Antipodosis, Jaschhof, 2016
|
publication ID |
https://doi.org/10.5852/ejt.2016.192 |
|
publication LSID |
lsid:zoobank.org:pub:5C461741-852C-4AEB-9DA3-31B92BB23777 |
|
DOI |
https://doi.org/10.5281/zenodo.3852519 |
|
persistent identifier |
https://treatment.plazi.org/id/B8BF652D-AFFF-418C-829E-608741C68503 |
|
taxon LSID |
lsid:zoobank.org:act:B8BF652D-AFFF-418C-829E-608741C68503 |
|
treatment provided by |
Valdenar (2020-05-21 13:33:03, last updated 2024-11-29 15:54:05) |
|
scientific name |
Antipodosis |
| status |
gen. nov. |
Genus Antipodosis View in CoL gen. nov.
urn:lsid:zoobank.org:act:B8BF652D-AFFF-418C-829E-608741C68503
Type species
Antipodosis australis View in CoL gen. et sp. nov., described below.
Diagnosis
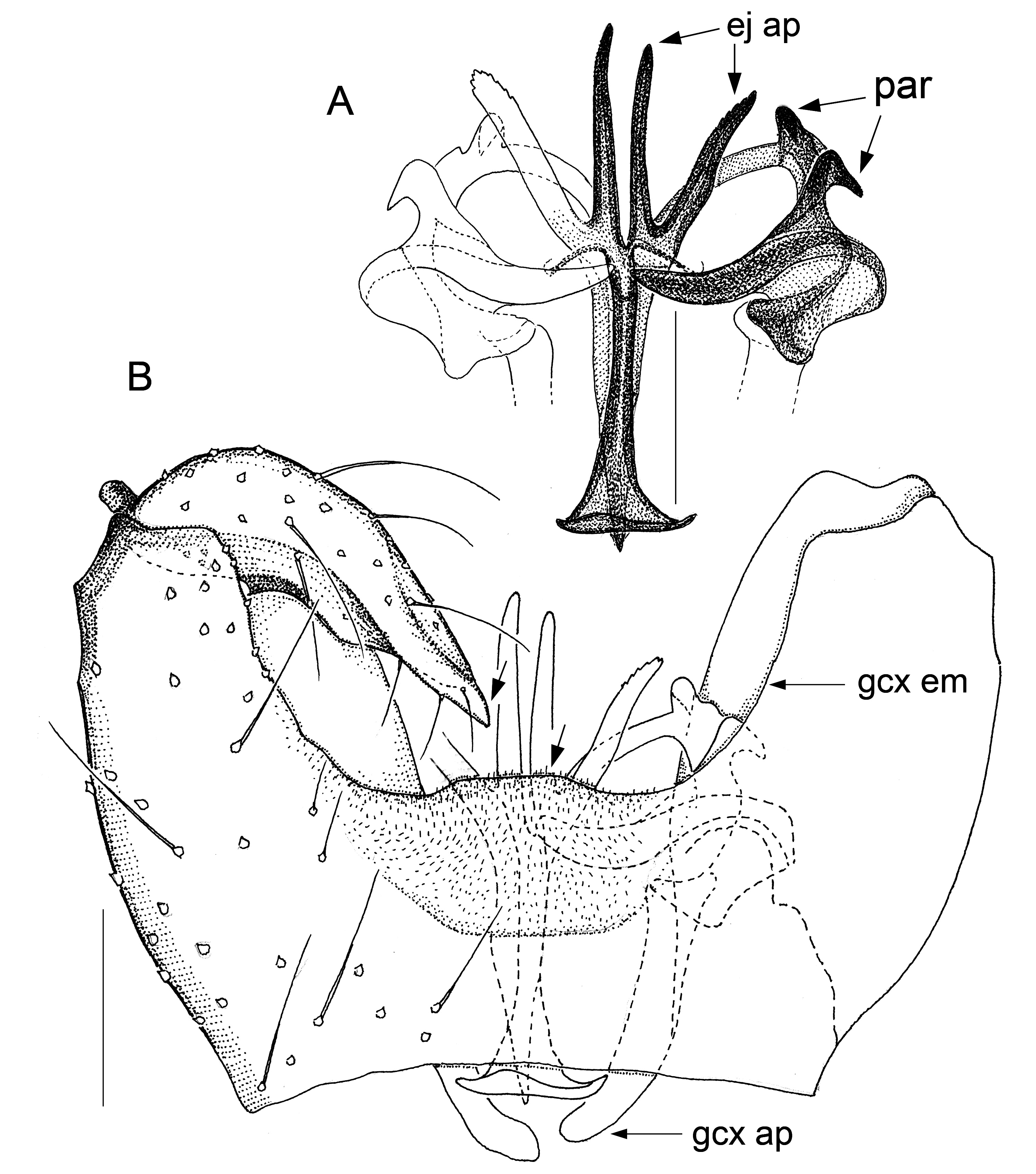
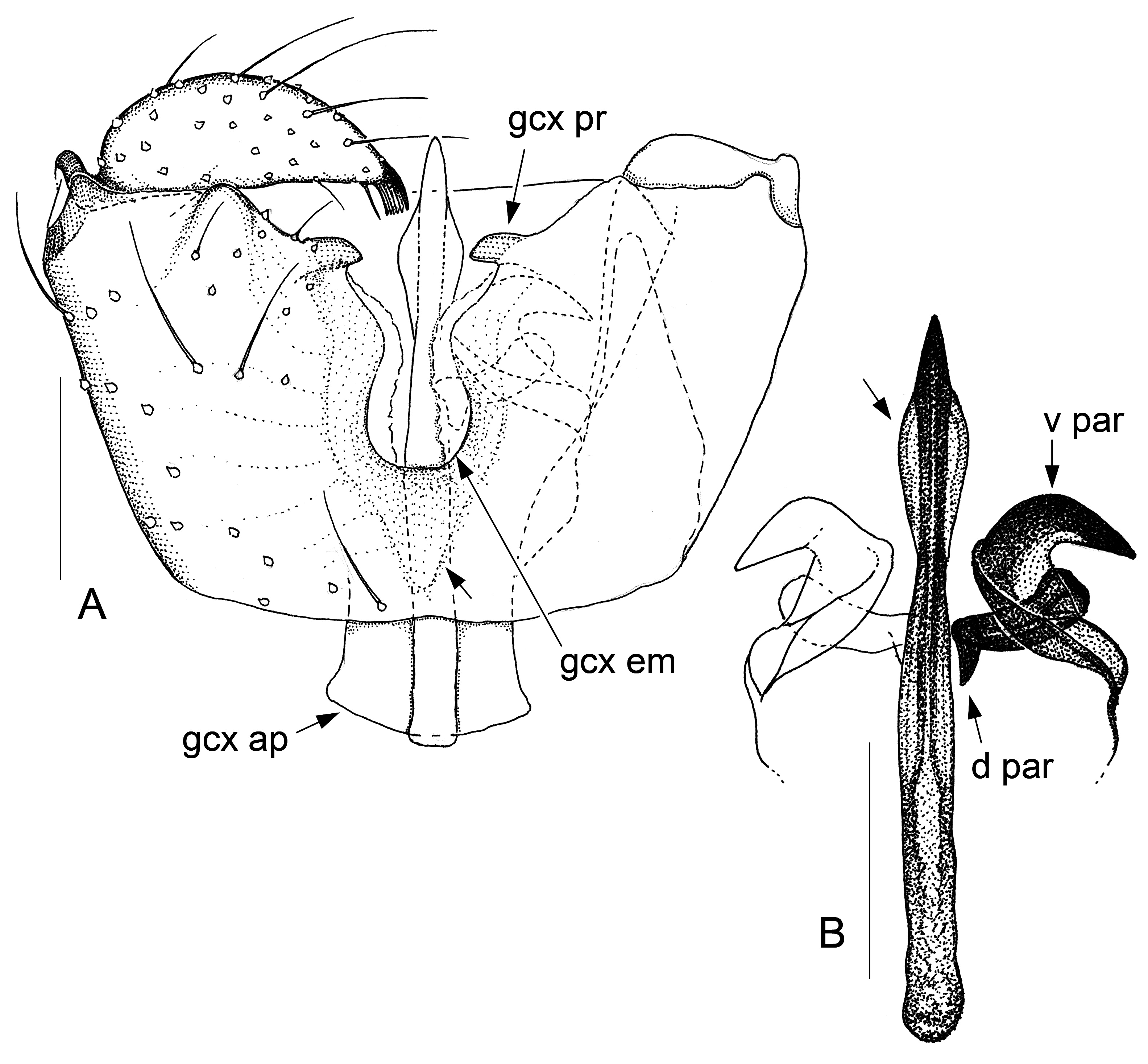
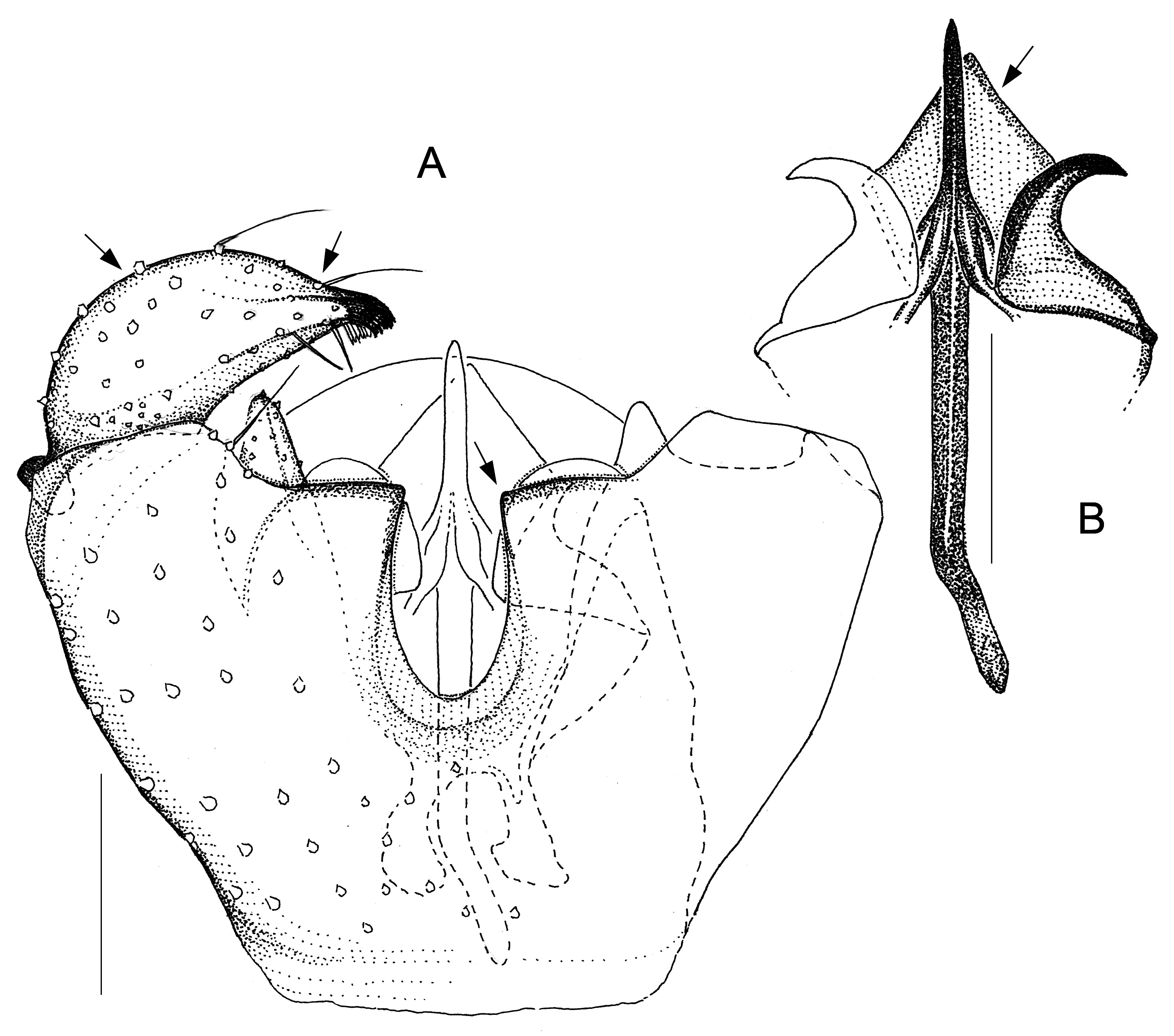
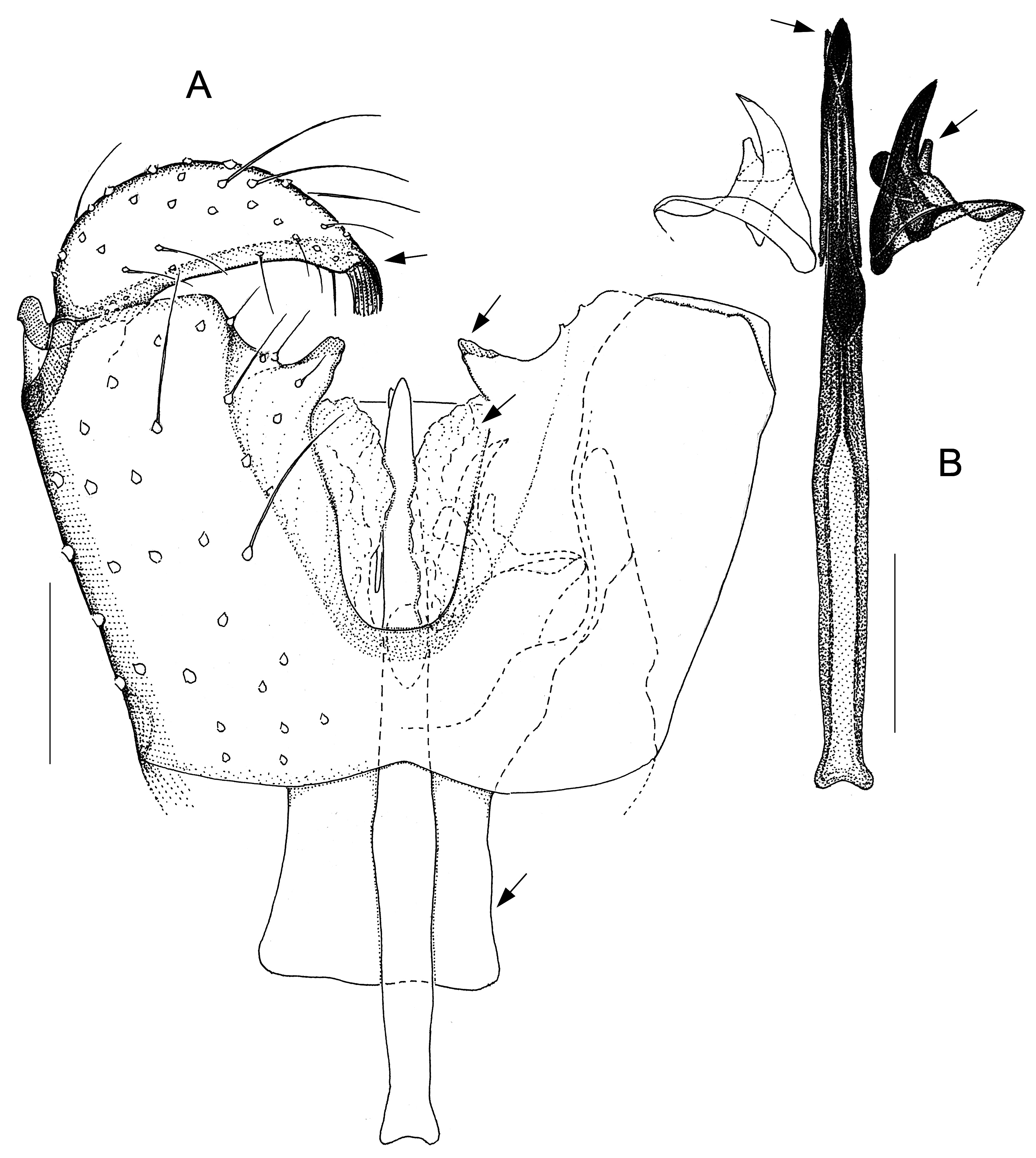
