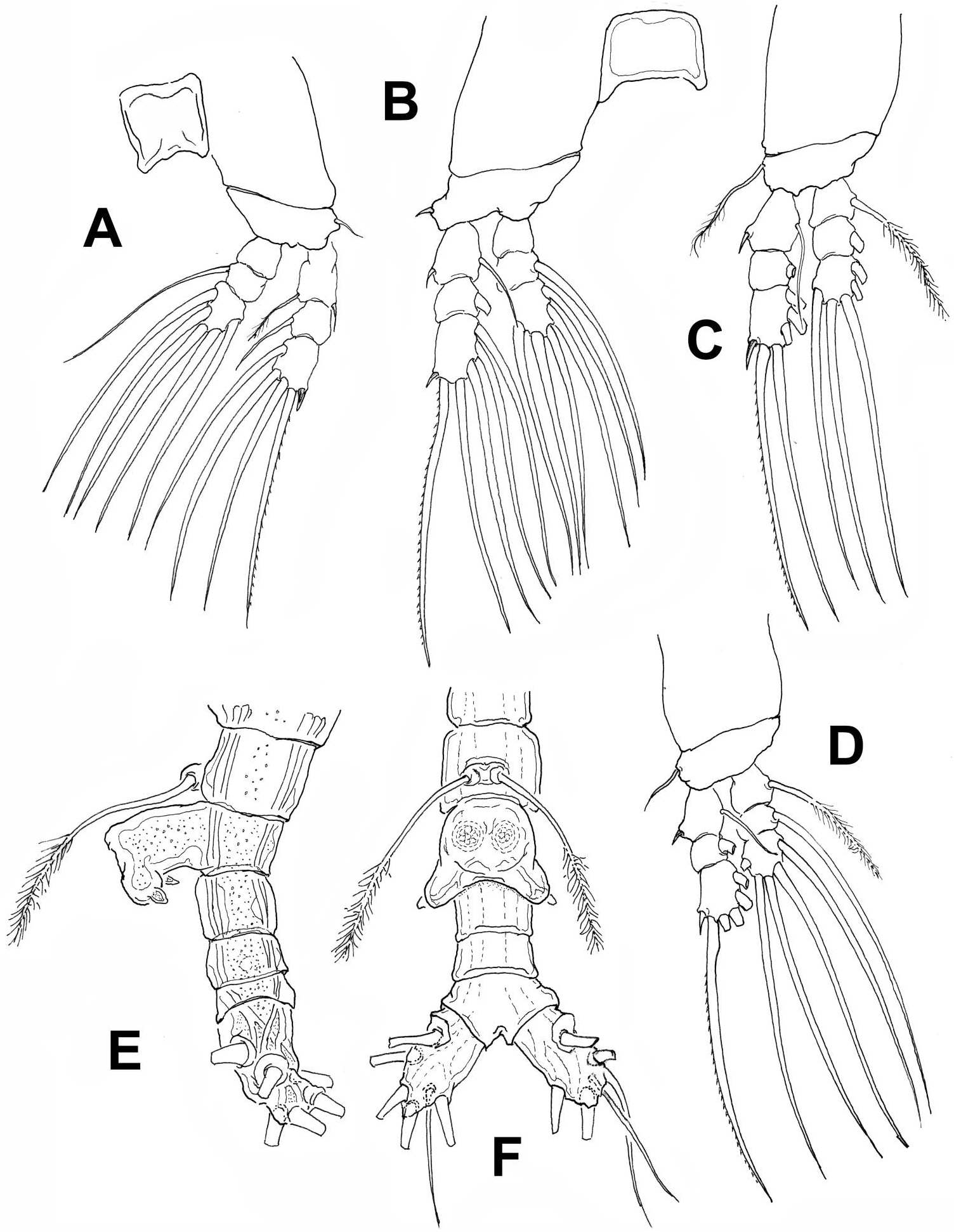
Incorporated first pedigerous somite and succeeding three free pedigerous somites each bearing pair of biramous swimming legs. Pedigerous somites 2–4 together accounting for 34% of total body length in dorsal view. Intercoxal sclerites of legs 1–4 rectangular. Basis with lateral seta on legs 1–4; on leg 3, this seta about 2.5 times longer and slightly thicker than in other legs. Endopodites and exopodites of swimming legs 1–4 triarticulated, outer margins of exopodites hirsute. Ramus setae all biserially plumose except outer spiniform seta on exopodal segments 1 and 3, outer apical exopodal seta, with row of spinules, and inner seta of first exopodal segment, these latter being short and smooth ( Figure 3A–D View Figure 3 ).
Armature formula of swimming legs is given in Table 1.
Fifth leg represented by a pair of long biserially setulated setae each arising from small basal lobe on ventral surface of fifth pedigerous somite. Fifth legs’ setae, when stretched backwards, reaching posterior end of anal somite. Genital apparatus strongly built, with enlarged base protruding ventrally; genital apparatus widely cylindrical, distally branched to form two short, rounded lappets each bearing subdistal rounded process posteriorly directed ( Figure 3E,F View Figure 3 ).
