Aonides orensanzi, Radashevsky, Vasily I., 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4019.1.22 |
|
publication LSID |
lsid:zoobank.org:pub:88F2DB05-58C4-4726-89D5-99302FABB908 |
|
DOI |
https://doi.org/10.5281/zenodo.4658126 |
|
persistent identifier |
https://treatment.plazi.org/id/5E51D737-FFDB-FFAA-FF4A-A0161AC4FD33 |
|
treatment provided by |
Plazi |
|
scientific name |
Aonides orensanzi |
| status |
sp. nov. |
Aonides orensanzi View in CoL n. sp.
( Figs 3 View FIGURE 3 , 4 View FIGURE 4 )
Type material. Queensland: Holotype: AM W.45226, MI QLD 2415. Paratypes: AM W.45220, MI QLD 2330 (3), MIMB 28111, MI QLD 2330 (2); AM W.45221, MI QLD 2360 (1); AM W.45222, MI QLD 2363 (1); AM W.45223, MI QLD 2373 (4); MIMB 28112, MI QLD 2373 (3); AM W.45225, MI QLD 2410 (2); MIMB 28113, MI QLD 2415 (1); AM W.45505, MI QLD 2435 (2).
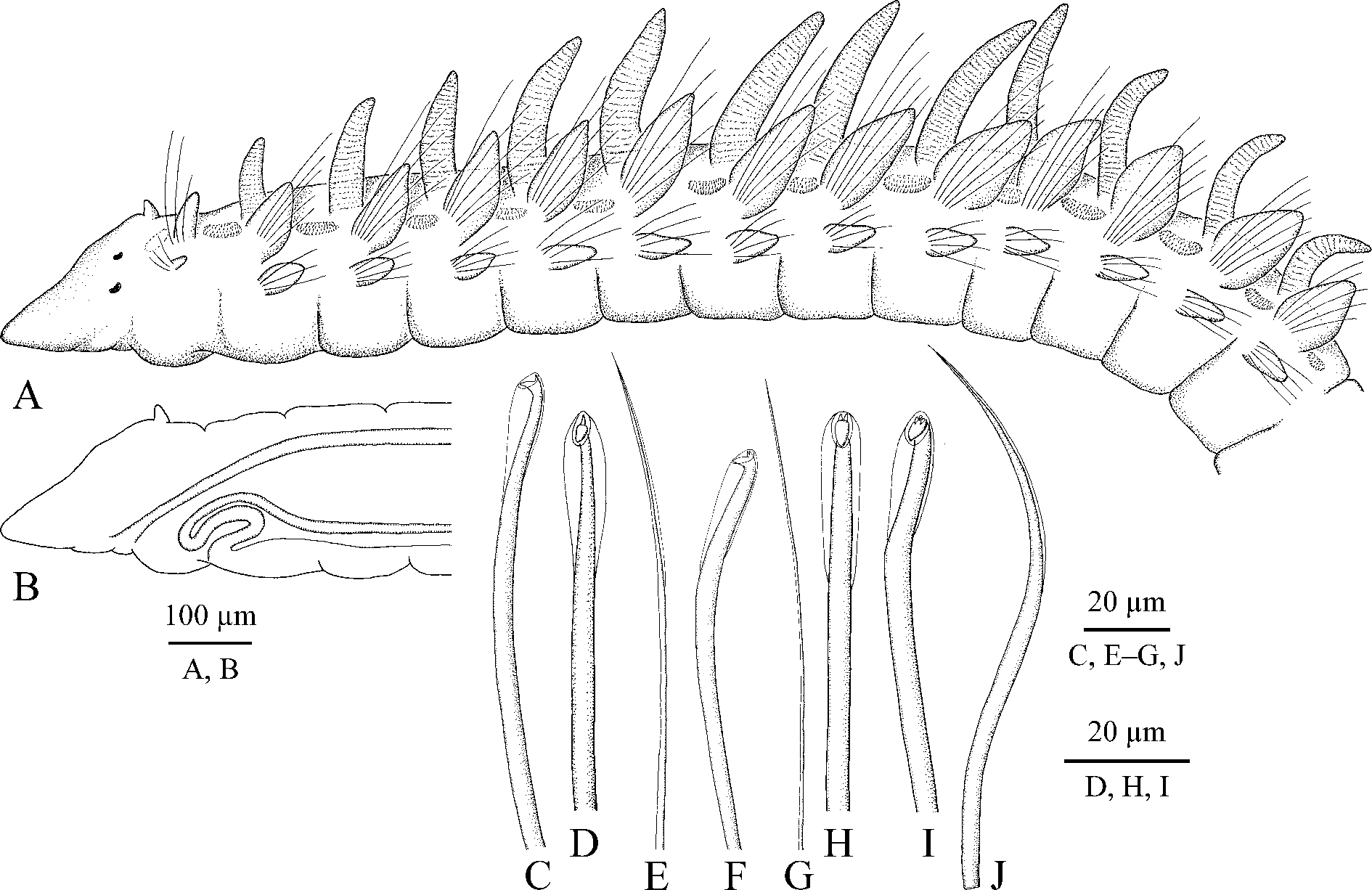
Adult morphology. Up to 12 mm long, 0.4 mm wide for 80 chaetigers; holotype a complete individual about 11 mm long for 77 chaetigers; smallest examined individual about 6 mm long for 50 chaetigers. No pigmentation on body and palps. Prostomium long, anteriorly sharply conical, posteriorly narrowed and pressed into chaetiger 1 but not extending over it as a caruncle. Small rounded knobs with short non-motile sensory cilia irregularly scattered on prostomium. Short finger-like antenna present on posterior most part of prostomium ( Fig. 3 View FIGURE 3 A). Ciliary bands or patches of nuchal organs absent on posterior sides of prostomium. Two pairs of red eyes arranged almost in a straight transverse line; lateral eyes slightly larger than median eyes ( Fig. 3 View FIGURE 3 A). Peristomium reduced to small ring around mouth. Palps as long as 5–15 chaetigers, with frontal longitudinal groove lined with fine cilia, and up to 25 short transverse ciliary bands regularly arranged on inner lateral surface; lateral bands fewer in small individuals; long cilia of bands beating towards distal end of palp.
Chaetiger 1 with capillaries and small postchaetal lamellae in both rami. Postchaetal lamellae on succeeding chaetigers elongated, leaf-like in both rami ( Fig. 3 View FIGURE 3 A). Dorsal crests, lateral pouches and ventral flaps absent.
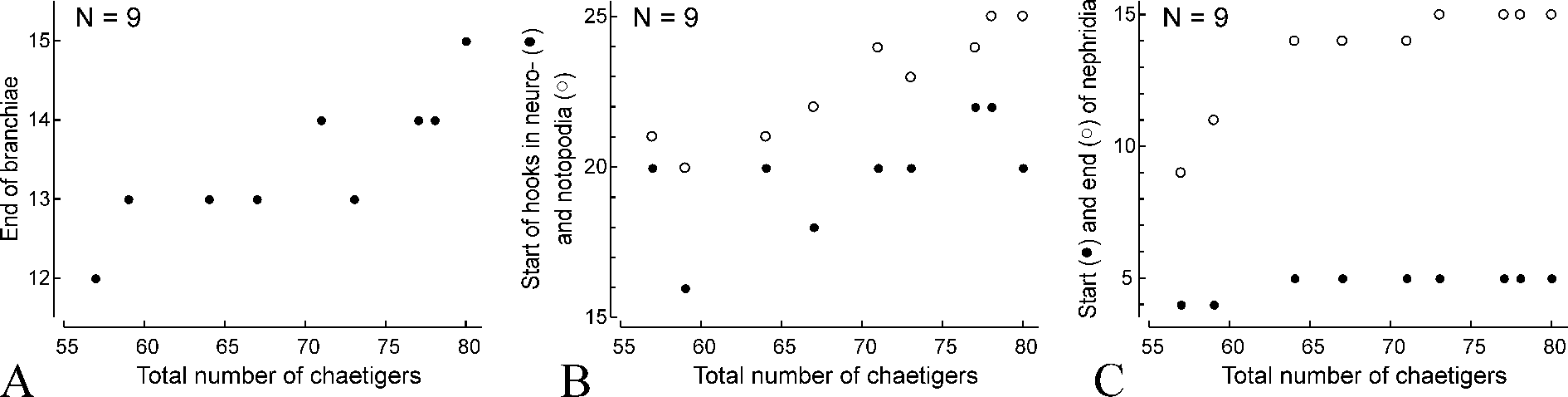
Hooks in notopodia from chaetigers 20–25 ( Fig. 4 View FIGURE 4 B), up to six in a series among capillaries. Hooks in neuropodia from chaetigers 16–22 ( Fig. 4 View FIGURE 4 B), up to six in a series, accompanied by 1–5 alternating capillaries and 1–3 inferior chaetae throughout. Alternating capillaries in anterior neuropodia with narrow limbation ( Fig. 3 View FIGURE 3 E), in posterior neuropodia alimbate, hair-like ( Fig. 3 View FIGURE 3 G). Inferior chaetae in anterior neuropodia capillaries, in posterior neuropodia of big mature individuals they gradually become larger and can be referred to as sabre chaetae ( Fig. 3 View FIGURE 3 J). Hooks in both rami in anterior parapodia bidentate, with upper tooth situated at almost right angle to main fang ( Fig. 3 View FIGURE 3 C, D). Hooks in posterior parapodia tridentate, with two small upper teeth situated side by side above main fang ( Fig. 3 View FIGURE 3 F, H), and occasionally quadridentate, with an additional median superior tooth ( Fig. 3 View FIGURE 3 I). Only outer hood present; no inner subdistal hood.
Branchiae up to 14 pairs, from chaetiger 2 to chaetiger 15, fewer in small individuals ( Figs 3 View FIGURE 3 A, 4A). Branchiae longest on chaetigers 5–7, up to three times as long as notopodial lamellae, gradually diminishing in length on succeeding chaetigers, free from lamellae, robust, flattened, with surfaces oriented perpendicular to body axis, with longitudinal bands of cilia along inner and outer edges. Afferent and efferent blood vessels of branchiae forming a loop and interconnected by radial capillaries giving branchiae annulate appearance.
Dorso-lateral dense bands of short cilia from chaetiger 1 to chaetiger 14, fewer in small individuals. Each band of cilia extending between successive notopodia; bands short and straight to slightly curved on anterior chaetigers, becoming longer and horse-shoe shaped on posterior chaetigers, with lateral sides of horse-shoe directed to midline of body. Short nototrochs present on branchiate chaetigers, each composed by single row of short cilia.
Pygidium usually with five cirri, comprising one pair of ventral cirri, a midventral cirrus and one pair of slightly longer and thicker dorsal cirri; one individual (of seven examined complete individuals with pygidia) with four cirri, and one individual with six pygidial cirri.
Oesophagus extending through 13–15 anterior chaetigers. Ventral buccal bulb below oesophagus extending to middle of chaetiger 2 ( Fig. 3 View FIGURE 3 B). Gizzard-like structure in digestive tract absent.
Main dorsal blood vessel transformed into gut sinus in anterior part of midgut. Heart body up to 10 µm in diameter inside main dorsal vessel extending from chaetigers 4–6 to chaetigers 13–15. Blood red, without elements.
Nephridia from chaetiger 4 in small individuals and from chaetiger 5 to chaetiger 15 in individuals with more than 60 chaetigers ( Fig. 4 View FIGURE 4 C).
Reproduction. Aonides orensanzi n. sp. is gonochoristic. Both in females and males gametes develop from chaetigers 16–17 throughout most part of body. Oogenesis is intraovarian. Vitellogenesis occurs when oocytes grow attached to segmental blood vessels. Vitellogenic intraovarian oocytes are up to 130 µm in diameter, with a germinal vesicle about 40 µm and a single nucleolus 13 µm in diameter. Oocyte envelope is 3–4 µm thick, with honey-combed external surface and a circle of about 30 vesicles associated with inner surface; each vesicle is about 10 µm in diameter. Sperm morphology and spermatogenesis are unknown.
Remarks. Adult Aonides orensanzi n. sp. appear similar to A. californiensis , A. mayaguezensis , A. oxycephala and A. trifida by the presence of an occipital antenna on the prostomium. The antenna was not described but was illustrated on the posterior edge of the prostomium in A. californiensis by Rioja (1947: fig. 11). Aonides orensanzi n. sp. differs from these species in having bi-, tri- and quadridentate hooks in same individual. Such variability of hook dentition is herein reported for the first time for Aonides .
Aonides orensanzi View in CoL n. sp. collected off Lizard Island in August had intraovarian vitellogenic oocytes up to 130 µm in diameter, each with a circle of about 30 vesicles associated with inner surface. Two circles of vesicles were described by Hannerz (1956), Sveshnikov (1967), and Lebsky (1970) in the oocytes and early larvae of A. oxycephala View in CoL and A. paucibranchiata View in CoL . One circle of vesicles instead of two in the oocytes of A. orensanzi View in CoL n. sp. may be due to incomplete development of the oocytes.
Etymology. The species is named in honour of Jose Maria (Lobo) Orensanz, biologist, colleague and a great man.
Habitat. Adults of A. orensanzi n. sp. were found in coral sand on coral reefs at 5–16 m depth. Worms were likely crawling free in the coral sand.
Distribution. Great Barrier Reef, Australia.
| MIMB |
Museum of the Institute of Marine Biology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.