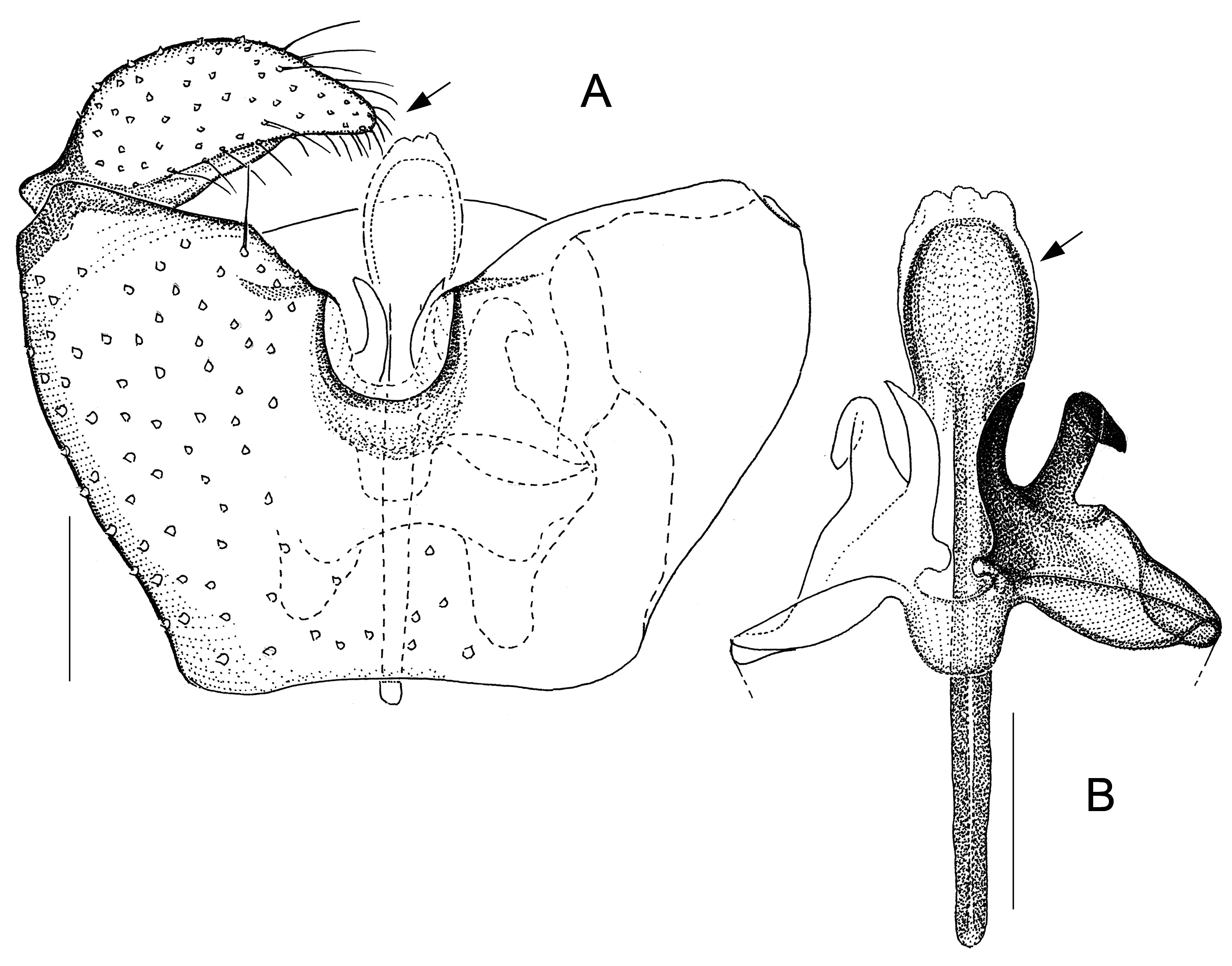
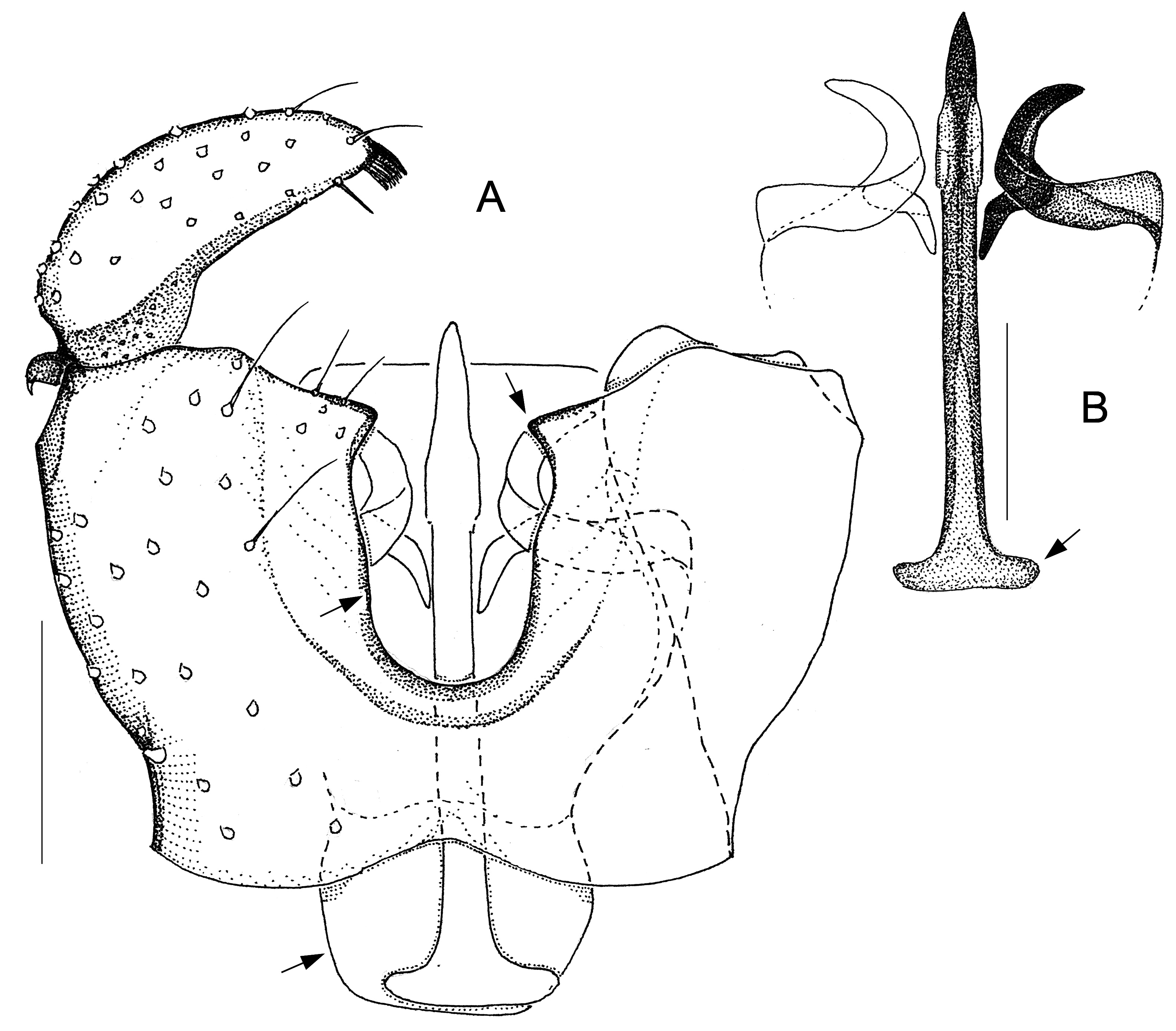
Larvae and females of Antipodosis gen. nov. are unknown, so this genus is based solely on characters of males. Antipodosis gen. nov. are small, inconspicuous Porricondylini , exhibiting the characters typical of that tribe and coming under the group of genera with 14 flagellomeres and without basitarsal spines (“group Aa” in Jaschhof & Jaschhof 2013). Antipodosis gen. nov. stands out from most other Porricondylini in that vein CuA 1 runs parallel to CuA 2 rather than approaching or joining it ( Fig. 1A View Fig versus 1B), and the eye bridge is longer dorsally (5–10 versus 2–3 ommatidia). The structure of the genitalia is genus-specific. Most notably, the two gonocoxal apodemes, which in other Porricondylini are long bars separated from one another ( Fig. 12 View Fig ), tend to merge into a single plate ( Fig. 2 View Fig ); parameres are typically present as two pairs (a ventral and a dorsal pair) of strongly sclerotized tusks interlinked with each other ( Fig. 2 View Fig ); and the ejaculatory apodeme, whose length exceeds that of the gonocoxites, is typically a strongly sclerotized rod with apical modifications.
Differential diagnosis
The genus Antipodosis gen. nov. is similar to Monepidosis ( Jaschhof & Jaschhof 2013) , differing from it as follows: the eye bridge is longer; sensory hairs (= setae with hooded sockets) on the flagellomeres are dispersed rather than aligned to form a single whorl ( Fig. 1C View Fig versus 1D); wings are wider (with length/width ratios usually <3.0); CuA 1 does not approach but runs parallel to CuA 2 ( Fig. 1A View Fig versus 1B); the gonostylar apex bears typically a pectinate tooth ( Figs 2A View Fig , 7A View Fig ), but never a large plate-like spine ( Figs 10 View Fig A–B, 11B); gonocoxites have a distinct ventral emargination ( Fig. 2A View Fig ) and have no central processes ( Fig. 10A View Fig ); and the anterior portions of the gonocoxal apodemes are either short and separated ( Fig. 6A View Fig ), or merged into a single, long plate ( Fig. 2A View Fig ).
Etymology
The name Antipodosis is composed of Antipod -, from the Latin antipodes, for ‘antipodes’, and the ending - osis, from Monepidosis , a closely related genus. Gender is feminine.
Other characters
BODY LENGTH. 1.7–2.3 mm.
HEAD. Postfrons asetose. Antenna longer than body. Scape and pedicel usually yellowish, lighter than flagellum. Circumfila on flagellomeres 1 to 11–14, evenly ring-shaped or slightly sinuous, in A. rotoiti gen. et sp. nov. with short posterior extensions. Neck of fourth flagellomere longer than node; node apart from circumfilum with microtrichia, short setae forming a basal whorl, numerous long sensory hairs ( Fig. 1C View Fig ). Palpus as long as head height or longer, 4 subcylindrical segments.
THORAX. Scutum with a few lateral and dorsocentral setae. Both anepisternum and anepimeron setose.
WING ( Fig. 1A View Fig ). Longer than body. Length/width 2.5–2.9 (in A. australis gen. et sp. nov. exceptionally 3.1). Costal cell narrow, reinforced. Rs strongly oblique, in line with R 5. M usually absent, in A. waipapa gen. et sp. nov. a remnant M present at wing margin.
LEGS. Densely covered with narrow scales. Claws crescent-shaped, with 1 large and 2–3 smaller teeth basally. Empodia rudimentary, as far as known.
ABDOMEN. Tergites 2–5(–6) varyingly strongly desclerotized at center, setae aligned in rows along margins; other tergites evenly sclerotized, setae aligned in 1 transverse row. Sternite 1 unsclerotized, asetose, other sternites evenly sclerotized, setae dispersed. Pleural membrane very sparsely setose.
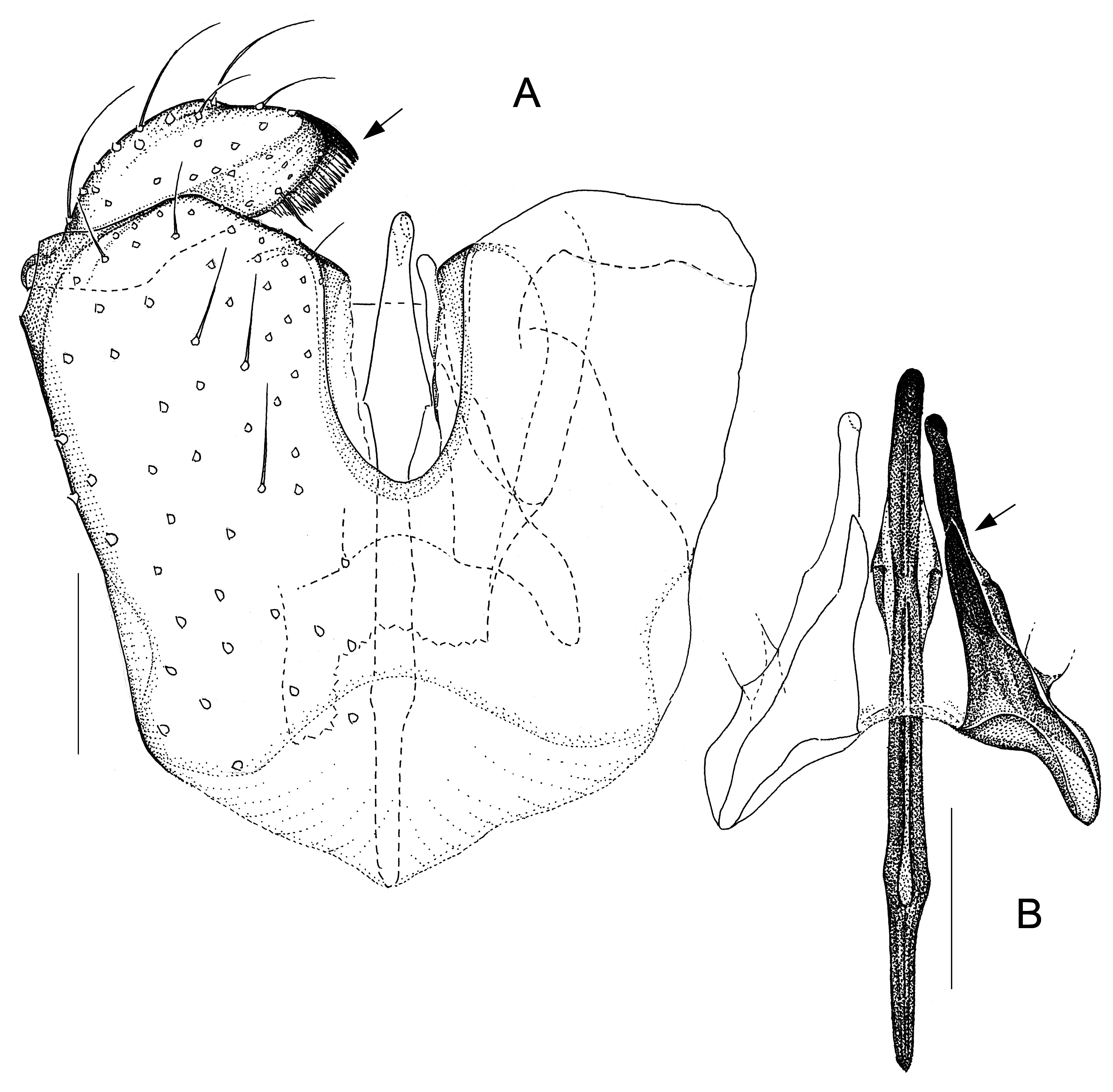
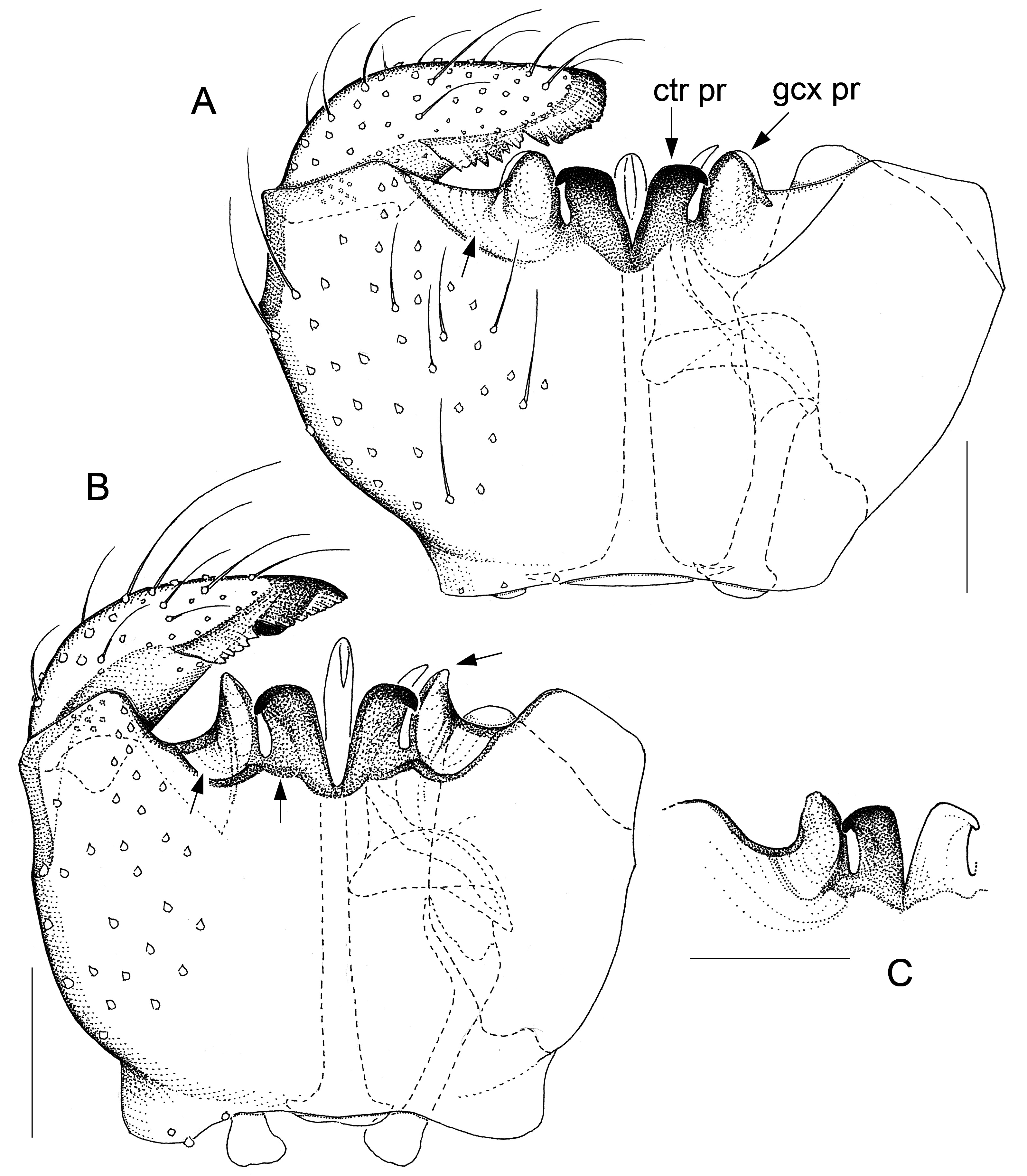
GENITALIA ( Figs 2–9 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig ). Ninth tergite either subtrapezoid or rounded posteriorly. Gonostylus either elongate, tapered towards apex, or flattened, with broadly rounded apex; typically a pectinate tooth apically, 1–2 bristles medially; basolateral apodeme large. Gonocoxites: ventral emargination approximately U-shaped, with glabrous, sclerotized rim; medial bridges sometimes with membranous outgrowths that occupy parts of the ventral emargination ( Figs 7A View Fig , 8A View Fig , 9A View Fig ). Ventral parameres usually bent laterally or dorsolaterally, occasionally interconnected mediobasally ( Figs 3B View Fig , 9B View Fig ); dorsal parameres usually bent dorsally (thus often hardly visible in ventral view); parameral apodemes usually large. Base of ejaculatory apodeme either widened (visible in ventral view, Fig. 5B View Fig ) or flattened (visible in lateral view). Accessory gland ducts, or their merging points with ejaculatory apodeme, distinct. Both hypoproct and cerci present as two setose, medially merged lobes (omitted in illustrations); cerci longer than hypoproct.
Distribution and phenology
The genus Antipodosis gen. nov. is to present knowledge endemic to New Zealand. Of eight species identified, two occur on the North Island and six on the South Island including Stewart Island. Almost
all the specimens known of Antipodosis gen. nov. were collected in native forest in austral summer (Nov.–Jan.).
Phylogenetic remarks
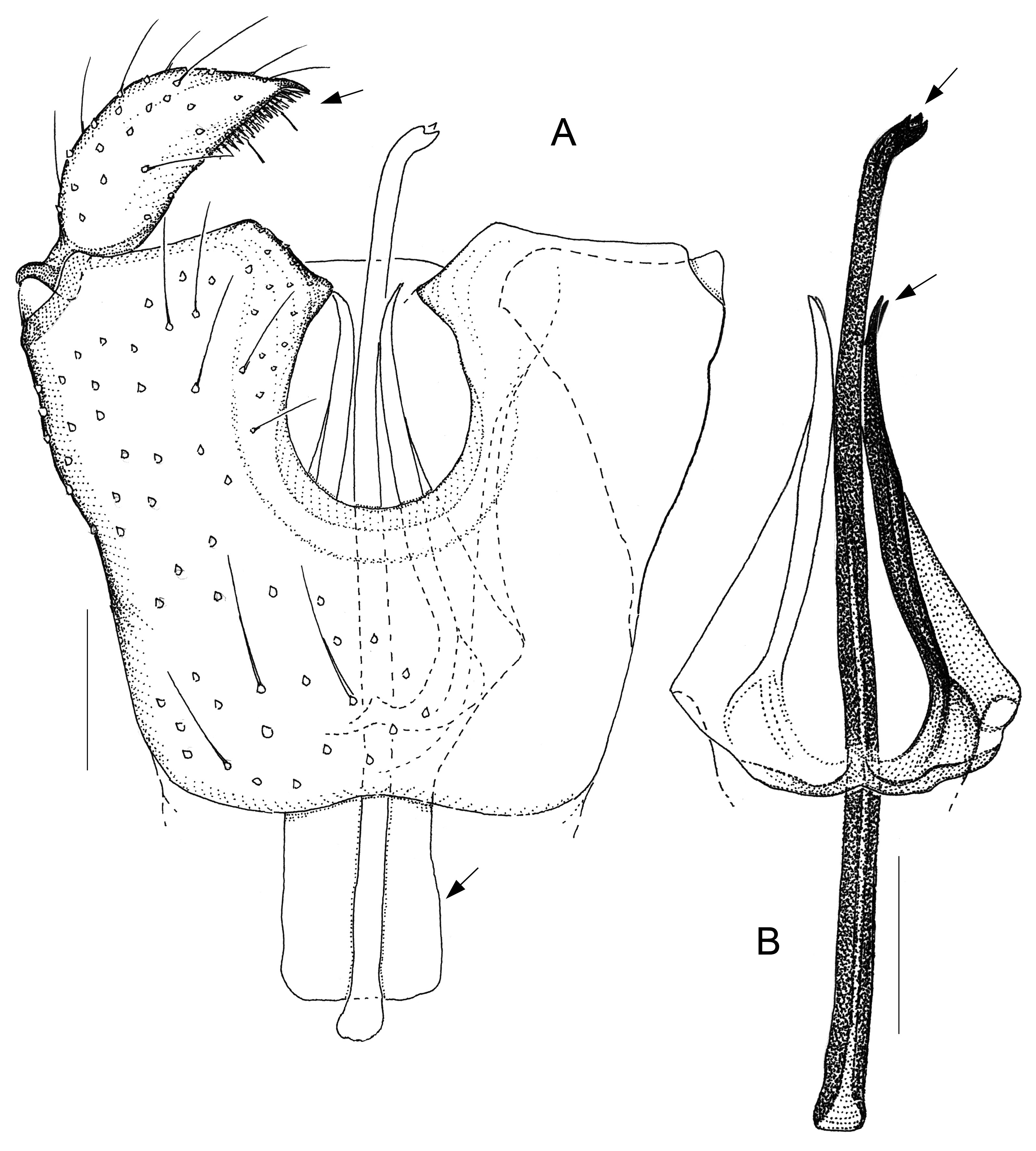
The peculiarity of Antipodosis gen. nov. that gonocoxal apodemes tend to merge into a large plate is a derived character state not known in other Porricondylini , thus an autapomorphy. From what is currently known of world Porricondylini , it appears that Antipodosis gen. nov. is most closely related to Monepidosis . Compelling evidence of this relationship is provided by the parameres and the ejaculatory apodeme, whose basic structure is identical in the two genera. Both are therefore combined in what is here called the Monepidosis group. The relationships of this group to other Porricondylini remain obscure for the time being. With respect to male morphology, Antipodosis gen. nov. has retained more ancestral traits than Monepidosis . For example, in Antipodosis gen. nov. the two gonocoxites are largely separated by the ventral emargination, whereas in Monepidosis they are lengthwise connected, with the connecting bridge even further modified to bear a pair of processes. Furthermore, in Antipodosis gen. nov. the gonostylar apex is equipped with a pectinate tooth of fine, separate spines, which is the structure found in many other Porricondylinae and Porricondylini , whereas in Monepidosis the spines are merged into a large, plate-like tooth that encircles much of the gonostylus’ distal half. Concurrent in Antipodosis gen. nov. and Monepidosis , the basic patterns of parameres and ejaculatory apodeme undergo various modifications, which may be so pronounced that the generic affiliations of the respective species are obscured (see Antipodosis elongata gen. et sp. nov., Fig. 3 View Fig ; Monepidosis shikokuensis sp. nov., Fig. 12 View Fig ). One may argue that such extreme variations are likely to evolve over long periods of time and both Antipodosis gen. nov. and Monepidosis might be phylogenetically old lineages.
Jaschhof M. & Jaschhof C. 2013. The Porricondylinae (Diptera: Cecidomyiidae) of Sweden, with notes on extralimital species. Studia Dipterologica Supplement 20: 1 - 392.
Fig. 1. Male morphology of Antipodosis gen. nov. and Monepidosis Mamaev, 1966. A. Wing of A. granvillensis gen. et sp. nov., holotype, setae omitted. B. Wing of M. shikokuensis sp. nov., holotype, setae omitted. C. Fourth flagellomere of A. rakiura gen. et sp. nov., holotype, lateral. D. Fourth flagellomere of M. scepteroides sp. nov., holotype, lateral. Scale lines: A–B = 0.50 mm, C–D = 0.05 mm.
Fig. 12. Monepidosis shikokuensis sp. nov., Ƌ, holotype. A. Parameres and ejaculatory apodeme, ventral. B. Genitalia, ventral. Scale lines: 0.05 mm.
Fig. 2. Antipodosis australis gen. et sp. nov., Ƌ, holotype. A. Genitalia, ventral. B. Parameres and ejaculatory apodeme, ventral. Scale lines: 0.05 mm.
Fig. 7. Antipodosis rotoiti gen. et sp. nov., Ƌ, holotype. A. Genitalia, ventral. B. Parameres and ejaculatory
Fig. 10. Monepidosis heterocera sp. nov., Ƌ. A. Genitalia, ventral, holotype. B. Genitalia, ventral (paratype). C. Processes at ventroposterior gonocoxal margin, ventral, paratype. Scale lines: 0.05 mm.
Fig. 6. Antipodosis rakiura gen. et sp. nov., Ƌ, holotype. A. Genitalia, ventral. B. Parameres and ejaculatory apodeme, ventral. Scale lines: 0.05 mm.
Fig. 9. Antipodosis waipapa gen. et sp. nov., Ƌ, holotype. A. Genitalia, ventral. B. Parameres and ejaculatory apodeme, ventral. Scale lines: 0.05 mm.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Bibionomorpha |
|
Family |
|
|
SubFamily |
Porricondylinae |
1 (by valdenar, 2020-05-21 13:33:03)
2 (by ExternalLinkService, 2020-05-21 13:38:16)
3 (by ExternalLinkService, 2020-05-21 13:48:34)
4 (by ExternalLinkService, 2020-05-21 15:37:29)
5 (by ExternalLinkService, 2020-05-26 03:40:53)
6 (by ExternalLinkService, 2022-01-29 21:36:07)
7 (by plazi, 2023-10-31 15:36:11)