Urosome consisting of five somites: fifth pedigerous somite, genital somite, two free postgenital somites, and anal somite. Ratio of lengths of urosomites 25: 16.2: 21.6: 19.3: 17.8 = 100. Caudal rami subrectangular, 0.055 mm long, moderately divergent, approximately twice as long as wide, each bearing six setae: three terminal, two outer, and one on inner margin. Dorsal medial seta shorter and more slender than other setae ( Figure 3E,F View Figure 3 ).
Host
Unknown.
Remarks
This species was originally described from Argentinian waters in the southern Atlantic at 49 ◦ S, 65 ◦ W ( Giesbrecht 1891), but only a brief diagnosis was published; the male was first illustrated from specimens collected in the Gulf of Naples ( Giesbrecht 1893). Based on specimens from Toulon Bay, Suárez-Morales (2000) described the male following upgraded standards. The specimen from Cahuita, Costa Rica was identified as Monstrilla grandis based on its possession of an elongate antennule which is close to 60% the length of the body, the presence of a fifth leg with a fused basal segment armed with a single long plumose seta, the shape and distal process of the genital apparatus, and the presence of six caudal setae.
After its description ( Giesbrecht 1891) this nominal species was subsequently recorded from different areas of the world including European, American, and Asian waters ( Ramírez 1971; Grygier 1995; Razouls 1996; Huang 2001; Somoue et al. 2005; Brylinski 2009). In the Northwestern Tropical Atlantic it has been previously found in the eastern Caribbean Basin; Fish (1962) reported one male and a female from off Barbados and Nutt and Yeaman (1975) found it in Puerto Rican waters. Despite the recent increase of the number of surveys and records of monstrillids from the Northwestern Tropical Atlantic ( Suárez-Morales 2011), it has not been observed elsewhere in this region. Its occurrence in the Cahuita National Park, western Caribbean Sea, represents an expansion of its known geographic range in the Americas. In South America it has been reported from Argentina ( Ramírez 1971), Chile ( Marín and Antezana 1985), and Brazil ( Dias 2005; Dias and Bonecker 2007a,b). Overall, records of this nominal species are concentrated in tropical and temperate waters of the Eastern Atlantic.
Valuable illustrated reports of the species are from the Black Sea ( Dolgopolskaya 1948), China ( Shen and Bai 1956), Argentina ( Ramírez 1971) and the Mediterranean ( Giesbrecht 1893; Suárez-Morales 2000). There are some characters present in the male Costa Rican specimen that differ slightly from those in other known records. The relative length of the antennules with respect to the total body length has some variation: 48.5% (Naples), 54.8% ( Argentina), 56.4% ( China), 57% (Black Sea), 60% ( Costa Rica) and 61.2% (Toulon). The oral papilla is located in slightly different positions where this information is available: 44% (Naples), 49% ( China), 53% (Toulon), and at 46% in the Costa Rican specimen. The relative lengths of the fourth antennular segment differ slightly: 25.9% (Naples), 20% (Black Sea), 23% ( China), 26.6% (Toulon), 24.4% ( Argentina) and 26.9% in the Costa Rican specimen. The body size is also somewhat variable, less than 0.8 mm (Naples, Black Sea, Costa Rica) up to more than 1.5 mm (Naples, Barbados, Argentina, Brazil, Scotland) (see Scott 1904; Dolgopolskaya 1948; Fish 1962; Ramírez 1971; Suárez-Morales 2000; Razouls et al. 2011). The cuticular ornamentation of the ventral surface of the cephalothorax was described only in the specimens from Toulon Bay ( Suárez-Morales 2000) and the pattern differs from the Costa Rica individual; in the former specimens, there is only one pair of strongly chitinized nipple-like processes versus three pairs of such structures (plus an additional middle one) in our specimen from Cahuita. Also, the Toulon specimens have a pair of protuberant processes on the inner and outer margins of the caudal rami; these have not been reported in any of the other examined records.
The length of the single fifth leg seta is similar in all cases, reaching the midlength of the caudal rami when stretched backwards. Also, in all cases the dorsal caudal seta (VII in Huys and Boxshall 1991) is thinner and shorter than the others. Some of the morphometric variations observed in this analysis could be attributed to the different techniques of fixation, handling or preservation, or to the illustration processes related to the illustrated records. The size and morphometric variations found in Monstrilla grandis even in geographically adjacent areas (i.e. the Mediterranean), its wide distribution and the idea that supposedly cosmopolitan species among the Monstrilloida could represent species complexes with slight morphological variations ( Suárez-Morales 2011; Grygier and Ohtsuka 1995) highlights the need for a deeper analysis to define whether this nominal species represents a species complex. In the light of this notion, it is suggested that the specimens reported by Ramírez (1971) from the Argentinian coast and probably some of the Brazilian specimens ( Dias and Bonecker 2007a,b) could be conspecific with the original type material and so will be useful to determine the status of records of Monstrilla grandis from other geographic regions.
| ECOSUR |
El Colegio de la Frontera Sur (Mexico) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |